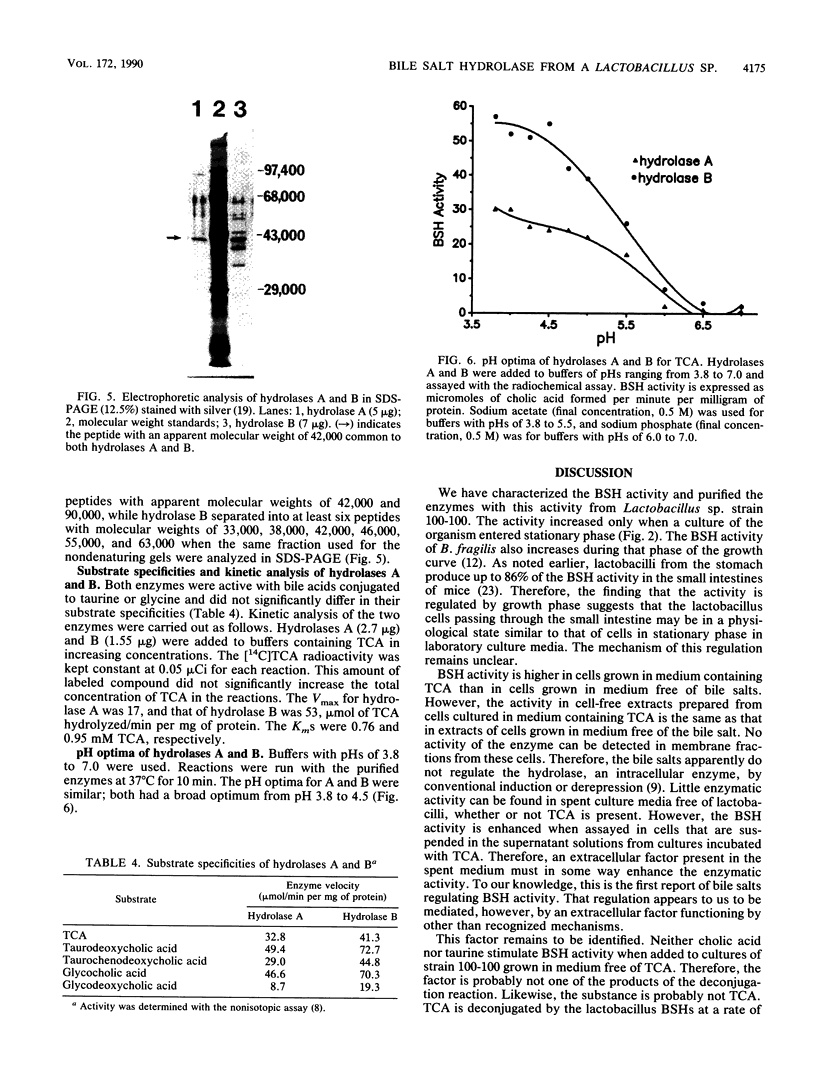
Abstract
We have characterized and purified the bile salt hydrolase from Lactobacillus sp. strain 100-100. Bile salt hydrolase from cells of the strain was purified with column and high-performance liquid chromatography. The activity was assayed in whole cells and cell-free extracts with either a radiochemical assay involving [14C]taurocholic acid or a nonradioactive assay involving trinitrobenzene sulfonate. The activity was detectable only in stationary-phase cells. Within 20 min after conjugated bile acids were added to stationary-phase cultures of strain 100-100, the activity in whole cells increased to levels three- to fivefold higher than in cells from cultures grown in medium free of bile salts. In cell-free extracts, however, the activity was about equal, 1.41 and 1.53 mumol/min per mg of protein, respectively, whether or not the cells have been grown with bile salts present. When supernatant solutions from cultures grown in medium containing taurocholic acid were used to suspend cells grown in medium free of the bile salt, the bile salt hydrolase activity detected in whole cells increased two- to threefold. Two forms of the hydrolase were purified from the cells and designated hydrolases A and B. They eluted from anion-exchange high-performance liquid chromatography in two sets of fractions, A at 0.15 M NaCl and B at 0.18 M NaCl. Their apparent molecular weights in nondenaturing polyacrylamide gel electrophoresis were 115,000 and 105,000, respectively. However, discrepancies existed in the apparent molecular weights and number of peptides detected in sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the two forms. Both had similar substrate specificities, highest on taurodeoxycholic and glycocholic acid, and pH optima between 3.8 and 4.5. The kinetic properties were also similar, with Vmaxs of 17 and 53 micromoles/min per mg of protein and Kms of 0.76 and 0.95 mM taurocholic acid for A and B, respectively. Therefore, whether the enzyme exists in two forms in the cells remains to be determined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aries V., Hill M. J. Degradation of steroids by intestinal bacteria. I. Deconjugation of bile salts. Biochim Biophys Acta. 1970 May 5;202(3):526–534. doi: 10.1016/0005-2760(70)90123-2. [DOI] [PubMed] [Google Scholar]
- Binder H. J., Filburn B., Floch M. Bile acid inhibition of intestinal anaerobic organisms. Am J Clin Nutr. 1975 Feb;28(2):119–125. doi: 10.1093/ajcn/28.2.119. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dickinson A. B., Gustafsson B. E., Norman A. Determination of bile acid conversion potencies of intestinal bacteria by screening in vitro and subsequent establishment in germfree rats. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(5):691–698. doi: 10.1111/j.1699-0463.1971.