Abstract
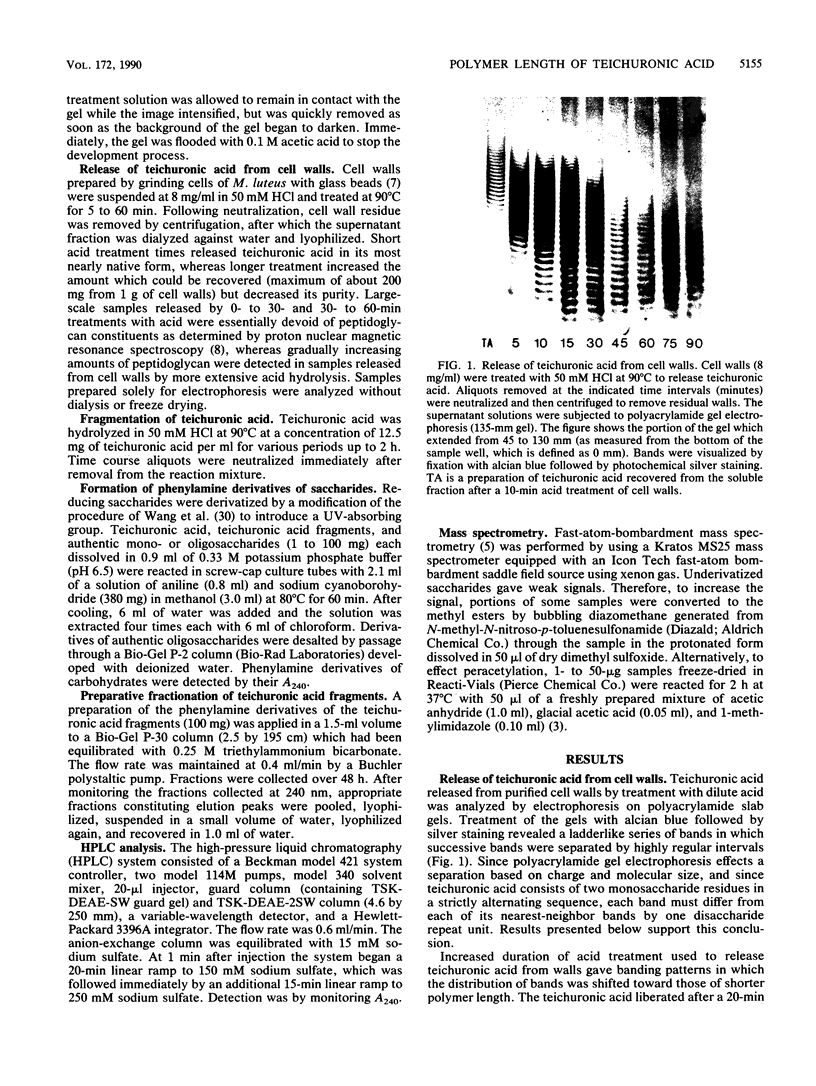
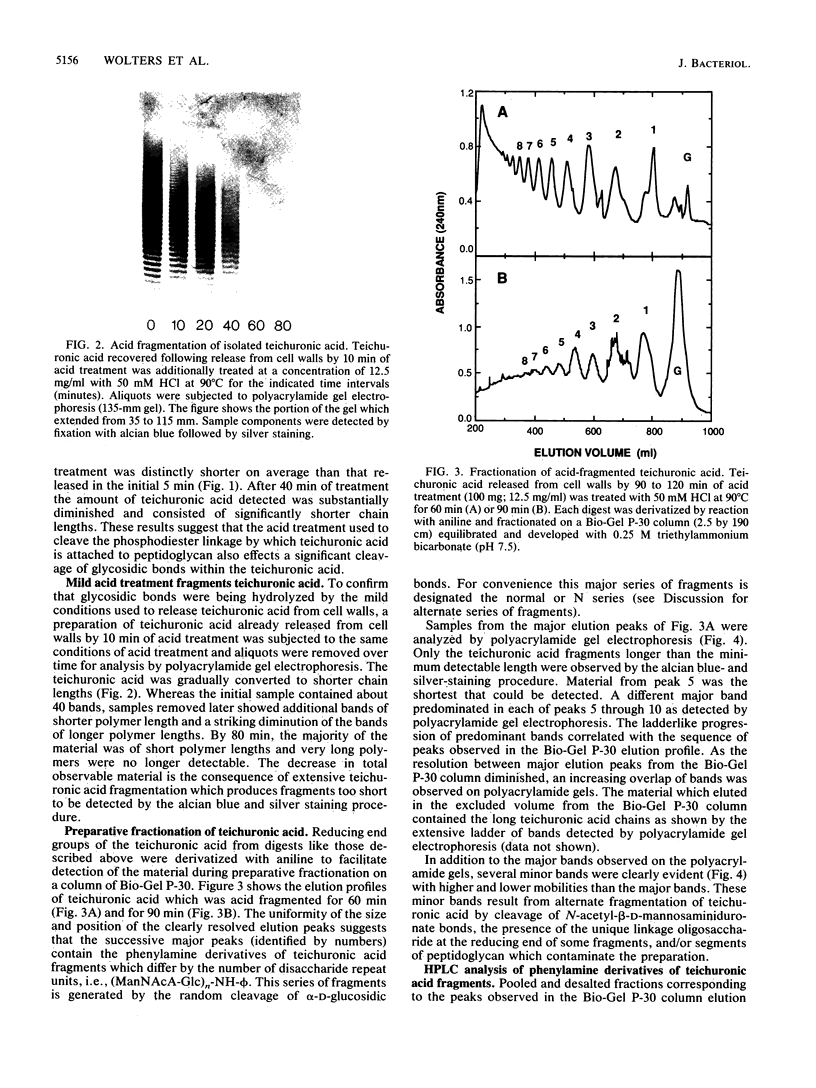
Teichuronic acid released from its phosphodiester linkage to peptidoglycan in the cell walls of Micrococcus luteus by mild acid treatment is resolved into a ladderlike series of bands by electrophoresis on polyacrylamide gels in the presence of borate. Each band of the ladder differs from its nearest neighbor by one disaccharide repeat unit, ----4)-2-acetamido-2-deoxy-beta-D-mannopyranuronosyl-(1----6)- alpha-D-glucopyranosyl-(1-. Acid-fragmented teichuronic acid, after conversion to the phenylamine derivative, was fractionated by preparative-scale molecular sieve column chromatography, which produced a series of elution peaks. Fast-atom-bombardment mass spectrometry of the smallest member of the series determined its molecular weight and established its identity as the phenylamine derivative of one disaccharide repeat unit of teichuronic acid. Homologous fractions of the same series were used to index the ladder of bands obtained by polyacrylamide gel electrophoresis from samples containing a more extensive distribution of polymer lengths. Nearly native teichuronic acid consists of polymers with a broad range of molecular sizes ranging from 20 to 55 disaccharide units. The most abundant species are those which have 25 to 40 repeat units. Prolonged treatment of teichuronic acid with the acid conditions used to release it from peptidoglycan causes gradual fragmentation of the teichuronic acid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BeMiller J. N. Acid-catalyzed hydrolysis of glycosides. Adv Carbohydr Chem Biochem. 1967;22:25–108. doi: 10.1016/s0096-5332(08)60151-4. [DOI] [PubMed] [Google Scholar]
- Campbell J. N., Leyh-Bouille M., Ghuysen J. M. Characterization of the Micrococcus lysodeikticus type of peptidoglycan in walls of other Micrococcaceae. Biochemistry. 1969 Jan;8(1):193–200. doi: 10.1021/bi00829a028. [DOI] [PubMed] [Google Scholar]
- Dell A. F.A.B.-mass spectrometry of carbohydrates. Adv Carbohydr Chem Biochem. 1987;45:19–72. doi: 10.1016/s0065-2318(08)60136-5. [DOI] [PubMed] [Google Scholar]
- FLODIN P., GREGORY J. D., RODEN L. SEPARATION OF ACIDIC OLIGOSACCHARIDES BY GEL FILTRATION. Anal Biochem. 1964 Aug;8:424–433. doi: 10.1016/0003-2697(64)90240-4. [DOI] [PubMed] [Google Scholar]
