Abstract
In common with other plant symbionts, Frankia spp., the actinomycete N2-fixing symbionts of certain nonleguminous woody plants, synthesize two glutamine synthetases, GSI and GSII. DNA encoding the Bradyrhizobium japonicum gene for GSII (glnII) hybridized to DNA from three Frankia strains. B. japonicum glnII was used as a probe to clone the glnII gene from a size-selected KpnI library of Frankia strain CpI1 DNA. The region corresponding to the Frankia sp. strain CpI1 glnII gene was sequenced, and the amino acid sequence was compared with that of the GS gene from the pea and glnII from B. japonicum. The Frankia glnII gene product has a high degree of similarity with both GSII from B. japonicum and GS from pea, although the sequence was about equally similar to both the bacterial and eucaryotic proteins. The Frankia glnII gene was also capable of complementing an Escherichia coli delta glnA mutant when transcribed from the vector lac promoter, but not when transcribed from the Frankia promoter. GSII produced in E. coli was heat labile, like the enzyme produced in Frankia sp. strain CpI1 but unlike the wild-type E. coli enzyme.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almassy R. J., Janson C. A., Hamlin R., Xuong N. H., Eisenberg D. Novel subunit-subunit interactions in the structure of glutamine synthetase. 1986 Sep 25-Oct 1Nature. 323(6086):304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
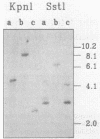
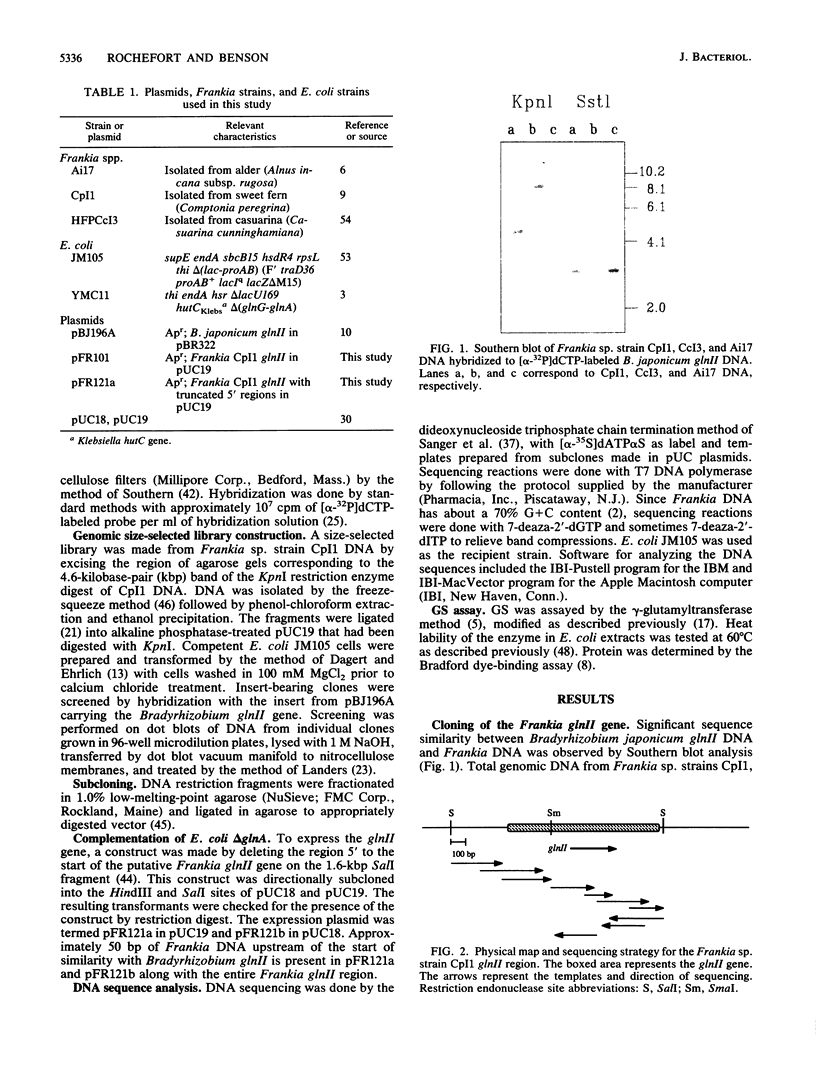
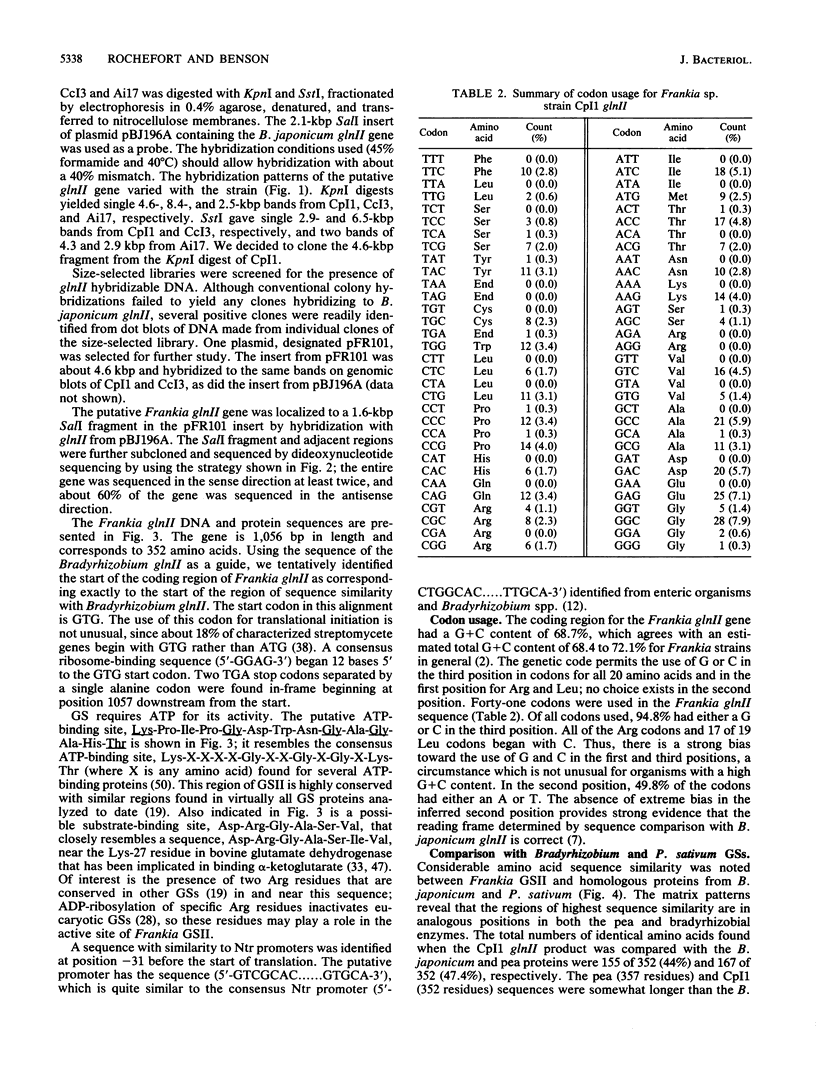
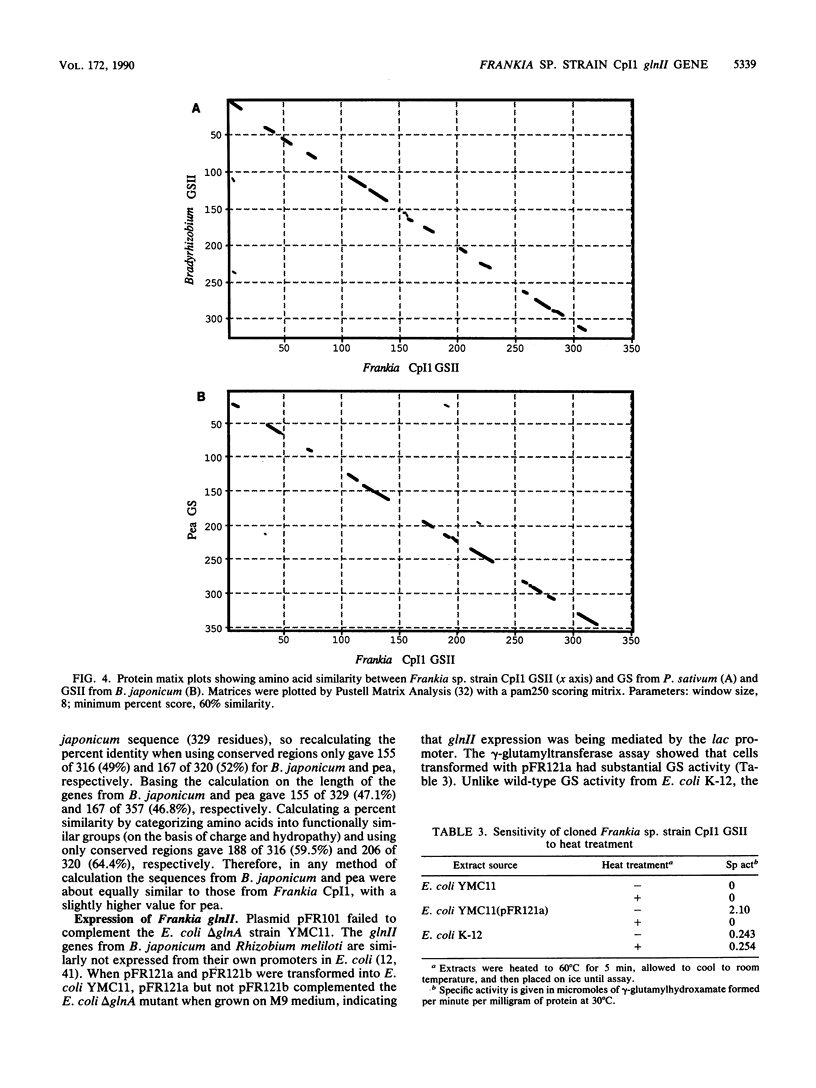
- Behrmann I., Hillemann D., Pühler A., Strauch E., Wohlleben W. Overexpression of a Streptomyces viridochromogenes gene (glnII) encoding a glutamine synthetase similar to those of eucaryotes confers resistance against the antibiotic phosphinothricyl-alanyl-alanine. J Bacteriol. 1990 Sep;172(9):5326–5334. doi: 10.1128/jb.172.9.5326-5334.