Introduction
To fight viral or bacterial infections effectively without endangering their own viability, vertebrate organisms rely on the ability of their adaptive immune system to distinguish self- from non-self agents. Among the many components of the adaptive immune system, the major CD4+ and CD8+ subsets of αβ T cells recognize antigens from these infectious organisms primarily in the form of peptides bound Major Histocompatibility Complex-encoded molecules (pMHCs). The cellular processes that generate and load peptides onto MHC molecules, then export the resulting pMHC ligands to the cell surface for recognition by the receptors of T cells, do not generally discriminate between pathogen-derived and self-derived proteins or translation products. This leaves the critical task of ligand discrimination to the T lymphocyte itself - T cells must be able to be efficiently activated by infected or professional antigen presenting cells (APCs) bearing foreign ligands, while remaining for the most part in a naïve state upon interaction with normal (uninfected) cells.
This sharp discrimination has been documented experimentally both with functional assays (involving responses spanning hours to days – e.g. cytokine secretion, cytotoxicity, proliferation) or signaling assays (dealing with responses in the minute to hour range – e.g. calcium influx, kinase activity). These assays revealed that a T cell expressing a particular αβ receptor shows distinct responses to structurally related pMHC ligands, with a rather clear separation between agonists (which trigger T cell activation and are typically associated with receptor ligands derived from proteins of infectious agents) and non-agonists (which fail to trigger T cell activation and comprise the bulk of ligands with peptides derived from broadly expressed self proteins) [1, 2]. This distinction can be refined with subcategories of (1) strong agonists, which trigger all functions of T cells, even at low presentation levels, (2) weak/partial agonists which trigger T cells only when presented at very high dose and may stimulate only some of a cell’s potential outputs, (3) antagonists, which not only fail to trigger functional responses from T cells but also may diminish activation induced by agonist ligands when the two ligands are presented on the same APC, (4) synergistic endogenous peptides, which enhance the response of T cells to small quantities of agonist peptides, but fail to trigger these cells on their own [3], and (5) null ligands.
A major challenge in the field of ligand discrimination by T cells is the construction of a detailed, quantitative, mechanistic model that accounts for the ability of small differences in TCR-ligand binding to give rise to the extremely wide divergence in the dose-responses characteristic of closely related pMHCs interacting with the same TCR. Such model building is made difficult by the need to ensure that mechanisms implemented to account for threshold setting in response to variant pMHCs are also compatible with other known properties of T cell activation (namely its sensitivity and speed [4]).
In this review, we will discuss how ligand discrimination by T cells came to be viewed as the result of kinetic thresholding rather than allosteric regulation and present recent models showing how incorporating differential signaling feedback pathways into a kinetic proofreading scheme can explain the bulk of available results and predict new behaviors that have been verified by experiment. We will also discuss how these schemes potentially provide a quantitative explanation for adaptation of the ligand discrimination properties of T cells during their differentiation and maturation.
1. Biophysics of T cell ligand discrimination
1.1 Ligand discrimination involving allostery: an Arlésienne with conceptual challenges
Activation of T lymphocytes is triggered by the engagement of their receptors (TCR) with pMHC ligands on the surface of antigen-presenting T cells. In contrast to co-evolved receptor-ligand pairs for which signaling secondary to ligand-induced conformational change can be easily understood to have been selected over long (evolutionarily relevant) time periods, there is no such genetic relationship between TCR fine specificity and ligand structure. This raises the question of whether it is likely that the agonist properties of pMHC ligand would depend on the capacity of the pMHC to induce a common structural change in the engaged receptor.
Soon after the primary structure of the TCR was determined, Rojo and Janeway showed that monoclonal antibodies raised against these receptors possessed differing capacities to activate a particular T cell [5]. This was true even after accounting for the extent of TCR binding and affinity of the antibodies. These investigators interpreted their data in terms of a requirement for specific conformational changes in the TCR upon ligand binding to achieve signaling capable of driving cellular activation, although other interpretations such as differential binding to sites that did or did not permit TCR association with CD4 coreceptors associated with the key kinase Lck offered other possible explanations of these data. Another intriguing study by Reich et al. monitored the interaction of soluble TCR and pMHC by dynamic light scattering [6]. These investigators showed that TCR-pMHC pairs could aggregate and that this clustering occurred only for pMHC with agonist potential. This specific aggregation was hypothesized to correspond to a specific conformational change in the TCR induced by agonist pMHC binding. However, this result has not been confirmed in other systems and has not been invoked in more recent models of TCR triggering. It also is compatible with an alternative interpretation in which only TCR-agonist pMHC binding aligns the components in such a way that weak conserved protein-protein interactions add to the avidity of association, stabilizing the oligomers.
Evidence for induced TCR conformation changes by agonist pMHC ligands was appealing because if agonist ligands (and only such ligands) were able to specifically induce structural alterations in the TCR complex that led to effective signaling, many questions about T cell biology would be solved at once. First, a T cell’s response upon ligand engagement could be understandably fast (conformational changes within protein complexes occur with characteristic timescales in the millisecond to sub-second range). Second, activation could be extremely sensitive (with only few agonist ligands sufficient to trigger the signaling cascade). Finally, activation could be very specific (if sequence variation in the loaded peptide were to enable or abrogate the specific conformational change, specificity would be completely defined at the level of the ligand-receptor interaction).
This molecular solution to the issue of ligand discrimination by T cells is appealing but remains an Arlésienne in the field (the myth of the Arlésienne relates the story of the Provençal village of Arles where tragedy strikes a young man enamored with a Dame -l’ Arlésienne- whose beauty is praised, but who is never to be seen). Even though many structures of TCR-pMHC pairs have been solved [7, 8], often showing large conformational changes of the ligand binding loops of the TCR during ligand-receptor association (consistent with a correspondingly large activation barrier [9, 10]), to our knowledge, no crystallographic study has yet identified a peptide-specific conformational change that would propagate to the cytoplasmic tail of the TCR, and/or to the TCR-associated chains and correlate with the functional potency of the ligand in activating T cells.
Ligand discrimination through conformational specificity would also have to resolve two crucial features of T cell biology: robustness despite receptor variability and flexibility during thymic positive selection. If T cell ligand discrimination were to rely on specific conformational changes, one would have to account for how it is conserved despite receptor variability. One could argue that there is no need of such robustness as positive selection in the thymus would weed out unresponsive T cell progenitors. But this very process of positive selection in turns poses a conceptual challenge to the possibility of ligand discrimination through specific conformational change: how would the TCR signaling machinery get triggered upon recognition of self-MHC in the thymus, while only agonist ligands (i.e. non-self) ligands (and only these) would induce the required specific conformational change in mature T cells in the periphery? One could conjecture that modifications of the TCR complex (such as the glycosylation of CD8 on thymocytes [11]) would achieve such signaling adjustment between self-recognition during thymic positive selection and self-ignorance in the periphery. Again, we are unaware of any evidence supporting this possibility.
We do not mean to imply that ligand-induced physical changes in the TCR signaling machinery as an ensemble may not be crucial to T cell stimulation. There may be critical alterations in the organization of elements of the TCR multiprotein complex that allow the translation of pMHC-TCR-engagement into kinase activation (an alternative being a molecularly simpler activation through TCR-clustering and/or TCR/CD4 or TCR/CD8 cross-linking). An example of such a change in the TCR complex was reported by Aivazian and Stern [12]: phosphorylation of the TCRζ unit was shown to correlate with membrane-deanchoring and unfolding of the ζ chain away from the inner plasma membrane lipid bilayer. Gil et al. [13, 14] have reported that Nck is recruited by CD3ε at the early stages of T cell activation, an event they attribute to a cryptic proline-rich region in CD3ε that undergoes a conformational change following TCR engagement by agonist pMHC.
