Abstract
Platelet/endothelial cell adhesion molecule (PECAM-1) is a cell adhesion molecule of the immunoglobulin superfamily that plays a role in a number of vascular processes including leukocyte transmigration through endothelium. The presence of a specific 19– amino acid exon within the cytoplasmic domain of PECAM-1 regulates the binding specificity of the molecule; specifically, isoforms containing exon 14 mediate heterophilic cell–cell aggregation while those variants missing exon 14 mediate homophilic cell–cell aggregation. To more precisely identify the region of exon 14 responsible for ligand specificity, a series of deletion mutants were created in which smaller regions of exon 14 were removed. After transfection into L cells, they were tested for their ability to mediate aggregation. For heterophilic aggregation to occur, a conserved 5–amino acid region (VYSEI in the murine sequence or VYSEV in the human sequence) in the mid-portion of the exon was required. A final construct, in which this tyrosine was mutated into a phenylalanine, aggregated in a homophilic manner when transfected into L cells. Inhibition of phosphatase activity by exposure of cells expressing wild type or mutant forms of PECAM-1 to sodium orthovanadate resulted in high levels of cytoplasmic tyrosine phosphorylation and led to a switch from heterophilic to homophilic aggregation. Our data thus indicate either loss of this tyrosine from exon 14 or its phosphorylation results in a change in ligand specificity from heterophilic to homophilic binding. Vascular cells could thus determine whether PECAM-1 functions as a heterophilic or homophilic adhesion molecule by processes such as alternative splicing or by regulation of the balance between tyrosine phosphorylation or dephosphorylation. Defining the conditions under which these changes occur will be important in understanding the biology of PECAM-1 in transmigration, angiogenesis, development, and other processes in which this molecule plays a role.
Platelet/endothelial cell adhesion molecule (PECAM-1, CD31)1 is a 130-kD integral membrane glycoprotein of the immunoglobulin superfamily expressed on endothelial cells, platelets and leukocytes (Newman et al., 1990; reviewed in DeLisser et al., 1994a and Newman, 1997). Although the full range of physiologic functions of PECAM-1 are not yet known, evidence exists to support a role for this molecule in leukocyte transmigration through endothelium (Muller et al., 1993; Vaporciyan et al., 1993; Bogen et al., 1994), release of leukocytes from bone marrow (Leavesley et al., 1994), cardiovascular development (Baldwin et al., 1994; Pinter et al., 1997), and angiogenesis (DeLisser et al., 1997; Matsumura et al., 1997).
PECAM-1 has been noted to function as both an adhesion molecule (Albelda et al., 1991; Muller et al., 1992; DeLisser et al., 1993) and a signal transduction molecule (Tanaka et al., 1992; Piali et al., 1993; Berman and Muller, 1995). It is somewhat unique as a cell adhesion molecule, however, in that conditions have been defined (see below) in which PECAM-1 mediates homophilic interactions (DeLisser et al., 1994b ; Yan et al., 1995) or heterophilic interactions to ligands such as heparin-containing proteoglycans (DeLisser et al., 1993; Watt et al., 1993), the integrin αvβ3 (Piali et al., 1995; Buckley et al., 1996), and an unidentified ligand on activated T cells (Prager et al., 1996). Although we believe that increased cell surface density, through its ability to favor homodimer formation, may be a factor in regulating PECAM-1 function (Sun et al., 1996), the molecular mechanisms that define this ligand specificity are still unknown.