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Feighner S. D., Dashkevicz M. P. Subtherapeutic levels of antibiotics in poultry feeds and their effects on weight gain, feed efficiency, and bacterial cholyltaurine hydrolase activity. Appl Environ Microbiol. 1987 Feb;53(2):331–336. doi: 10.1128/aem.53.2.331-336.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari A., Pacini N., Canzi E. A note on bile acids transformations by strains of Bifidobacterium. J Appl Bacteriol. 1980 Oct;49(2):193–197. doi: 10.1111/j.1365-2672.1980.tb05117.x. [DOI] [PubMed] [Google Scholar]
- Gilliland S. E., Speck M. L. Deconjugation of bile acids by intestinal lactobacilli. Appl Environ Microbiol. 1977 Jan;33(1):15–18. doi: 10.1128/aem.33.1.15-18.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal-Srivastava R., Hylemon P. B. Purification and characterization of bile salt hydrolase from Clostridium perfringens. J Lipid Res. 1988 Aug;29(8):1079–1085. [PubMed] [Google Scholar]
- Hylemon P. B., Stellwag E. J. Bile acid biotransformation rates of selected gram-positive and gram-negative intestinal anaerobic bacteria. Biochem Biophys Res Commun. 1976 Apr 19;69(4):1088–1094. doi: 10.1016/0006-291x(76)90484-8. [DOI] [PubMed] [Google Scholar]
- Kobashi K., Nishizawa I., Yamada T., Hase J. A new hydrolase specific for taurine-conjugates of bile acids. J Biochem. 1978 Aug;84(2):495–497. doi: 10.1093/oxfordjournals.jbchem.a132152. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Masuda N. Deconjugation of bile salts by Bacteroids and Clostridium. Microbiol Immunol. 1981;25(1):1–11. doi: 10.1111/j.1348-0421.1981.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Midtvedt T. Microbial bile acid transformation. Am J Clin Nutr. 1974 Nov;27(11):1341–1347. doi: 10.1093/ajcn/27.11.1341. [DOI] [PubMed] [Google Scholar]
- Midtvedt T., Norman A. Bile acid transformations by microbial strains belonging to genera found in intestinal contents. Acta Pathol Microbiol Scand. 1967;71(4):629–638. doi: 10.1111/j.1699-0463.1967.tb05183.x. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Stellwag E. J., Hylemon P. B. Purification and characterization of bile salt hydrolase from Bacteroides fragilis subsp. fragilis. Biochim Biophys Acta. 1976 Nov 8;452(1):165–176. doi: 10.1016/0005-2744(76)90068-1. [DOI] [PubMed] [Google Scholar]
- Tannock G. W., Dashkevicz M. P., Feighner S. D. Lactobacilli and bile salt hydrolase in the murine intestinal tract. Appl Environ Microbiol. 1989 Jul;55(7):1848–1851. doi: 10.1128/aem.55.7.1848-1851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldere J., Robben J., De Pauw G., Merckx R., Eyssen H. Isolation and identification of intestinal steroid-desulfating bacteria from rats and humans. Appl Environ Microbiol. 1988 Aug;54(8):2112–2117. doi: 10.1128/aem.54.8.2112-2117.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]