However, it is unclear how these reported conformational changes can be specific for the agonist (foreign-derived) ligands that trigger peripheral mature lymphocytes, based solely on the nature of the pMHC-TCR interaction. Positive selection of thymocytes involves ligands that are non-agonists for mature T cells. Yet these pMHC ligands activate the TCR signaling machinery and many if not all of the same downstream pathways (involving Lck, ZAP-70, LAT, SLP-76, and so on) in immature T cells, as do agonists capable of activating mature T cells. Hence, conformational changes in the TCR machinery, if these occur upon ligand binding, cannot account on their own for functional discrimination. Absent crystallographic evidence for an allosteric mechanism, we and others in the field have focused on a more biophysical view that involves kinetic and dynamic explanations for ligand discrimination.
1.2 Dynamics of ligand discrimination
Fifteen years of biophysical characterization [15] suggest that the best predictor of the quality of activation for a T cell bearing a particular receptor (TCR) interacting with a particular ligand (pMHC) is the lifetime of this ligand-receptor complex (we will refer to this experimental result as the “lifetime dogma”). These lifetimes have been typically estimated by monitoring the interaction of purified-soluble pMHC and TCR with the surface-plasmon resonance (SPR) technique. It is important to note that there are some experimental observations that challenge this “lifetime dogma”. In particular, many groups have reported examples of pMHC-TCR pairs whose lifetime according to the general ‘rules’ for such correlations was either too long to be a non-agonist yet failed to stimulate a T cell response or too small to be a good agonist yet evoked measurable responses. One must immediately point out that all these counter examples to the common rules involve room-temperature measurements of the pMHC-TCR interaction. Due to the extensive contact surface and substantial conformational changes of binding domain loops involved in pMHC-TCR association/dissociation, one can anticipate large thermodynamic characteristics (enthalpy, entropy and heat capacity), such that an extrapolation of room-temperature measurements to physiological temperature is at best hazardous. A more thorough study of this temperature effect has been presented by Krogsgaard et al. [16]. The authors showed that the lifetime of the 2B4 TCR interacting with I-Ek associated with either of two variants of the MCC peptide, as measured at room temperature with purified soluble proteins, did not correlate well with the functional potency of these pMHCs in terms of IL-2 production by the 2B4 T cells. The authors then systematically measured the temperature dependence of these pMHC-TCR interactions, pointing out that large heat capacities were associated with poor complex lifetimes at room temperature. Krogsgaard et al. originally used a phenomenological multiplication of lifetime and heat capacity to restore the correlation between thermodynamic and functional data [16]. Revisiting these data, Qi et al. computed corrections for the lifetime τ1/2(2D) of membrane-bound molecules compared to the lifetime τ1/2(3D) of ligand/receptor complexes in solution [17]: τ1/2(2D)= τ1/2(3D)*exp(−B.ΔCp) where ΔCp is a heat-capacity change and B is a constant parameter. Qi et al. derived this formula from first principle statistical physics by taking into account limited vibrational modes for proteins on membranes. Note that the same formula could have been derived from a simpler thermodynamic argument (extrapolating free energy at room temperature and at 37°C would have yielded a comparable result). Qi et al. applied this correction for the lifetime of the pMHC-TCR complex (at 37°C on membranes instead of room temperature in solution) and reconciled the hierarchy of lifetimes with the hierarchy of functional activation [17].
A second set of challenges to the lifetime dogma comes from measurements based on the use of tetramer debinding to estimate of the lifetime of pMHC-TCR complexes [18, 19]. Tetramers are oligomers of pMHC that are used routinely to stain specific T cell clones. Crawford et al. showed that, for these molecular constructs, the staining levels correlated linearly with the lifetime of a particular TCR-pMHC as measured by SPR [20]. However, there are potential problems associated with the generalization of this observation to other systems and the underlying details of pMHC tetramer - TCR binding/debinding remain murky [21]. For example, Cameron et al. [22] reported that specific staining of T cells with pMHC tetramers correlated with endocytosis of these TCR ligands (in turn, this endocytosis may itself be activation-driven).
We report here two intriguing experimental facts related to the “lifetime dogma”. First, when reviewing a list of pMHC-TCR lifetimes [15], we noticed that, if there were a lifetime threshold to distinguish non-agonist ligands from agonist ligands, this threshold could not be universal for all T cell clones. For example, SIINFEKL/H-2Kb (an agonist) and EIINFEKL/H-2Kb (a non-agonist) bind to OT-1 TCR at room temperature for 31.5s and 10.6s respectively [23]; for the 3L2 TCR clone, Hb64-76/I-Ek (an agonist) binds for 10.8s while Hb64-I72-76/I-Ek (a non-agonist) binds for 2.3–3.4s [24]: thus, a lifetime of 10s corresponds to an agonist for 3L2 TCR but to a non-agonist for OT-1 TCR (a simple explanation for this discrepancy could be that 3L2 expressed a class-II-restricted TCR while OT-1 expresses a TCR that is class-I- restricted, but that still removes the discrimination from the TCR-pMHC interaction per se to some factor distinguishing CD4 and CD8 T cells, including the coreceptors, which have been claimed to bind with very different affinities to their respective MHC ligands). Our second observation is that the ratio of lifetimes between a typical agonist and the best non-agonist is about three to five (see Table 2 in [15]). These observations are of course mostly circumstantial and lump together experimental results derived from different systems by different groups. Yet they raise three fundamental issues that need to be addressed quantitatively: namely, is there a lifetime threshold for pMHC-TCR interaction that enables functional ligand discrimination? If so, how is this threshold defined by the dynamics of cell signaling biochemistry? Finally, is this threshold tunable for different lymphocyte differentiation states or is it set by the structure of the TCR?
1.3. Speed, sensitivity and specificity: the S3 characteristics of T cell activation
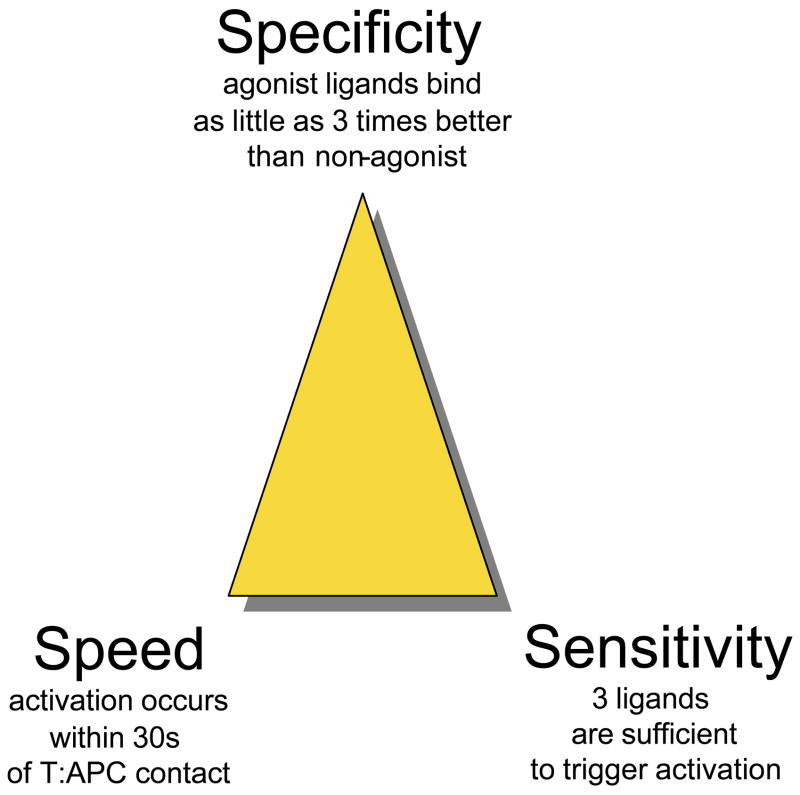
Successful theoretical models for T cell signaling must simultaneously account for the exquisite specificity of pMHC discrimination, the high sensitivity of activation (a few ligands being sufficient to trigger the response), and the speed of the biochemical response (calcium or ERK elevations have been documented to occur within a few seconds of initiating TCR occupancy). Here we discuss briefly these core S3 characteristics (for Specificity, Speed and Sensitivity) that are critical to the function of T cells in the adaptive immune response.