Recent analysis of PECAM-1 expression in the developing mouse embryo has revealed the presence of multiple isoforms of murine PECAM-1 (muPECAM-1) that appear to result from the alternative splicing of exons encoding cytoplasmic domain sequences (exons 10–16) (Baldwin et al., 1994). Forms of human PECAM-1 (huPECAM-1) (Goldberger et al., 1994) and bovine PECAM-1 (Osawa et al., 1997) with alternatively spliced cytoplasmic domains have also been identified. To investigate the functional consequences of alternatively spliced muPECAM-1 cytoplasmic domains, L cells were transfected with cDNA for each variant and their ability to promote cell aggregation was compared (Yan et al., 1995). In this assay, full-length muPECAM-1 and all three isoforms containing exon 14 behaved like full-length huPECAM-1 in that they mediated heterophilic aggregation. In contrast, all muPECAM-1 variants missing exon 14 exhibited homophilic aggregation. Exon 14 thus appeared to be an important modulator of the ligand and adhesive interactions of the extracellular domain of muPECAM-1. These observations were confirmed and expanded in a recent study examining the role of exon 14 in the function of huPECAM-1. Similar to the results with muPECAM-1, all L cell lines expressing mutants of huPECAM-1 lacking exon 14 exhibited homophilic aggregation while all those expressing huPECAM-1 possessing exon 14 aggregated in a heterophilic fashion (Sun et al., 1996).
These data indicate that exon 14 of the cytoplasmic domain contains specific information that regulates the ligand choice of PECAM-1 in transfected L cells. Although alterations in the cytoplasmic domain have clearly been shown to affect ligand binding of many cell adhesion molecules (CAMs) with regard to affinity, to our knowledge, alternative splicing of a cytoplasmic domain has not been reported to regulate ligand specificity of a CAM.
A key question raised by our findings is how changes in one exon of the cytoplasmic domain of PECAM-1 can dramatically affect the binding characteristics of the molecule. Exon 14 is a small exon that encodes 19 amino acids (Kirschbaum et al., 1994). A large portion of the amino acids in this exon are highly conserved in mice (ALGTRATETVYSEIRKVDP) and humans (DLGKKDTETVYSEVRKAVP) (Baldwin et al., 1994). Within this exon, there are five amino acids that could potentially serve as phosphorylation sites, including one tyrosine in the context of a possible SH2 binding domain (YSEI or YSEV). The carboxyl end of the exon has a proline, as well as a number of highly charged amino acids. It is currently unknown which sequences in this exon are regulatory. The purposes of this investigation were to more precisely identify the region of exon 14 responsible for ligand specificity and to use this information to try to begin to understand the mechanism by which this part of the molecule might regulate function. Our experiments suggest that the key residue of exon 14 is the tyrosine at amino acid 686 and that either loss of this tyrosine from exon 14 or its phosphorylation results in a change in ligand specificity from heterophilic binding to homophilic binding. These results imply that vascular cells could thus determine whether PECAM-1 functions as a heterophilic or homophilic adhesion molecule by processes such as alternative splicing or by regulation of the balance between tyrosine phosphorylation or dephosphorylation.
Materials and Methods
Construction of PECAM-1 Cytoplasmic Domain Mutants
A series of mutant muPECAM-1 constructs were produced in which portions of exon 14 were removed or mutated (see Fig. 1). The altered forms of PECAM-1 were constructed from the full-length wild-type murine form of PECAM-1 cDNA subcloned into the PcDNA/Neo vector (Yan et al., 1995). This full-length cDNA served as the template for the sequence overlap extension (SOE) polymerase chain reactions (Horton et al., 1990).
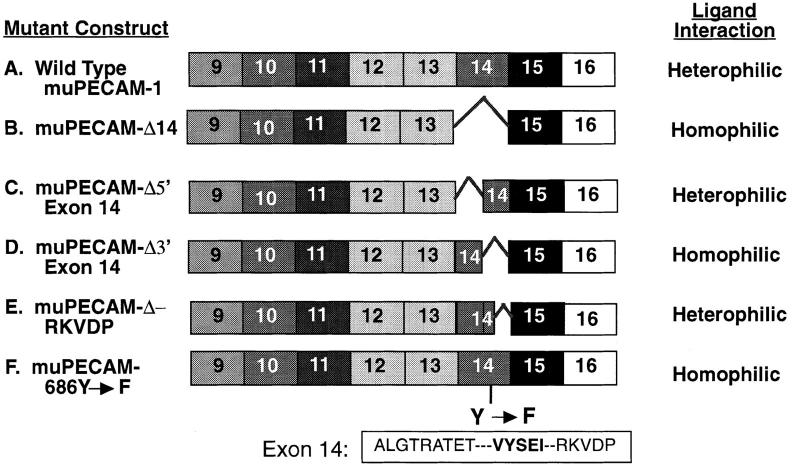
Figure 1.