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R. A., Janssen K. A., Resnick A. D., Blumenberg M., Foor F., Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977 Feb;129(2):1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D. R. Isolation of frankia strains from alder actinorhizal root nodules. Appl Environ Microbiol. 1982 Aug;44(2):461–465. doi: 10.1128/aem.44.2.461-465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Callaham D., Deltredici P., Torrey J. G. Isolation and Cultivation in vitro of the Actinomycete Causing Root Nodulation in Comptonia. Science. 1978 Feb 24;199(4331):899–902. doi: 10.1126/science.199.4331.899. [DOI] [PubMed] [Google Scholar]
- Carlson T. A., Guerinot M. L., Chelm B. K. Characterization of the gene encoding glutamine synthetase I (glnA) from Bradyrhizobium japonicum. J Bacteriol. 1985 May;162(2):698–703. doi: 10.1128/jb.162.2.698-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T. A., Martin G. B., Chelm B. K. Differential transcription of the two glutamine synthetase genes of Bradyrhizobium japonicum. J Bacteriol. 1987 Dec;169(12):5861–5866. doi: 10.1128/jb.169.12.5861-5866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Darrow R. A., Knotts R. R. Two forms of glutamine synthetase in free-living root-nodule bacteria. Biochem Biophys Res Commun. 1977 Sep 23;78(2):554–559. doi: 10.1016/0006-291x(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Stadtman E. R. Some kinetic properties of Bacillus subtilis glutamine synthetase. J Biol Chem. 1970 Oct 25;245(20):5206–5213. [PubMed] [Google Scholar]
- Edmands J., Noridge N. A., Benson D. R. The actinorhizal root-nodule symbiont Frankia sp. strain CpI1 has two glutamine synthetases. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6126–6130. doi: 10.1073/pnas.84.17.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. T., Parker J. R., Goodman H. J., Jones D. T., Woods D. R. Molecular analysis of a novel glutamine synthetase of the anaerobe Bacteroides fragilis. J Gen Microbiol. 1989 Dec;135(12):3271–3279. doi: 10.1099/00221287-135-12-3271. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kumada Y., Takano E., Nagaoka K., Thompson C. J. Streptomyces hygroscopicus has two glutamine synthetase genes. J Bacteriol. 