Speed
T cells must be able to sense activating stimuli quickly: the average time that T cells spend in the vicinity of one particular antigen presenting cells in vivo is limited to allow efficient scanning of all antigen-presenting cells (innate signaling may focus the motion of naïve T cells towards activated APC [25], but that does not alleviate the need to limit time spent in futile T:APC interactions). Based on intravital imaging data [26–28], T cells spend 1–5 min with dendritic cells if they fail to find pMHC ligands engendering a ‘stop’ signal [29], and their activation (at least in terms of integrin upregulation that stops motion and promotes effective synapse formation) must be at least that fast.
Sensitivity
T cells have been shown to respond to very few (<10) ligands on the membrane of a presenting cell [3, 30]: this characteristic is in fact critical for identifying pathogens early and efficiently during an infection.
Specificity
specificity is the hallmark of lymphocyte activation in the adaptive immune system, hence it has been the focus of theoretical efforts. As pointed out in the previous section, a three- to five-fold decrease in the pMHC-TCR lifetime distinguishes a non-agonist from an agonist ligand, with a corresponding difference in potency of at least 104 [4] (the potency being defined by the minimal number of ligands that trigger a response). Note that this sharp discrimination appears restricted to T lymphocytes: receptors on B cells have been reported to show a linear correlation between the potency of a particular ligand and its receptor binding capabilities i.e. changing the ligand affinity to the receptor by a factor of 10 does scale with a change of 10 in potency [31].
High sensitivity in T cell responses was first seen in “slow” functional tests (cytotoxicity assays are typically run over 1–3 hr [30]) but this characteristic has been confirmed in signaling tests (calcium response integrated over 10 min [3] or MAPK phosphorylation response after 3min [4]). In the latter assay, specificity was directly tested and revealed that 105 altered peptide ligands (whose binding to TCR had been measured to be at most 5 times worse than the strong agonist at room temperature) were still not sufficient to induce any MAPK phosphorylation. Hence, S3 characteristics must be reconciled together in any satisfactory theoretical model of T cell activation (Figure 1). Moreover, an acceptable model of T cell activation should account for the existence of antagonism (whereby the presentation of large quantity of sub-threshold ligands can inhibit the response to agonist ligands), the role of endogenous ligands (which can amplify the response to agonist ligands), as well as the tuning of ligand discrimination during thymic development or maturation (more on this below). In the following section, we will review critically theoretical models of T cell activation for their ability to handle all S3 characteristics and more.
Figure 1.
golden triangle of T cell activation. T cells display speed, sensitivity and specificity (S3 characteristics) in their activation upon engagement with pMHC ligands. This cartoon is presented to illustrate the minimum requirements a model of T cell activation should fulfill.
2. Theoretical understanding of ligand discrimination
For the sake of quantitatively understanding T cell ligand discrimination, theoreticians are thus left with the “lifetime dogma”, with the TCR-pMHC lifetime as the single biophysical parameter determining the functional activation of T cells. In fact, as the lifetime dogma emerged, specific quantitative models were proposed showing how exquisite ligand discrimination could be achieved when there were only “minute” biophysical differences in pMHC-TCR lifetimes between agonists and non-agonists.
2.1. Kinetic proofreading and its limitations
The first conceptual leap towards understanding ligand discrimination at a mechanistic level was made by McKeithan in 1995 [32], who applied the concept of kinetic proofreading to pMHC-TCR interactions. In this model, the interaction of pMHCs with TCRs activates kinases that phosphorylate TCR-associated chains. Upon dissociation of the pMHC-TCR complex, phosphatases efficiently strip the TCR-associated chains of their phosphates. Proofreading is set by kinase-mediated phosphorylation whose kinetics “clocks” the lifetime of interactions between TCR and pMHC in the face of otherwise excess levels of competing tyrosine phosphatase activity. The quantity of fully-phosphorylated TCR-associated chains ultimately scales with the quantity of pMHC convolved with the quality of TCR-pMHC as a power law with the number of proofreading steps as an exponent (see protocol S1 in [4]). In other words, by adding proofreading steps, the TCR machinery would amplify in a non-linear manner the difference between two pMHC-TCR pairs based on the lifetime of their interactions.
This original attempt at explaining T cell ligand discrimination through a kinetic proofreading model has been the blueprint for many models of kinetic thresholding [32–34]. Yet, in their most straightforward incarnations, these models are not compatible with the S3 characteristics of T cell activation: one can increase specificity by adding kinetic proofreading steps (there are 20 potential kinetic proofreading steps associated with the 20 tyrosine phosphorylations of sites in the ITAM-containing chains of the TCR complex – 12 in the ζ dimer, 4 in the two epsilon chains, and 2 each in the delta and gamma chains), but this occurs at the expense of speed (each proofreading step must be slow enough to discriminate between agonist pMHC and non-agonist pMHC binding to TCR –see protocol S1 in [4]). Moreover, proofreading is limited because ZAP70 kinase binds tightly to fully phosphorylated ITAMs, preventing rapid dephosphorylation upon pMHC debinding, introducing a lag in the reverse direction that lessens the discrimination gap between good and poor pMHC binders. Finally, all signals filtered by kinetic proofreading schemes are greatly attenuated as one proceeds downstream in the pathway, while T cell responses rely on strong molecular responses (signaling amplitudes can be estimated to be 3 million molecular events – e.g. calcium ions entering the cytoplasm or phosphorylation of MAPK - per cell after 3min), and these responses can be triggered by very few ligands (perhaps as few as 1 [3]): it is extremely difficult to account for the ability of a single ligand to elicit such a robust signaling response by a kinetic proofreading scheme alone. For all these reasons, this algorithm alone is by itself insufficient to explain the S3 characteristics of T cell activation.
2.2 Spatial segregation of signaling proteins
A striking observation in the field of T cell biology is the existence of immunological synapses in lymphocytes: upon activation, T cells reorganize their plasma membrane, under optimized conditions generating a high concentration of TCRs in the center of the contact zone surrounded by a ring of larger adhesion proteins [35, 36]. The functional role of the immunological synapses remains a subject a debate (Does it enhance T cell activation by segregating CD45 away from concentrating signaling receptors [37, 38]? Or does it limit overstimulation by enhancing internalization, inactivation, and degradation of engaged TCRs [39])?). Despite these competing views, some have tried to apply the characteristics of the synapse involving long distances (microns) and time scales (minutes) to molecular models in which kinases and phosphatases segregate in the T:APC zones to yield activation.
All of these models fail to account for the speed of T cell activation as they required 60 min [40], 30 min [41], or 2000s [38] of integration time to register substantial ligand discrimination. Even though these models offer an appealing idea, their implementation falls short of reconciling the speed and specificity of characteristic of the T cell signaling response. More specifically, the timescales associated with membrane diffusion (the passive process associated with membrane phase segregation) are too slow (typically D<0.1μm2/s) to help discriminate between ligands whose binding lifetimes are in the second range. Thus, macroscopic segregation of signaling proteins in the T:APC area operates on too long a timescale to enforce ligand discrimination. More recent claims that microclusters are involved in the early signaling events in T cells and that these small units coalesce to form larger synaptic structures are more compatible with the relevant spatiotemporal scale, but the molecular organization of these microclusters is unresolved at present and no models of ligand discrimination have been developed based on their characteristics.
2.3 Differential feedback signaling
Most kinetic proofreading and membrane segregation models rely on a fixed-timescale discriminator (a tonic kinase activity for the former, membrane diffusion for the latter) to achieve ligand discrimination. These fixed-time discriminators seriously limit the ability of the signaling machinery to amplify minute differences in pMHC-TCR lifetime over a large range of pMHC density.
Following the work of Stefanova et al. [42], we implemented a model based on a classical kinetic proofreading scheme with differential signaling feedbacks to account for the S3 characteristics of T cell activation [4]. The conceptual improvement of our model is that competition between signaling feedbacks dynamically generates a robust bifurcation in the signaling capabilities of the receptor pool when confronted with pMHC ligands with only small differences in their receptor’s binding lifetime.