Cytoplasmic domain mutants of muPECAM-1. The PECAM-1 cytoplasmic domain is encoded by exons 9–16. A series of muPECAM-1 mutants in which specific exons encoding regions of the cytoplasmic domain had been deleted were constructed and expressed in L cells as described in Materials and Methods. The extracellular domain and transmembrane regions were wild type and the same for each construct. The figure indicates: the name of each construct; the regions of exon 14 that were deleted or mutated (note: muPECAM-Δ5′ exon 14 is deleted in the sequence ALGRTRATET; muPECAM-Δ3′ exon 14 is deleted in the sequence VYSEIRKVDP); and the aggregation behavior of murine L cells stably expressing each construct.
The following two outside primers were used as the external primers for these reactions: a sense primer (primer A) (5′-1395TATGAAAGCAAAGAGTGA1412-3′) flanking the BstEII restriction site within the 6th extracellular Ig-like domain, and an antisense primer (primer B) (5′-2253 CGAATGCATCCAGGAATCGGCTGCTCT2235-3′) complimentary to a region 70-bp downstream from the stop codon of muPECAM-1 and carrying an NsiI recognition sequence to facilitate subcloning. For each of the mutants described below a set of inside primers was constructed, each spanning the area of deletion or mutation and each having substantial overlap. The sense inside primer was used in a PCR reaction with primer B to create a fragment that would become the 3′ end of the cytoplasmic domain. The antisense inside primer was used in a PCR reaction with primer A to create a fragment that would become the 5′ end of the cytoplasmic domain. Each of these two fragments were then reacted together in a PCR reaction to create a longer fragment. These SOE products were then digested with BstEII and NsiI and ligated into PcDNA/neo vector containing muPECAM-1 previously cut with the same restriction enzymes.
The following pairs of primers were used as inside primers in the initial PCR to create the following mutants:
PECAM-1-Δ5′ exon 14 (Missing ALGTRATET in Exon 14).
Sense primer (5′-2134 EXON14ACAGAGACG2142EXON15ATTCTCATGGAAAA-3′); Antisense primer (5′-EXON15CATGAGATT2142 EXON14CGTCTCTGTGGCTC2129-3′).
PECAM-1-Δ3′ exon14 (Missing VYSEIRKVDP in Exon 14).
Sense primer (5′-EXON13CCTCACCAAEXON14GTGTACAGTGAGAT-3′); Antisense primer (5′-EXON14ACTGTACACEXON13TTGGTGAGGCTC-3′).
PECAM-1-ΔRKVDP (in Exon 14).
Sense primer (5′-2148 EXON14CAGTGAGATCEXON15AATCTCATGGAAAAC2187-3′); Antisense primer (5′2182 EXON15CCATGAGATTEXON 14GATCTC ACTGTA CAC2145-3′).
PECAM-1-686Y→F (in Exon 14).
Sense primer (5′-2279GAGACGGTGTTCAGTGACATCCGGAAG2305-3′); Antisense primer (5′-GATCTCACTGAACACCGTCTCTGTGGC-3′).
The resulting ligation products were transformed into competent MC1061.p3 cells. Initially, antibiotic resistant colonies were screened by digestion of miniprep plasmid DNA using the enzyme HincII or BsmaI. These enzymes would lose a restriction site if a portion of exon 14 was deleted. Other mutants, in which we could not take advantage of this tactic, were simply checked for insert size by digestion with BstEII and NsiI. All positive clones were then confirmed by sequence analysis using an antisense Sp6 primer 3′ of the mutation site. Candidate clones finally were tested for efficient protein translation by transfection into Cos-1 cells before going on to permanent transfections of L cell fibroblasts.