- Gassner G. T., Dickie J. P., Hamerski D. A., Magnuson J. K., Anderson J. S. Teichuronic acid reducing terminal N-acetylglucosamine residue linked by phosphodiester to peptidoglycan of Micrococcus luteus. J Bacteriol. 1990 May;172(5):2273–2279. doi: 10.1128/jb.172.5.2273-2279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase S., Matsushima Y. Structural studies on a glucose-containing polysaccharide obtained from cell walls of Micrococcus lysodeikticus. 3. Determination of the structure. J Biochem. 1972 Nov;72(5):1117–1128. doi: 10.1093/oxfordjournals.jbchem.a129999. [DOI] [PubMed] [Google Scholar]
- Hase S., Matsushima Y. The structure of the branching point between acidic polysaccharide and peptidoglycan in Micrococcus lysodeikticus cell wall. J Biochem. 1977 May;81(5):1181–1186. [PubMed] [Google Scholar]
- Hildebrandt K. M., Anderson J. S. Biosynthetic elongation of isolated teichuronic acid polymers via glucosyl- and N-acetylmannosaminuronosyltransferases from solubilized cytoplasmic membrane fragments of Micrococcus luteus. J Bacteriol. 1990 Sep;172(9):5160–5164. doi: 10.1128/jb.172.9.5160-5164.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanaga Y., Park J. T. Studies on the cell walls of Micrococcus lysodeikticus. Fractionation of the nondialyzable components from a lysozyme digest of cell walls. Biochemistry. 1972 Oct 10;11(21):4006–4012. doi: 10.1021/bi00771a026. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Hoger J. H., Ratnayake J. H., Anderson J. S. Characterization of three intermediates in the biosynthesis of teichuronic acid of Micrococcus luteus. Arch Biochem Biophys. 1984 Dec;235(2):679–691. doi: 10.1016/0003-9861(84)90244-3. [DOI] [PubMed] [Google Scholar]
- Johnson S. D., Lacher K. P., Anderson J. S. Carbon-13 nuclear magnetic resonance spectroscopic study of teichuronic acid from Micrococcus luteus cell walls. Comparison of the polysaccharide isolated from cells with that synthesized in vitro. Biochemistry. 1981 Aug 4;20(16):4781–4785. doi: 10.1021/bi00519a039. [DOI] [PubMed] [Google Scholar]
- Kato K., Iwata S., Matsuda T., Kotani S. Isolation of glucosamine 6-phosphate from the cell walls of Micrococcus lysodeikticus. Biken J. 1978 Jun;21(2):63–67. [PubMed] [Google Scholar]
- Liu T. Y., Gotschlich E. C. Muramic acid phosphate as a component of the mucopeptide of Gram-positive bacteria. J Biol Chem. 1967 Feb 10;242(3):471–476. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Van Keuren M. L. Silver staining methods for polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:230–239. doi: 10.1016/s0076-6879(83)96021-4. [DOI] [PubMed] [Google Scholar]
- Min H., Cowman M. K. Combined alcian blue and silver staining of glycosaminoglycans in polyacrylamide gels: application to electrophoretic analysis of molecular weight distribution. Anal Biochem. 1986 Jun;155(2):275–285. doi: 10.1016/0003-2697(86)90437-9. [DOI] [PubMed] [Google Scholar]