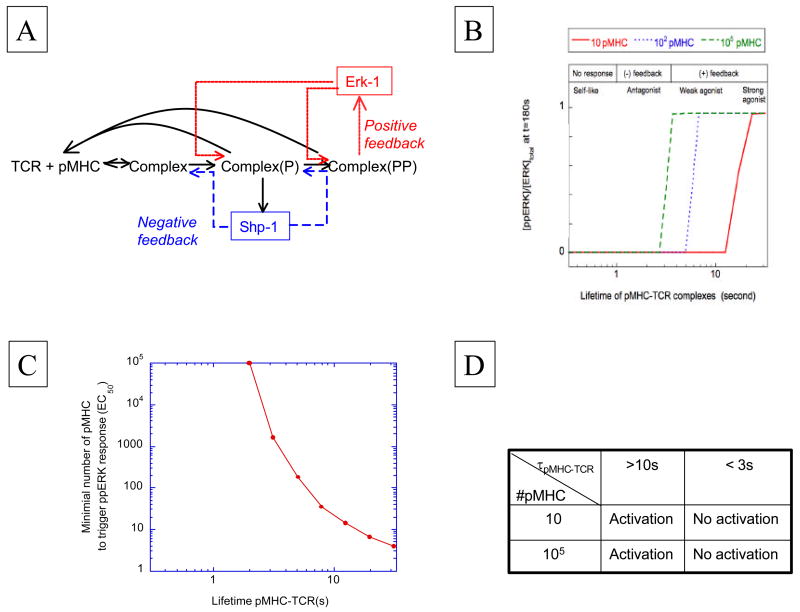
Briefly, this model proposed that engagement of TCR by pMHC ligands triggers a src-family kinase (Lck) that add phosphates to ITAM-containing TCR-associated chains, while also modifying a phosphatase (SHP-1) that is recruited to Lck associated with the TCR complex. This recruited phosphatase acts as a negative feedback by dephosphorylating the activation site of the kinase as well as phosphate-modified TCR-associated chains involved in the initial TCR-driven signaling response (Figure 2A). The ultimate pro-stimulatory event in this scheme is the digital activation of the MAPK pathway, which acts as a positive signaling feedback (or feedforward stimulus) by inhibiting the action of the SHP-1 phosphatase [42] (in other words, providing positive feedback by way of negative feedback on a negative feedback). We showed that this model achieves ligand discrimination with all of the S3 characteristics accounted for through a combination of kinetic proofreading and competition between two regulatory circuits linked to TCR engagement (figure 2B).
Figure 2.
model of TCR signaling response through differential activation of kinase/phosphatase feedbacks (from [4]). A) Sketch of the signaling network: early TCR engagement with pMHC yields src-kinase activation and phosphorylation of TCR-associated chains. This same kinase activates SHP-1 phosphatase as a negative feedback, while the TCR chain phosphorylation promotes ERK-1 kinase activation that provides a positive feedback. B) Output from the computer model in [4] for different numbers of pMHC: simulation of ERK response with differential feedback is compatible with the speed/sensitivity/specificity of T cell activation. C) Non-linear dependence of pMHC potency on the lifetime of pMHC-TCR complex. D) Table recapitulating the results of the model in [4] for 3-min MAP kinase response.
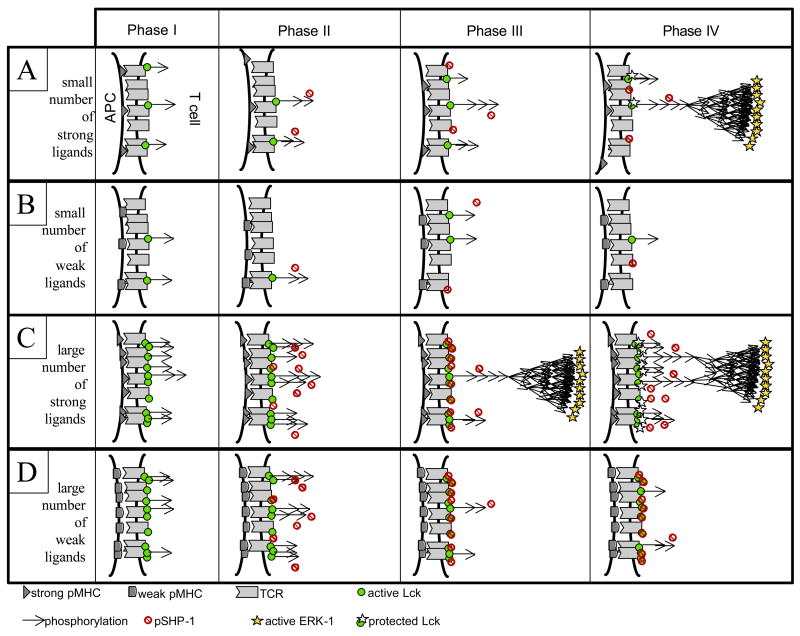
To illustrate the dynamics of T cell signaling, in Figure 3 we present schematics of four time phases for four stimulatory conditions (small/large quantities of good/poor ligands), as generated by our model. At low doses of ligands, simple kinetic proofreading of ITAM phosphorylation suffices for discrimination between good and poor ligands in terms of their ability to trigger the MAPK cascade (Figure 3A and 3B). In contrast, for large numbers of poor ligands, negative feedback acts to prevent spurious activation of the MAPK pathway (Figure 3D). Agonist ligands do trigger this negative feedback (especially at high dose) but they also manage to engage the MAPK cascade before the negative feedback can desensitize a large fraction of the available TCR - this overriding positive effect ultimately dictates the functional outcome (maintenance of T cell signaling) (Figure 3C). Hence negative feedback through SHP-1 phosphatase activity is crucial for abrogating responsiveness only for large quantities of poor ligands; positive feedback through ERK-1 kinase activity is crucial for responsiveness to large quantities of good ligands. Note that in such a scheme, we expect the dynamic range of response in terms of ITAM phosphorylation to be severely compressed between agonist and non-agonist, while the dynamic range for MAPK response would be large: ligand discrimination thus appears only quantitative at the level of ITAM phosphorylation, while it becomes qualitative at the level of MAPK phosphorylation and T cell activation.
Figure 3.
sketch of four phases in T cell signaling for four different types of activation. Each phase was delineated as a time window in our computer simulation [4]. For small numbers of ligands, kinetic proofreading and nonlinear delays in MAPK activation are sufficient to create an all-or-none discrimination between strong (row 1 – phase IV) and weak ligands (row 2 – phase IV). For large numbers of ligands, negative feedback through SHP-1 activation (Phase III) gets activated: for weak ligands, this feedback abrogates the signaling response (row 4 – phase IV) while for strong ligands, this feedback is insufficient to block all signaling, allowing the activation of MAPK cascade (row 3 – phase III), subsequent protection from pSHP-1 and signal maintenance (row 3 – phase IV).
The concept of differential signaling feedbacks also sheds light on a “natural” hierarchy of pMHC in terms of functional activation of T cells. In Figure 2C, we present an output of our model showing what number of ligands (EC50) are necessary to trigger the MAPK pathway for a given pMHC-TCR-interaction time (τ). Note that this curve is presented in log-log format; hence, our model predicts a very significant non-linearity between EC50 and τ. This also illustrates the natural hierarchy among pMHC ligands: agonists trigger the T cell as soon as a few ligands are presented whereas non-agonists fail to trigger the T cell even when 105 are presented on an APC (Figures 2B & 2D). The range of pMHC-TCR lifetimes in between these two extreme signaling outcomes is very narrow and corresponds to the partial or weak agonists. Consequently, our model explains how a continuum of pMHC-TCR lifetimes is interpreted by the TCR machinery to generate qualitative differences in signaling/functional response (from agonists, to weak/partial agonists, antagonists and null ligands as the lifetime decreases). It makes obsolete the need for digital differences between pMHC effects on the TCR (such as hypothetical conformational changes) to explain their functionality. Ultimately, our model shows how a digital threshold can be set by the TCR signaling machinery to discriminate between pMHC ligands solely based on the lifetime of the pMHC-TCR complex.