Tissue Culture and Transfection of L Cells
Murine L cells were cultured in RPMI medium with 10% FBS plus glutamine, and antibiotics. The procedure to transfect PECAM-1 cDNA into these cells has been previously described in detail (DeLisser et al., 1994b ). Briefly 105 L cells were plated on a 100-mm tissue culture plate the night before transfection. The day of the transfection the cells were washed with PBS and fed DME with 10% FBS. Using the CaPO4 method of transfection, 10 μg of muPECAM-1 cDNA and 14 μg of calf thymus DNA were combined with BBS and CaCl2 to form a DNA precipitate. This precipitate was added to the cells in culture and allowed to incubate ∼20 h. After 20 hours the cells were washed three times with PBS and fed RPMI medium with 10% FBS. 24 h after transfection, the cells were split 1:10, and 48 h after transfection the cells were fed G418 selection medium (0.5 mg/ml). The G418-resistant colonies were grown up and tested by FACS® for surface expression of PECAM-1.
To isolate cells with higher expression levels of PECAM-1, cells were sorted using magnetic beads coupled with anti–PECAM-1 antibody. The night before sorting, the transfected L cells were trypsinized and replated in the original tissue culture dishes. On the day of sorting, the transfected L cells were washed with PBS twice and dissociated with enzyme-free cell dissociation solution (Specialty Media, Inc., Lavallette, NJ). The cell pellet was resuspended and incubated in 400 μl of rat anti–mouse PECAM-1 primary antibody (mAb 390) for 1 h on a nutator at 4°C. 1 h later, the cells were spun down, washed with PBS twice, resuspended, and then incubated in 400 μl of RPMI medium with 1% FBS that contained 10 μl of Dynabeads M-450 coated with sheep anti–rat IgG (Dynal, Inc. Great Neck, NY) for 30 min on a nutator at 4°C. After 30 min, the bead-coated cells were resuspended in 3 ml of PBS, transferred into a snap cap tube, and concentrated using a Dynal Magnetic Particle Concentrator (Dynal, Inc.). The sorted cells were washed with PBS three times and cultured in RPMI medium with 10% FBS plus G418.
In addition to the muPECAM-1 L cell lines, experiments were also conducted with L cells transfected with full length huPECAM-1 and with a truncated form of huPECAM containing the extracellular and transmembrane domains along with exon 9, 10, and 14, PECAM-1(+)9,10,14 (Sun et al., 1996).
Antibodies
The following antibodies were used: (a) anti–muPECAM-1 mAb 390 generated in rat after immunization with mouse 32D leukocyte cell line screened against muPECAM-1Δ12,15 (Baldwin et al., 1994); (b) anti– huPECAM-1 polyclonal antibody “Houston” (Albelda et al., 1991); and (c) anti–huPECAM-1 mAb 1.3 generously provided by P. Newman (Blood Center of Southeastern Wisconsin, Milwaukee, WI).
FACS® Analysis.
L cells transfected with muPECAM-1 isoforms were nonenzymatically removed from a T-25 tissue culture flask, washed with PBS, and treated with mAb 390 for 1 h at 4°C. The primary antibody was then removed and the cells were washed with PBS. A dilution of 1:200 of fluorescein isothiocyanate–labeled goat anti–rat secondary antibody was added to the cells for 30 min at 4°C. The cells were then washed again in PBS and flow cytometry was performed using an Ortho Cytofluorograph 50H cell sorter equipped with a 2150 data handling system (Ortho Instruments, Westwood, MA). All cell lines established had an approximate mean fluorescence of 110–125 (a level comparable to that seen on endothelial cells) and were >90% positive for muPECAM-1.
Sodium Orthovanadate Treatment
A working solution at 92 mg/ml of sodium orthovanadate (Sigma Chemical Co., St. Louis, MO) was boiled and cooled. The appropriate amount was added to RPMI/10% FBS to a final concentration of 0.5 mM. The L cells were then incubated for the indicated times in this vanadate-containing media. The time of incubation ranged from overnight exposure to 1-h exposure.