1990 Sep;172(9):5343–5351. doi: 10.1128/jb.172.9.5343-5351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon J. M., Nakas J. P. Isolation and Characterization of Frankia sp. Strain FaC1 Genes Involved in Nitrogen Fixation. Appl Environ Microbiol. 1987 Oct;53(10):2321–2327. doi: 10.1128/aem.53.10.2321-2327.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. B., Chapman K. A., Chelm B. K. Role of the Bradyrhizobium japonicum ntrC gene product in differential regulation of the glutamine synthetase II gene (glnII). J Bacteriol. 1988 Dec;170(12):5452–5459. doi: 10.1128/jb.170.12.5452-5459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Watkins P. A., Stanley S. J., Purnell M. R., Kidwell W. R. Inactivation of glutamine synthetases by an NAD:arginine ADP-ribosyltransferase. J Biol Chem. 1984 Apr 25;259(8):5100–5104. [PubMed] [Google Scholar]
- Noridge N. A., Benson D. R. Isolation and nitrogen-fixing activity of Frankia sp. strain CpI1 vesicles. J Bacteriol. 1986 Apr;166(1):301–305. doi: 10.1128/jb.166.1.301-305.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Orr J., Haselkorn R. Regulation of glutamine synthetase activity and synthesis in free-living and symbiotic Anabaena spp. J Bacteriol. 1982 Nov;152(2):626–635. doi: 10.1128/jb.152.2.626-635.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A high speed, high capacity homology matrix: zooming through SV40 and polyoma. Nucleic Acids Res. 1982 Aug 11;10(15):4765–4782. doi: 10.1093/nar/10.15.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasched I., Jörnvall H., Sund H. Studies of glutamate dehydrogenase. Identification of an amino group involved in the substrate binding. Eur J Biochem. 1974 Feb 1;41(3):603–606. doi: 10.1111/j.1432-1033.1974.tb03302.x. [DOI] [PubMed] [Google Scholar]