Even though ERK response is digital and sensitive, other responses (such as cytokine secretion) are not. Hence, we want to stress that the ppERK response is a necessary condition for downstream functional activation, but not a sufficient one. T cells are, in a sense, “green-lighted” towards building other signaling responses upon abrogation of the SHP-1-associated negative feedback by activation of ERK-associated positive feedback: for example, ITAM phosphorylation is stabilized and can trigger other responses (e.g. long-term calcium response and cytoskeletal rearrangements). In other words, even though the ppERK response is digital, there is room for a wide hierarchy of effector functions among T cells, depending on other signaling events [43].
2.4 Additional challenges in modeling ligand discrimination by T cells
Our model accurately “predicted” the existence of antagonism, the paradoxical capacity of better-binding pMHC to show more potent antagonism up to a threshold lifetime of TCR binding, as well as the possible hyperresponsiveness of cells with low levels of SHP-1 [4]. On the other hand, it did not test the possible involvement of endogenous ligands in T cell activation. As T cells scan the surface of antigen-presenting cells in vivo, they are confronted with a complex array of pMHCs derived from pathogens as well as from the proteins encoded by the host’s genome. This issue is quite challenging to address quantitatively because of the diverse roles reported for endogenous ligands in T cell signaling. Some groups have argued that self-ligands tune T cells towards desensitization, anergy and/or tolerance [44–47]. On the other hand, recent findings have shown that self-pMHC ligands can sustain the sensitivity of the TCR signaling machinery [48] and during exposure to agonist ligands, enhance T cell responses at low foreign pMHC densities [48–51]. A possible reconciliation of these divergent results can be proposed by taking into account the different experimental conditions and different timescales under consideration [52].
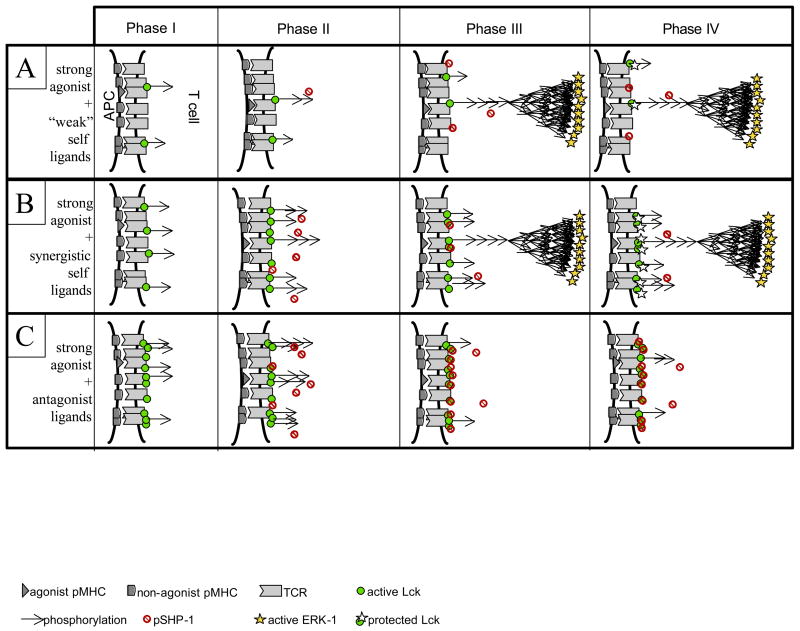
As to the specific issue of how self-ligands can act in synergy with agonists, two different models have been proposed. Li et al. [53] suggested that the formation of pseudo-dimers between endogeneous pMHC, non-self agonist pMHC, TCRs and CD4/8 coreceptors account for the enhancing role of endogenous ligands in T cell activation. Although this computer model displays the appropriate endogenous-enhancement of T cell activation, it fails to account for the proper ligand discrimination, as a “large” quantity of sub-threshold ligands (>3000) could still trigger the T cells. Alternatively, using the schematics of Figure 3, we can illustrate how our differential signaling model could account for diverse and even opposing effects associated with non-agonist ligands, including antagonism and synergy (Figure 4). Based on the model, we can distinguish three categories of non-agonist ligands according to their TCR-binding capabilities. Category A ligands do not engage TCR for any substantial time and fail to induce any ITAM phosphorylation (row A). They do not contribute significantly to the response to a small quantity of strong agonists. Category C ligands are better TCR-binders that induce tyrosine kinase activation well enough that they stimulate a robust SHP-1 phosphatase negative feedback response (row C - phase III) that limits the response of the same cell to the small available quantity of good ligands (row C – phase IV). These non-agonists therefore act as antagonists. Ligands with in-between TCR binding capabilities (category B) may engage TCRs enough to induce some ITAM phosphorylation, but not enough to antagonize the response of good ligands through extensive SHP-1 recruitment (row B – phase III; probably representative of most positively selecting self pMHC ligands present in the periphery). In this case, once the agonist ligand triggers MAPK activation, ‘synergistic’ self-pMHCs can trigger additional TCRs by taking advantage of the Lck protection against SHP-1 deactivation afforded by agonist-induced, MAPK-mediated Lck modification, adding to the stimulus provided by the agonist pMHCs alone (row B - phase IV). Overall, our model anticipates that adding non-agonist ligands can have three effects on the signaling induced by a small number of agonist ligands: null (category A), synergistic (category B) or antagonistic (category C). We conjecture that this hierarchy maps directly onto a hierarchy of binding capabilities of non-agonist pMHC to TCR, a suggestion that will require additional experimental testing to confirm or deny.
Figure 4.
differential effect of non-agonist ligands. Depending on the strength of pMHC-TCR interaction, a non-agonist ligand can have different influences on the signaling of a strong ligands: null (row #1), synergistic (row #2), or antagonistic (row #3). We anticipate that synergism will not be observed in the ERK response but rather on signaling responses that depend on protection of the TCR signaling complexes from premature inactivation (such as PLCγ activation and the ensuing Ca2+ response, which in turn depend on early phosphorylation status of the TCR-associated chains). Antagonism occurs when the non-agonist ligands bind TCR so well that robust SHP-1 activation occurs earlier and forbids ERK activation.
Other features beyond self-ligand synergy will be important to model (e.g. synapse formation and serial triggering), but they imply larger timescales that requires even more complex models (in particular, spatial considerations in protein distribution become crucial). Ultimately, a more complete model of T cell activation must also take into account the role of costimulation (e.g. CD28 and CTLA-4) and chemokine/cytokine signaling to account for the synergy between the innate and adaptive immune systems [54].
3. Tunability of ligand discrimination in T cell activation
As argued above, it is difficult to reconcile with allosteric models of discrimination the ability of a pMHC ligand to be a non-agonist for a mature T cell but an agonist mediating positive selection for a developing thymocyte with the same TCR. Rather, these data imply that the relationship between the biophysical parameters of pMHC-TCR interaction and agonist activity is determined by the differentiation state of the T cell and that the discrimination threshold is actively altered during intrathymic development to give rise to this changing response to weak and strong TCR-binding pMHCs.
There are reasons to also consider whether post-thymic differentiation processes also modify the ligand discrimination properties of T cells. If it were perfect, thymic selection would secure the release of an “ideal” repertoire of T cells (T cells that can be activated by pathogen-derived pMHCs but are not overtly responsive to self-derived pMHCs). However, it is clear that negative selection is imperfect and models of peripheral tolerance emphasize the role of micro-environments (e.g. costimulatory molecules, cytokines) in lymph nodes and other secondary lymphatic organs in limiting potential auto-immune catastrophes resulting from T cell engagement with self ligands able to signal beyond the activation threshold of the mature T cell. Such T cells may receive a detectable signal upon engagement with some self pMHC ligands, but, in the absence of inflammation, this activation would lead to anergy or tolerance. A non-exclusive view of peripheral tolerance is that aside from the influence of cosignals on T cell fate in the periphery upon self-recognition, there is a cell-autonomous effect of self-pMHC engagement. Such recognition might alter the signaling machinery to biochemically ‘erase’ the potential of self ligands to generate an activating signal in the mature lymphocytes: this process is classically called ‘tuning’. We will review some of the experimental studies suggesting such behavior among T cells, discuss how our differential feedback model can shed some light on this process, and present a conjecture on the mechanism of tunability.