Immunoprecipitation and Immunoblotting
Nonionic detergent cellular extracts were prepared by adding small volumes of TNC (0.01 M Tris acetate, pH 8.0, 0.5% NP-40, 0.5 mM Ca2+) with 2 mM PMSF and 100 mM vanadate to cells that had been trypsinized and washed. Cells were lysed on ice for 20 min. The resulting extracts were preabsorbed for 1 h at 4°C with protein G–conjugated Sepharose beads (Pharmacia Fine Chemicals, Piscataway, NJ). After removal from the beads, the precleared supernatants were transferred to another eppendorf with fresh protein G–Sepharose beads and 2 μl of Houston polyclonal antiserum was added for each 100 μl of lysate. Immunoprecipitation was carried out at 4°C for 2 h. After the immunoprecipitation, the beads were washed five times with DOC wash (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 5% deoxycholate, and 0.1% SDS). The sample was then dissolved in loading buffer (62.5 mM Tris base, 2% SDS, 10% glycerol, pH 6.8), and electrophoresed on a 6% polyacrylamide gel.
After resolving on the SDS-PAGE gel, the proteins were transferred to nitrocellulose membranes. The membranes were blocked for at least 1 h in 4% BSA/PBS/.02% azide. Blots were then incubated in either 1 μg/ml mouse anti-phosphotyrosine mAb (clone 4G10; Upstate Biotechnology Inc., Lake Placid, NY) or in the polyclonal anti–huPECAM-1 antiserum at a dilution of 1:700 for 1 h at room temperature. After extensive washing for 1 h, the blots were incubated in HRP donkey anti–mouse Ig (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) at room temperature for 1 h. After washing, the blots were developed with the chemiluminescence reagent (New England Nuclear, Boston, MA) according to the manufacturer's instructions.
Aggregation of L Cell Transfectants
The aggregation assays used in these studies have been described previously in detail by DeLisser et al. (1993). Briefly, stable L cell transfectants that had been plated (0.8–1 × 106 cells/75-cm2 flask) and grown overnight were nonezymatically removed. The cells were washed twice in 10 mM EDTA in PBS, pH 7.2, and twice in HBSS without divalent cations. After washing, the cells were resuspended at a final concentration of 0.8–1 × 106 cells/ml in HBSS with or without 1 mM calcium. Where indicated, the polyclonal anti–huPECAM-1 antibody Houston was added at a final concentration of 100 μg/ml. After the cells had been dispersed into a single cell suspension, 1-ml aliquots were transferred to wells in a 24-well nontissue culture plastic tray (Costar Corp., Cambridge, MA) that had been previously blocked with 2% BSA in HBSS for 1 h and washed thoroughly with HBSS immediately before use. The nontissue culture-treated trays containing the suspended L cells were rotated on a gyratory platform at 100 rpm, for 45 min at 37°C. Aggregation was quantified by examining representative aliquots from each sample on a hemocytometer grid using phase contrast optics. The number of single cells (cells in aggregates of 3 or less) versus those present in aggregates of greater than three cells were counted from four 1-mm squares. At least 400 cells were counted from each sample. Data were expressed as the percent total cells present in aggregates.
Mixed Aggregation Assay
To determine if the muPECAM-1–dependent L cell aggregation was mediated by homophilic or heterophilic mechanisms, mixed aggregation assays were performed as described in detail (DeLisser et al., 1993). In these experiments, L cell aggregation was performed by mixing nontransfected and transfected L cells, with the nontransfected L cells fluorescently labeled before mixing. After the nontransfected cells had been washed once with EDTA, they were resuspended in HBSS to a total volume of 1 ml. 1 ml of rhodamine-conjugated dye solution at a final concentration of 1 mM (Sigma Chemical Co.), in a buffer provided by the manufacturer, was added, followed by incubation at room temperature for 5–10 min. Labeling was terminated by adding an equal volume of FBS and washing the cells in HBSS. The second EDTA wash and the two HBSS washes were then performed as described above. Each set of cells, one labeled, the other unlabeled, were resuspended at 0.8–1 × 106 cells/ml. 500-μl aliquots of each were combined in the wells of the 24-well nontissue culture plate and allowed to aggregate as described above. After the aggregation was complete, the cells were viewed under epifluorescence. The number of fluorescent cells in each aggregate of a given size was counted. Quantitative analysis of the aggregating cell populations was performed as described by Sieber and Roseman (1981).