- Rossbach S, Schell J, de Bruijn F J. The ntrC gene of Agrobacterium tumefaciens C58 controls glutamine synthetase (GSII) activity, growth on nitrate and chromosomal but not Ti-encoded arginine catabolism pathways. Mol Gen Genet. 1987 Oct;209(3):419–426. doi: 10.1007/BF00331144. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatters R. G., Kahn M. L. Glutamine synthetase II in Rhizobium: reexamination of the proposed horizontal transfer of DNA from eukaryotes to prokaryotes. J Mol Evol. 1989 Nov;29(5):422–428. doi: 10.1007/BF02602912. [DOI] [PubMed] [Google Scholar]
- Somerville J. E., Shatters R. G., Kahn M. L. Isolation, characterization, and complementation of Rhizobium meliloti 104A14 mutants that lack glutamine synthetase II activity. J Bacteriol. 1989 Sep;171(9):5079–5086. doi: 10.1128/jb.171.9.5079-5086.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stauffer G. V., Plamann M. D., Stauffer L. T. Construction and expression of hybrid plasmids containing the Escherichia coli glyA genes. Gene. 1981 Jun-Jul;14(1-2):63–72. doi: 10.1016/0378-1119(81)90148-7. [DOI] [PubMed] [Google Scholar]
- Steggles A. W. A rapid procedure for creating nested sets of deletions using mini-prep plasmid DNA samples. Biotechniques. 1989 Mar;7(3):241–242. [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedler F. C., Shreve D. S., Fisher K. E., Merkler D. J. Complementarity of regulation for the two glutamine synthetases from Bacillus caldolyticus, an extreme thermophile. Arch Biochem Biophys. 1981 Oct 1;211(1):276–287. doi: 10.1016/0003-9861(81)90455-0. [DOI] [PubMed] [Google Scholar]
- Wedler F. C., Shreve D. S., Kenney R. M., Ashour A. E., Carfi J., Rhee S. G. Two glutamine synthetases from Bacillus caldolyticus, an extreme thermophile. Isolation, physicochemical and kinetic properties. J Biol Chem. 1980 Oct 10;255(19):9507–9516. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Rossbach S., Schneider M., Ratet P., Messmer S., Szeto W. W., Ausubel F. M., Schell J. Rhizobium meliloti 1021 has three differentially regulated loci involved in glutamine biosynthesis, none of which is essential for symbiotic nitrogen fixation. J Bacteriol. 1989 Mar;171(3):1673–1682. doi: 10.1128/jb.171.3.1673-1682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]