3.1 Tuning during T cell development
CD4+CD8+ thymocytes are responsive in terms of ERK phosphorylation to endogenous (self-derived) ligands and this activation is necessary to their full differentiation. After release to the periphery, the same T cells lack this overt biochemical self-responsiveness. In making such a comparison, we believe it is crucial to focus on early events in a T cell’s response towards a given spectrum of ligands (such as ERK1/2 phosphorylation or CD69 upregulation) when evaluating changes in TCR-signaling coupling during differentiation. This is because analysis of more functional outcomes (cytokine secretion, cytotoxicity, proliferation) are complicated by the potential effects of gene remodeling differences between the immature and mature cells and by changes in the cell’s capacity to perceive and respond to non-antigen-associated factors such as costimuli, cytokines, etc.
The simplest view of this ‘erasure’ of overt responsiveness to positively selecting ligands that do not also induce subsequent negative selection [55, 56] is that the maturing T cells increase the threshold necessary for activation in a global sense. Under such conditions, weak TCR binders such as the self pMHCs involved in positive selection, would never provide enough input to reach the new threshold, whereas foreign pMHC that are good receptor binders would do so when they are available at a high enough density on APC. However, it is clear that such a simple scheme demands that the T cell lose potential sensitivity for the foreign agonists as compared to the immature thymocytes. This makes little biological sense – such a view suggests that positive selection evolved in such a way as to decrease the ability of the adaptive immune system to detect foreign antigens. As it turns out, such a sacrifice is not necessary nor does it occur. Several groups [57, 58] have clearly shown that as a T cell matures, it maintains the same sensitivity to foreign agonist ligands while at the same time completely losing the capacity to show a functional response to weak TCR binders. Furthermore, at the level of proximal TCR signaling, the agonists retain their ability to generate fully phosphorylated TCRζ ITAMs and induce ZAP70 activation, whereas the weak binders change the pattern and not just extent of early signaling, producing an excess of hypo-phosphorylated TCRζ and failing to activate ZAP70. These findings are not compatible with an across-the-board decrease in sensitivity of the TCR to transmit signals, but rather clearly indicate that the threshold for discrimination between ligands of distinct TCR binding capacity has been altered. Very recent data indicate that, as presumed but not directly tested in the earlier studies, this occurs without measurable change in the lifetimes of the pMHC interactions with the TCR [59]. Given these results, one must conclude that maturation changes how ligand-engaged TCRs couple to the signaling apparatus, or perhaps more precisely, how the signaling machinery of the T cell process signals downstream of initial kinase activation.
What might these changes be? Stark et al. [60] have pointed out that there are different TCR signaling networks yielding ERK1/2 activation (namely through RAS-GRDP and GRB2), with different characteristics (transient or sustained activation), so one could imagine how the same input (pMHC engagement by TCR) and the same signaling output (phosphorylated ERK1/2) might be connected through different signaling pathways. Consequently, the tunability of ligand discrimination in maturing T cells would correspond to a qualitative “rewiring” of the TCR signaling machinery. The central issue is how particular biochemical changes (for example, switching from Ras-GRDP in the thymus to GRB2 in peripheral T cells) actually effect the necessary functional changes (positive selection with self pMHC at the DP stage vs. self restriction in the naïve stage in the periphery). This theme will be elaborated below and specific biochemical changes suggested that might contribute to such tuning not of triggering threshold but of ligand discrimination in signal propagation both among thymocytes and peripheral T cells.
3.2 Tunability during T cell differentiation in the periphery
Recent experimental results suggest that T cells may modify their ligand discrimination threshold in the periphery. The direction of this change in some but not other studies appears consistent with theoretical considerations positing the generation of peripheral tolerance through reduced responsiveness to self induced by chronic exposure to self-ligands, without loss of useful sensitivity to foreign stimuli. This concept of tuning of T cell responsiveness in the periphery came from Grossman and colleagues [45–47, 61]. The core model (the tunable-adaptation threshold model or TAT) argues that long-term adaptation to tonic levels of self-induced TCR signaling desensitizes the receptor response just enough to prevent overt responses to this self-ligand landscape, while preserving the capacity of the T cells to respond to acute contact with more potent foreign ligands.
Experimental testing of the TAT model came through adoptive transfers of T cells into TAP−/− mice with a defect in presentation of Class I pMHC [62] or into mice with abolished expression of MHC-II [45]: T cells, left in the latter pMHC-deficient environment for at least a week were shown to gain increased signaling and functional responsiveness towards foreign pMHC upon rechallenge. Quite in contrast to these reports, Stefanova et al. [48] as well as the Davis group [3, 49] have shown that T cells can take advantage of endogenous ligands to boost T cells’ response towards agonist ligands.
It is likely that major differences in experimental settings account for these divergent results. In the two cases in which loss of self pMHC contact induced gain of responsiveness in T cells [45, 62], these cells had been kept in lymphopenic environments for substantial time periods during which proliferation and differentiation into an effector/memory cell state occurred. As compared to naïve cells, previously-activated cells of this type are known to be more responsive to antigen and to require less costimulation, consistent with what these two groups reported. In contrast, the experiments showing a positive contribution of self to T cell sensitivity or responsiveness [3, 48, 49] were done under acute settings, involved cells from normal animals, or examined cells within a few hours of adoptive transfer rather than many days or weeks, that is, prior to lymphopenia-induced proliferation and changes in phenotype.
Thus, under normal circumstances, in animals with intact lymphoid compartments, self-ligands tend to make a net positive contribution to the responsiveness of naïve T cells, rather than blunting their response to maintain tolerance. This is not to say that a more subtle form of self-induced TAT does not occur under physiological circumstances: available data and the illustrations above for synergistic self-ligands (Figure 3B) indicate that exposure to these pMHCs will induce a small amount of SHP-1 recruitment to the TCR pool, just sufficient to prevent effective responses by poor TCR binders. This is quite consistent with the TAT model. However, because of the ability of agonist-induced MAPK activation to override this low level of negative regulation, there is a net positive effect of TCR engagement by self pMHCs apparently due to pre-association of key signaling molecules with the receptor that enhances activation of MAPK pathway.
Another set of experiments has addressed the issue of tunable-activation threshold upon constant agonist pMHC stimulation. Singh et al. established a model in which different levels of agonist ligands were chronically presented in vivo: these ligands induced naïve T cells in lymphopenic environments to proliferate, then enter a stable state with a low turnover rate and decrease their responsiveness to the same pMHC ligand to an extent that depended on the level of chronic pMHC presentation [63]. In particular, chronic stimulation with constant low levels of antigen left the cells more responsive (in terms of signaling, cytokine production or proliferative capabilities upon re-challenge) than did exposure to (four-fold) higher levels of the same antigen. In these circumstances, strong agonist ligands for naïve cells became partial agonists for cells chronically exposed to low amounts of agonist ligand in vivo (such T cells showed a characteristic transient ERK response) whereas the same pMHC acted as a null/weak ligand for cells exposed chronically to higher amounts of agonist (showing a diminished ERK response). This sliding scale of pMHC responsiveness depending on the receptor occupancy history of the T cells matched the predictions of the Tunable-Activation Threshold model. Recent developments demonstrated the role of other T cells in adjusting the tuning process (through extrinsic regulatory functions) for chronically-activated T cells [64]. Understanding quantitatively the underlying mechanisms of this tuning remains challenging, even more so because this adaptation of the TCR machinery does not apply for all functional outputs: responsiveness in terms of CD69 upregulation or actin polymerization was not modulated with chronic exposure to antigen [63].