Results
The Region of Exon 14 That Regulates Heterophilic Aggregation Is within 5 Amino Acids in the 3′ Portion of Exon 14
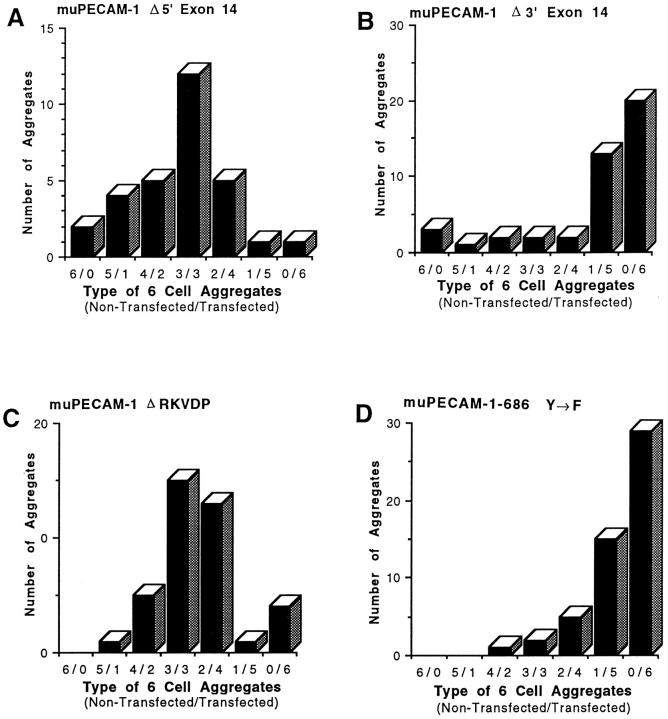
Since the full-length form of muPECAM-1 (Fig. 1 A) mediates heterophilic aggregation and a form missing exon 14 mediates homophilic aggregation (Fig. 1 B), we initially designed two additional muPECAM-1 constructs in which either the 9 amino acids from the NH2-terminal portion of exon 14 (muPECAM-Δ5′ exon 14; Fig. 1 C) or the 10 amino acids from the carboxyl-terminal portion of exon 14 (muPECAM-Δ3′ exon 14; Fig. 1 D) were removed. These constructs were sequenced and transfected into L cells. After selection in G418, cells expressing the constructs were enriched using magnetic beads and at least three separate cell lines with equivalent muPECAM-1 expression selected.
Fig. 2 demonstrates the cell composition of aggregates when these cell lines were subjected to mixed aggregation studies. The construct lacking the NH2-terminal portion of exon 14, muPECAM-Δ5′ exon 14, showed a bell-shaped distribution pattern indicating heterophilic aggregation (Fig. 2 A) similar to that seen with full-length muPECAM-1 (Yan et al., 1995). In contrast, the construct lacking the carboxyl-terminal portion of exon 14, muPECAM-Δ3′ exon 14, showed a pattern consistent with homophilic aggregation (Fig. 2 B) similar to that seen with the muPECAM-1 mutant lacking exon 14 (Yan et al., 1995). These studies indicate that the region of exon 14 that regulates heterophilic aggregation is within the last 10 amino acids (VYSEIRKVDP) of the carboxyl portion of exon 14.
Figure 2.
