Abstract
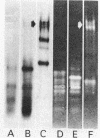
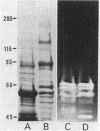
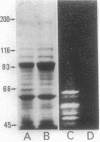
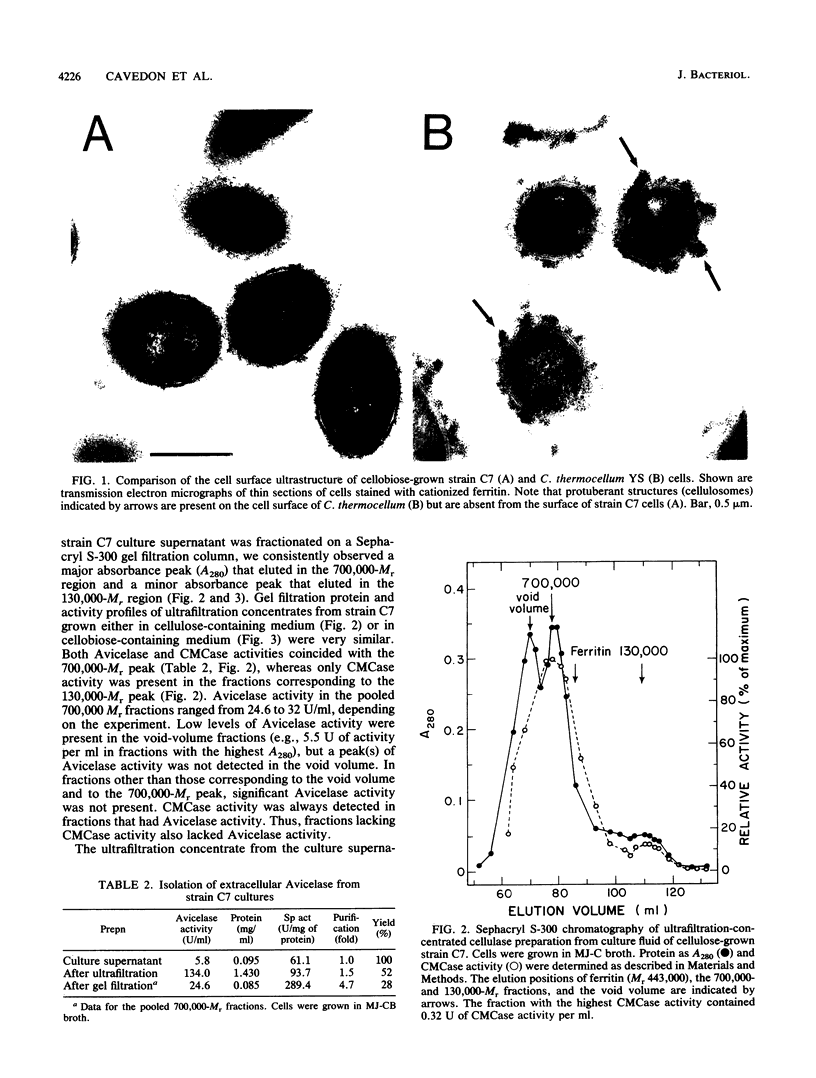
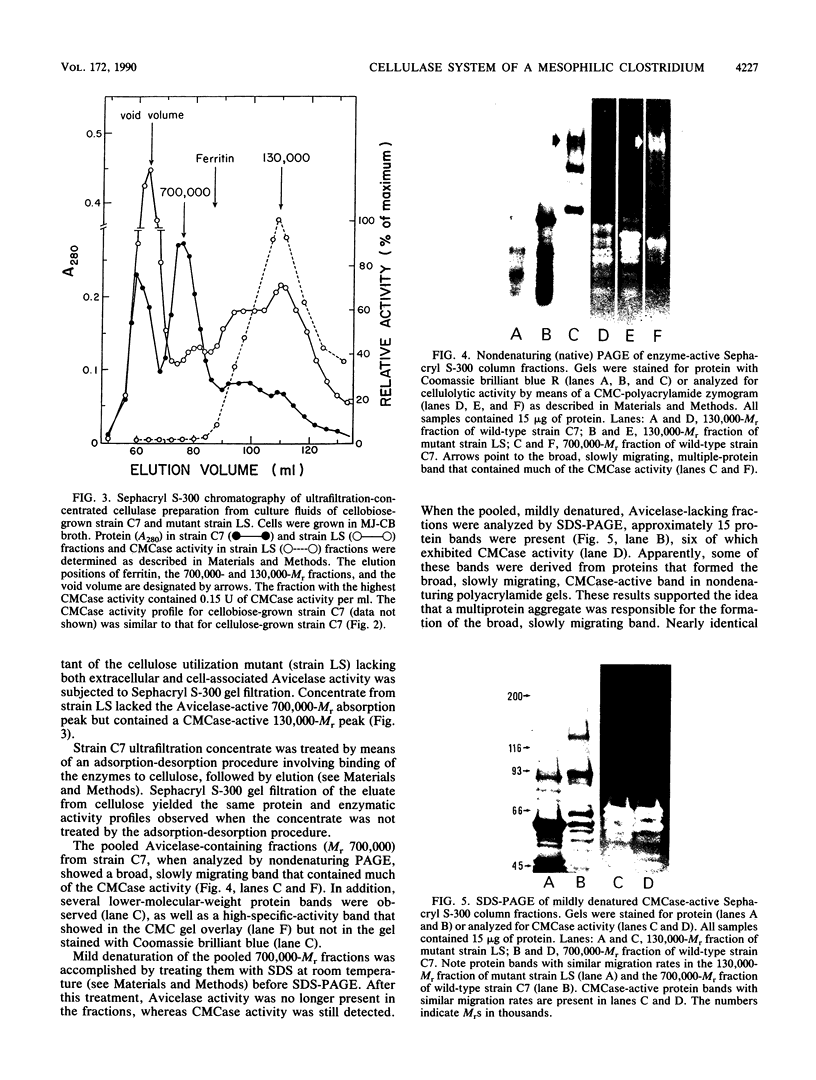
The enzymatic activity responsible for crystalline cellulose degradation (Avicelase activity) by a mesophilic clostridium (strain C7) was present in culture supernatant fluid but was not detected in significant amounts in association with whole cells or in disrupted cells. Cells of the mesophilic clostridium lacked cellulosome clusters on their surface and did not adhere to cellulose fibers. The extracellular cellulase system of the mesophilic clostridium was fractionated by Sephracryl S-300 gel filtration, and the fractions were assayed for Avicelase and carboxymethylcellulase activities. The Avicelase activity coincided with an A280 peak that eluted in the 700,000-Mr region. Nondenaturing polyacrylamide gel electrophoresis and sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis of the 700,000-Mr fractions showed that Avicelase was present as a multiprotein aggregate that lost the ability to hydrolyze crystalline cellulose when partially dissociated by sodium dodecyl sulfate treatment. Proteins resulting from the partial dissociation of the aggregate retained carboxymethylcellulase activity. An Avicelase-deficient mutant of strain C7 (strain LS), which was not capable of degrading crystalline cellulose, lacked the Avicelase-active 700,000-Mr peak. The results indicated that an extracellular 700,000-Mr multiprotein complex, consisting of at least 15 proteins, is utilized by the mesophilic clostridium for the hydrolysis of crystalline cellulose. At least six different endo-1,4-beta-glucanases may be part of the cellulase system of strain C7. Sephacryl S-300 column fractions, corresponding to an A280 peak in the 130,000-Mr region, contained carboxymethylcellulase-active proteins that may serve as precursors for the assembly of the Avicelase-active complex by the mesophilic clostridium.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Lamed R. Ultrastructure of the cell surface cellulosome of Clostridium thermocellum and its interaction with cellulose. J Bacteriol. 1986 Sep;167(3):828–836. doi: 10.1128/jb.167.3.828-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer E. A., Setter E., Lamed R. Organization and distribution of the cellulosome in Clostridium thermocellum. J Bacteriol. 