Recently, experimental work in our lab unraveled another manifestation of tuning in T cell activation, namely a transient hypersensitivity of T cells towards self-like ligands. Using naïve lymphocytes that have been activated ex vivo for one day and rested after APC removal for five days, or activated ex vivo and expanded for 5 days [4], responsiveness towards sub-threshold ligands was tested. The 5C.C7 clone was shown to gain responsiveness towards MCC102G/I-Ek (a non-agonist for naïve 5C.C7 T cells) and the OT-1 clone was shown to gain responsiveness towards EIINFEKL/H-2Kb. This hypersensitivity of T cells was also shown to be transient, as memory-type T cells regained a spectrum of responsiveness similar to that of the initial naïve T cells. These observations emphasize that ligand discrimination is not set for T cells endowed with a given TCR, but rather can be tuned/rewired depending on the differentiation state.
The functional relevance as well as the underlying molecular mechanism for this transient hypersensitivity in T cells is still under analysis. One change contributing to this hypersensitivity is the down-regulation of SHP-1 phosphatase (the main component of TCR negative feedback unraveled by Stefanova et al. [42]. Re-establishment of near resting state levels of SHP-1 through retroviral infection of T cells corrected the transient hypersensitivity towards non-agonist ligands, without affecting the T cell’s responsiveness towards agonist ligands (a prediction of our computer model [4]). What triggers SHP-1 downregulation remains unknown, but is clearly worth investigating because of this phenomenon’s implications with respect to autoimmunity and therapeutic responses to self antigens such as in cancer treatment. More generally, the observation that the up/downregulation of a single key signaling component (SHP-1) can abrogate or allow self-responsiveness suggests the existence of master regulators in ligand discrimination.
3.3 Tunability and the differential feedback model
As discussed in the first part of this section, the existence of differential feedbacks in TCR signaling provides a major step forward in understanding ligand discrimination at the biochemical level. A computer simulation of these feedback effects illustrated how the threshold of pMHC-TCR lifetime in terms of activation of T cells can be set by the detailed properties of the kinetic competition between the positive and negative pathways. A consequence of this feature of TCR signal control is that modest up and downregulation of signaling protein levels in the feedback pathways can be anticipated to modulate in dramatic ways the threshold of ligand discrimination.
This leads us to propose a simple algorithm for enforcing positive/negative selection and peripheral tolerance that also optimizes ligand discrimination and foreign antigen sensitivity. First, low levels of a key inhibitory signaling component (e.g. SHP-1) or high levels of a key stimulatory signaling component (e.g. Lck) will endow DP thymocytes with responsiveness to endogenous ligands. Following initiation of positive selection through such signaling, increased expression of the inhibitory or decreased expression of the stimulatory enzymes can modify the effective discrimination threshold for effective signaling until responsiveness to endogenous ligands is lost, preventing negative selection by the pMHCs involved in positive selection and producing a self-recognizing but not overtly self-reactive T cell for release to the periphery. This is an optimal algorithm for TCR response tuning, at least at the conceptual level, as it allows any pMHC binding to the TCR more avidly than endogenous ligands to be stimulatory, offering the repertoire the widest range of foreign ligand sensitivity in the periphery. It is especially attractive because the loss of self-reactivity does not come at the expense of foreign ligand sensitivity, as would a more general blunting of the T cell signaling capacity of a cell. Such global blunting would prevent self-responses but also diminish sensitivity for low levels of foreign ligands, an undesirable outcome of the tuning process and one that is not seen experimentally [48]. This algorithm easily accounts for the differential change in signaling properties of a given T cell undergoing maturation. Some experimental evidence is also consistent with this algorithm: SHP-1 has been shown to be low in DP thymocytes [65]; Lck association with the TCR and with CD4 also is high in DP thymocytes [58]. These two observations correlate well with hypersensitivity of DP thymocytes: quantitative analysis and computer modeling will further test this correlation and help identify critical kinetic components in the tuning of T cell ligand discrimination in the thymus.
Conclusion
In conclusion, we have reviewed several quantitative challenges to our understanding of ligand discrimination in T cells. Although conformational changes associated with the engagement of TCR with agonist pMHC have been suggested by many groups, they remain a working hypothesis needing experimental confirmation and must be accompanied by an explanation for how thymocytes and mature T cells with identical TCRs respond differently to the same pMHC ligand. Kinetic thresholding is the more dominant concept in the field, allowing minute kinetic differences to be discriminated by the TCR signaling machinery in terms of triggering functional responses. The main challenge to a kinetic threshold model remains understanding in a quantitative manner how the TCR signaling machinery sets a threshold in terms of the quality of pMHC-TCR engagement. We have introduced here a core concept in this regard, namely the alteration of the relative concentrations of key components of the signaling network that impact downstream signal propagation in highly non-linear fashion due to the circuitry of the cell. Further detailed elaboration on this core concept should reconcile many divergent observations in T cell signaling: extreme sensitivity and specificity of pMHC recognition, existence of antagonism by sub-threshold ligands, signal enhancement by endogenous ligands, and more. A primary benefit of developing an improved quantitative model of kinetic thresholding built on this core concept will be a better understanding of how and to what extent self/non-self discrimination varies in T cells as they undergo various developmental events. Beyond the issue of threshold setting per se, such modeling will also allow us to address the central issue of robustness in maintaining a cell below the activation threshold (“How does a T cell clonal population achieve effective ligand discrimination while each individual cell differs in the level of expression of its signaling components?”) while permitting flexibility (“How does one T cell switch from self-responsiveness to self-restriction during development?”). In other words, it will become possible to understand in a detailed mechanistic manner the control of ligand discrimination by T cells at all stages of differentiation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis MM, et al. Ligand recognition by alpha beta T cell receptors. Annual Review of Immunology. 1998;16:523–44. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 2.Germain RN, Stefanova I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annual Review of Immunology. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- 3.Irvine DJ, et al. Direct observation of ligand recognition by T cells. Nature. 2002;419(6909):845–9. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 4.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback controls of digital ERK response. PLoSBiology. 2005;3(11):e356. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rojo JM, Janeway CA., Jr The biologic activity of anti-T cell receptor V region monoclonal antibodies is determined by the epitope recognized. Journal of Immunology. 1988;140(4):1081–8. [PubMed] [Google Scholar]
- 6.Reich Z, et al. Ligand-specific oligomerization of T-cell receptor molecules. Nature. 1997;387(6633):617–20. doi: 10.1038/42500. [DOI] [PubMed] [Google Scholar]
- 7.Garcia KC, Teyton L, Wilson IA. Structural basis of T cell recognition. Annu Rev Immunol. 1999;17:369–97. doi: 10.1146/annurev.immunol.17.1.369. [DOI] [PubMed] [Google Scholar]
- 8.Ding YH, et al. Four A6-TCR/peptide/HLA-A2 structures that generate very different T cell signals are nearly identical. Immunity. 1999;11(1):45–56. doi: 10.1016/s1074-7613(00)80080-1. [DOI] [PubMed] [Google Scholar]
- 9.Housset D, Malissen B. What do TCR-pMHC crystal structures teach us about MHC restriction and alloreactivity? Trends Immunol. 2003;24(8):429–37. doi: 10.1016/s1471-4906(03)00180-7. [DOI] [PubMed] [Google Scholar]
- 10.Reiser JB, et al. CDR3 loop flexibility contributes to the degeneracy of TCR recognition. Nat Immunol. 2003;4(3):241–7. doi: 10.1038/ni891. [DOI] [PubMed] [Google Scholar]
- 11.Daniels MA, et al. CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity. 2001;15(6):1051–61. doi: 10.1016/s1074-7613(01)00252-7. [DOI] [PubMed] [Google Scholar]
- 12.Aivazian D, Stern LJ. Phosphorylation of T cell receptor zeta is regulated by a lipid dependent folding transition. Nat Struct Biol. 2000;7(11):1023–6. doi: 10.1038/80930. [DOI] [PubMed] [Google Scholar]