Effect of deletion of exon 14 or mutation of tyrosine in exon 14 on muPECAM-1–dependent aggregation. Mixed aggregation studies were performed with L cell transfectants expressing muPECAM-1–Δ5′ exon 14 (A), muPECAM- 1-Δ3′ exon 14 (B), muPECAM–1-ΔRKVDP (C) or muPECAM-1–686Y→ F (D) in which equal numbers of nontransfected and transfected L cells were mixed together after fluorescent labeling of one of the cell lines. After incubation, the number of labeled cells within each six-cell aggregate was counted. muPECAM-1-Δ5′ exon 14 (A) and muPECAM-1-ΔRKVDP (C) formed aggregates composed primarily of mixtures of nontransfected and transfected cells consistent with a heterophilic interaction while for muPECAM-1-Δ3′ exon 14 (B) and muPECAM-1-686Y→ F (D) the majority of the aggregates consisted only of transfected cells, indicative of homophilic binding. The data is representative of at least two experiments. All the transfectants described above expressed PECAM-1 at comparable levels by FACS® analysis (mean [log] fluorescence intensities of 100–120).
This carboxyl end of exon 14 appeared to have two potential functional regions. The first was a 5–amino acid region (VYSEI) that places tyrosine in the context of a potential SH2 binding site (Songyang et al., 1993). The second region (RKVDP) was a highly charged region with a terminal proline. To localize the regulatory region more precisely, an additional construct was made in which only the last 5 amino acids in the carboxyl end of exon 14 were removed (muPECAM-ΔRKVDP, Fig. 1 E). After transfecting and isolating L cells expressing this construct at comparable levels, multiple clones were tested in mixed aggregation assays. As shown in Fig. 2 C, these cells aggregated in a heterophilic manner.
Since the muPECAM-Δ3′ exon 14 mutant showed a pattern characteristic of homophilic aggregation, while the muPECAM-DRKVDP aggregated in a heterophilic manner, these data indicate that the presence of the VYSEI region was the critical region to confer heterophilic binding.
Mutation of a Single Amino Acid in Exon 14 (the Tyrosine at AA686) Can Convert Heterophilic to Homophilic Binding
As mentioned above, the VYSEI sequence fits criteria for a potential SH2 binding domain. To determine if this tyrosine was important in the ability of this sequence to regulate PECAM-1 function, an additional construct was made in which the tyrosine at amino acid 686 was mutated to a phenylalanine (muPECAM-1-686Y→ F, Fig. 1 F).
After transfecting and isolating L cells expressing this construct at comparable levels, multiple clones were tested in mixed aggregation assays. As shown in Fig. 2 D, these cells aggregated in a homophilic manner. This experiment indicates that mutation of a single amino acid in exon 14 (the tyrosine at AA686) can convert heterophilic to homophilic binding.
PECAM-1 Can Be Phosphorylated on Tyrosine after Treatment with Sodium Orthovanadate
The results described above indicate that the tyrosine in exon 14 plays a role in the ligand specificity of PECAM-1. It was therefore of interest to study the effects of enhanced tyrosine phosphorylation on PECAM-1 function.
PECAM-1 is known to be phosphorylated primarily on serine and threonine residues (Newman et al., 1992; Zhender et al., 1992). However, recent studies have identified conditions where tyrosine phosphorylation has also been observed (Modderman et al., 1994; Lu et al., 1996; Newman, 1997). To examine the status of tyrosine phosphorylation in L cells transfected with full-length PECAM-1, cells were immunoprecipitated with a polyclonal antibody against huPECAM (Houston) and transferred to nitrocellulose where they were then immunoblotted with either an mAb to huPECAM-1 (mAb 1.3) or an mAb recognizing phosphotyrosine. HuPECAM-1 was used in these studies since an antibody capable of immunoblotting murine PECAM was not available and we have observed no differences in the behavior of muPECAM and huPECAM with regard to the presence or absence of exon 14 (DeLisser et al., 1993; Yan et al., 1995; Sun et al., 1996).
As shown in Fig. 3 (lane 1), under resting conditions, L cells expressing huPECAM-1 show very low levels of tyrosine phosphorylation. Treatment of cells with the tyrosine phosphatase inhibitor, sodium orthovanadate, however, prevented dephosphorylation and resulted in very high steady state levels of PECAM-1 tyrosine phosphorylation (Fig. 3, lane 2). As a control, treatment of L cells expressing a mutant form of huPECAM-1 lacking the exons 11–16 (PECAM-Δ11–16) showed no evidence of tyrosine phosphorylation before (Fig. 3, lane 3) or after (Fig. 3, lane 4) treatment with sodium orthovanadate.