- Nasir-ud-Din, Lhermitte M., Lamblin G., Jeanloz R. W. The phosphate diester linkage of the peptidoglycan polysaccharide moieties of Micrococcus lysodeikticus cell wall. J Biol Chem. 1985 Aug 25;260(18):9981–9987. [PubMed] [Google Scholar]
- PERKINS H. R. A polymer containing glucose and aminohexuronic acid isolated from the cell walls of micrococcus lysodeikticus. Biochem J. 1963 Mar;86:475–483. doi: 10.1042/bj0860475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlik J. G., Rogers H. J. Selective extraction of polymers from cell walls of gram-positive bacteria. Biochem J. 1973 Mar;131(3):619–621. doi: 10.1042/bj1310619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen S., Häyrinen J., Finne J. Polyacrylamide gel electrophoresis of the capsular polysaccharides of Escherichia coli K1 and other bacteria. J Bacteriol. 1988 Jun;170(6):2646–2653. doi: 10.1128/jb.170.6.2646-2653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr T. E., Levy G. N., Stark N. J., Anderson J. S. Initial reactions in biosynthesis of teichuronic acid of Micrococcus lysodeikticus cell walls. J Biol Chem. 1977 May 25;252(10):3460–3465. [PubMed] [Google Scholar]
- Stark N. J., Levy G. N., Rohr T. E., Anderson J. S. Reactions of second stage of biosynthesis of teichuronic acid of Micrococcus lysodeikticus cell walls. J Biol Chem. 1977 May 25;252(10):3466–3472. [PubMed] [Google Scholar]
- Traxler C. I., Goustin A. S., Anderson J. S. Elongation of teichuronic acid chains by a wall-membrane preparation from Micrococcus luteus. J Bacteriol. 1982 May;150(2):649–656. doi: 10.1128/jb.150.2.649-656.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. E., Cowman M. K. Cationic dye binding by hyaluronate fragments: dependence on hyaluronate chain length. Arch Biochem Biophys. 1985 Feb 15;237(1):253–260. doi: 10.1016/0003-9861(85)90276-0. [DOI] [PubMed] [Google Scholar]
- Wang W. T., LeDonne N. C., Jr, Ackerman B., Sweeley C. C. Structural characterization of oligosaccharides by high-performance liquid chromatography, fast-atom bombardment-mass spectrometry, and exoglycosidase digestion. Anal Biochem. 1984 Sep;141(2):366–381. doi: 10.1016/0003-2697(84)90057-5. [DOI] [PubMed] [Google Scholar]
- Yamada M., Hirose A., Matsuhashi M. Association of lack of cell wall teichuronic acid with formation of cell packets of Micrococcus lysodeikticus (luteus) mutants. J Bacteriol. 1975 Aug;123(2):678–686. doi: 10.1128/jb.123.2.678-686.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]