1985 Aug;163(2):552–559. doi: 10.1128/jb.163.2.552-559.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cavedon K., Leschine S. B., Canale-Parola E. Characterization of the extracellular cellulase from a mesophilic clostridium (strain C7). J Bacteriol. 1990 Aug;172(8):4231–4237. doi: 10.1128/jb.172.8.4231-4237.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich G. G., Goerlitz D. F., Bourell J. H., Eisen G. V., Godsy E. M. Liquid chromatographic procedure for fermentation product analysis in the identification of anaerobic bacteria. Appl Environ Microbiol. 1981 Nov;42(5):878–885. doi: 10.1128/aem.42.5.878-885.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Madia A., Demain A. L. Chemically Defined Minimal Medium for Growth of the Anaerobic Cellulolytic Thermophile Clostridium thermocellum. Appl Environ Microbiol. 1981 Apr;41(4):1060–1062. doi: 10.1128/aem.41.4.1060-1062.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Sakajoh M., Halliwell G., Madia A., Demain A. L. Saccharification of Complex Cellulosic Substrates by the Cellulase System from Clostridium thermocellum. Appl Environ Microbiol. 1982 May;43(5):1125–1132. doi: 10.1128/aem.43.5.1125-1132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leschine S. B., Canale-Parola E. Mesophilic cellulolytic clostridia from freshwater environments. Appl Environ Microbiol. 1983 Sep;46(3):728–737. doi: 10.1128/aem.46.3.728-737.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschine S. B., Holwell K., Canale-Parola E. Nitrogen fixation by anaerobic cellulolytic bacteria. Science. 1988 Nov 25;242(4882):1157–1159. doi: 10.1126/science.242.4882.1157. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Mayer F., Coughlan M. P., Mori Y., Ljungdahl L. G. Macromolecular Organization of the Cellulolytic Enzyme Complex of Clostridium thermocellum as Revealed by Electron Microscopy. Appl Environ Microbiol. 1987 Dec;53(12):2785–2792. doi: 10.1128/aem.53.12.2785-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw J. E., Leschine S. B., Canale-Parola E. Anaerobic cellulolytic bacteria from wetwood of living trees. Appl Environ Microbiol. 1985 Oct;50(4):807–811. doi: 10.1128/aem.50.4.807-811.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]