- 13.Gil D, et al. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109(7):901–12. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 14.Gil D, et al. T cell receptor engagement by peptide-MHC ligands induces a conformational change in the CD3 complex of thymocytes. J Exp Med. 2005;201(4):517–22. doi: 10.1084/jem.20042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gascoigne NR, Zal T, Alam SM. T-cell receptor binding kinetics in T-cell development and activation. Expert Rev Mol Med. 2001;2001:1–17. doi: 10.1017/S1462399401002502. [DOI] [PubMed] [Google Scholar]
- 16.Krogsgaard M, et al. Evidence that structural rearrangements and/or flexibility during TCR binding can contribute to T cell activation. Molecular Cell. 2003;12(6):1367–78. doi: 10.1016/s1097-2765(03)00474-x. [DOI] [PubMed] [Google Scholar]
- 17.Qi S, et al. Molecular flexibility can influence the stimulatory ability of receptor-ligand interactions at cell-cell junctions. Proc Natl Acad Sci U S A. 2006;103(12):4416–21. doi: 10.1073/pnas.0510991103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalergis AM, et al. Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat Immunol. 2001;2(3):229–34. doi: 10.1038/85286. [DOI] [PubMed] [Google Scholar]
- 19.Rosette C, et al. The impact of duration versus extent of TCR occupancy on T cell activation: a revision of the kinetic proofreading model. Immunity. 2001;15(1):59–70. doi: 10.1016/s1074-7613(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 20.Crawford F, et al. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8(6):675–82. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 21.Wang XL, Altman JD. Caveats in the design of MHC class I tetramer/antigen-specific T lymphocytes dissociation assays. J Immunol Methods. 2003;280(1–2):25–35. doi: 10.1016/s0022-1759(03)00079-6. [DOI] [PubMed] [Google Scholar]
- 22.Cameron TO, et al. Cutting edge: detection of antigen-specific CD4+ T cells by HLA-DR1 oligomers is dependent on the T cell activation state. J Immunol. 2001;166(2):741–5. doi: 10.4049/jimmunol.166.2.741. [DOI] [PubMed] [Google Scholar]
- 23.Alam SM, et al. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10(2):227–37. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 24.Kersh GJ, et al. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity. 1998;9(6):817–26. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 25.Castellino F, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440(7086):890–5. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 26.Stoll S, et al. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296(5574):1873–6. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 27.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427(6970):154–9. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 28.Miller MJ, et al. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc Natl Acad Sci U S A. 2004;101(4):998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dustin ML. Stop and go traffic to tune T cell responses. Immunity. 2004;21(3):305–14. doi: 10.1016/j.immuni.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Sykulev Y, et al. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4(6):565–71. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 31.Fleire SJ, et al. B cell ligand discrimination through a spreading and contraction response. Science. 2006;312(5774):738–41. doi: 10.1126/science.1123940. [DOI] [PubMed] [Google Scholar]
- 32.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci U S A. 1995;92(11):5042–6. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabinowitz JD, et al. Altered T cell receptor ligands trigger a subset of early T cell signals. Immunity. 1996;5(2):125–35. doi: 10.1016/s1074-7613(00)80489-6. [DOI] [PubMed] [Google Scholar]
- 34.Lord GM, Lechler RI, George AJ. A kinetic differentiation model for the action of altered TCR ligands. Immunology Today. 1999;20(1):33–9. doi: 10.1016/s0167-5699(98)01379-6. [DOI] [PubMed] [Google Scholar]
- 35.Monks CR, et al. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395(6697):82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 36.Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285(5425):221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 37.Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nat Immunol. 2006;7(8):803–9. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 38.Burroughs NJ, Lazic Z, van der Merwe PA. Ligand detection and discrimination by spatial relocalization: A kinase-phosphatase segregation model of TCR activation. Biophys J. 2006;91(5):1619–29. doi: 10.1529/biophysj.105.080044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee KH, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302(5648):1218–22. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 40.Chan C, George AJ, Stark J. Cooperative enhancement of specificity in a lattice of T cell receptors. Proc Natl Acad Sci U S A. 2001;98(10):5758–63. doi: 10.1073/pnas.101113698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi SY, Groves JT, Chakraborty AK. Synaptic pattern formation during cellular recognition. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6548–53. doi: 10.1073/pnas.111536798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefanova I, et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways.[see comment] Nature Immunology. 2003;4(3):248–54. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 43.Hemmer B, et al. Relationships among TCR ligand potency, thresholds for effector function elicitation, and the quality of early signaling events in human T cells. J Immunol. 1998;160(12):5807–14. [PubMed] [Google Scholar]
- 44.Kersh EN, Kersh GJ, Allen PM. Partially phosphorylated T cell receptor zeta molecules can inhibit T cell activation. J Exp Med. 1999;190(11):1627–36. doi: 10.1084/jem.190.11.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhandoola A, et al. Peripheral expression of self-MHC-II influences the reactivity and self-tolerance of mature CD4(+) T cells: evidence from a lymphopenic T cell model. Immunity. 2002;17(4):425–36. doi: 10.1016/s1074-7613(02)00417-x. [DOI] [PubMed] [Google Scholar]
- 46.Grossman Z, Paul WE. Autoreactivity, dynamic tuning and selectivity. Curr Opin Immunol. 2001;13(6):687–98. doi: 10.1016/s0952-7915(01)00280-1. [DOI] [PubMed] [Google Scholar]
- 47.Grossman Z, Singer A. Tuning of activation thresholds explains flexibility in the selection and development of T cells in the thymus. Proc Natl Acad Sci U S A. 1996;93(25):14747–52. doi: 10.1073/pnas.93.25.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420(6914):429–34. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 49.Krogsgaard M, et al. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434(7030):238–43. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 50.Wulfing C, et al. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat Immunol. 2002;3(1):42–7. doi: 10.1038/ni741. [DOI] [PubMed] [Google Scholar]
- 51.Yachi PP, et al. Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat Immunol. 2005;6(8):785–92. doi: 10.1038/ni1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hogquist KA, Starr TK, Jameson SC. Receptor sensitivity: when T cells lose their sense of self. Curr Biol. 2003;13(6):R239–41. doi: 10.1016/s0960-9822(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 53.Li QJ, et al. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse.[see comment] Nature Immunology. 2004;5(8):791–9. doi: 10.1038/ni1095. [DOI] [PubMed] [Google Scholar]
- 54.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–40. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 55.Guidos CJ, et al. T cell receptor-mediated negative selection of autoreactive T lymphocyte precursors occurs after commitment to the CD4 or CD8 lineages. J Exp Med. 1990;172(3):835–45. doi: 10.1084/jem.172.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2(5):309–22. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 57.Davey GM, et al. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188(10):1867–74. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lucas B, et al. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10(3):367–76. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- 59.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444(7120):724–9. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 60.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 61.Grossman Z, Paul WE. Adaptive cellular interactions in the immune system: the tunable activation threshold and the significance of subthreshold responses. Proc Natl Acad Sci U S A. 1992;89(21):10365–9. doi: 10.1073/pnas.89.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santori FR, Arsov I, Vukmanovic S. Modulation of CD8+ T cell response to antigen by the levels of self MHC class I. J Immunol. 2001;166(9):5416–21. doi: 10.4049/jimmunol.166.9.5416. [DOI] [PubMed] [Google Scholar]
- 63.Singh NJ, Schwartz RH. The strength of persistent antigenic stimulation modulates adaptive tolerance in peripheral CD4+ T cells. J Exp Med. 2003;198(7):1107–17. doi: 10.1084/jem.20030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh NJ, Chen C, Schwartz RH. The impact of T cell intrinsic antigen adaptation on peripheral immune tolerance. PLoS Biol. 2006;4(11):e340. doi: 10.1371/journal.pbio.0040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plas DR, et al. Direct regulation of ZAP-70 by SHP-1 in T cell antigen receptor signaling. Science. 1996;272(5265):1173–6. doi: 10.1126/science.272.5265.1173. [DOI] [PubMed] [Google Scholar]