Figure 3.
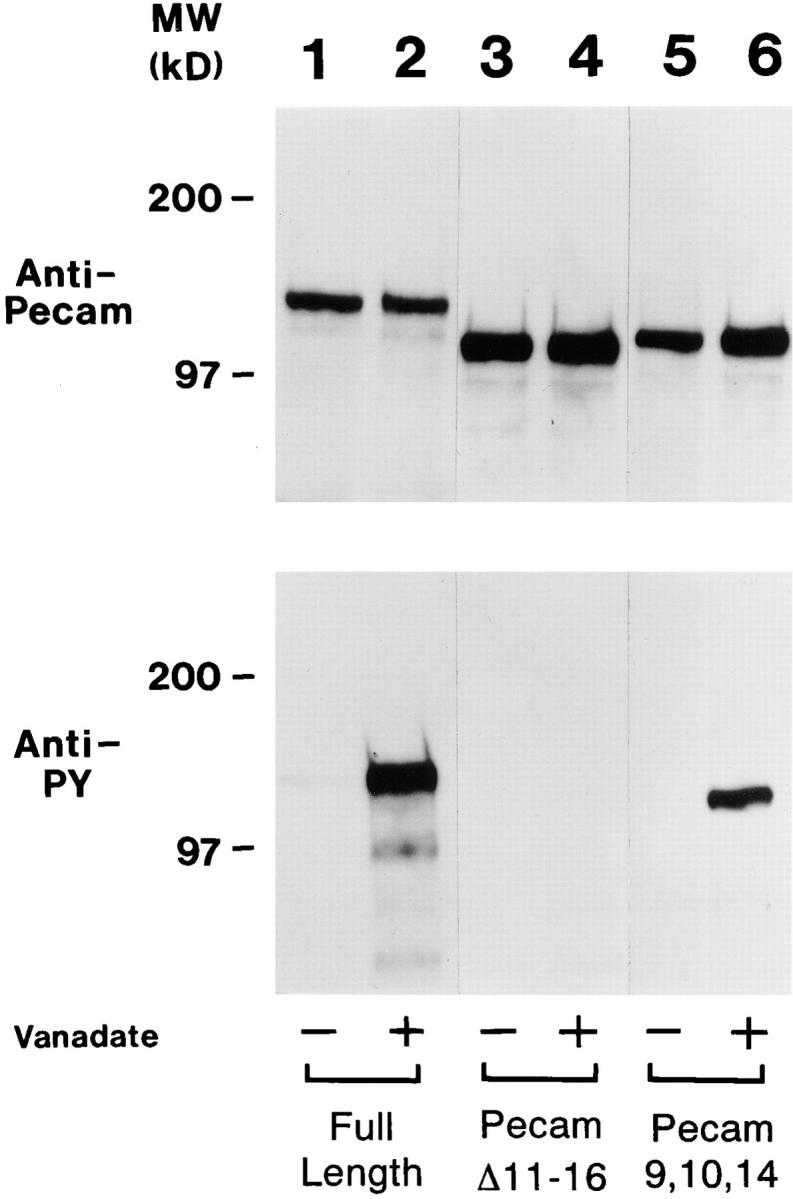
Immunoprecipitation and immunoblotting of PECAM-1 mutants with anti-PECAM and anti-phosphotyrosine antibodies. L cells expressing full-length huPECAM-1 (lanes 1 and 2), huPECAM-1 lacking exons 11–16 (PECAMΔ11–16, lanes 3 and 4), and huPECAM-1 lacking exons 11–16 with the addition of exon 14 (PECAM-9,10,14, lanes 5 and 6) were immunoprecipitated with a polyclonal antiserum directed against huPECAM-1, transferred to nitrocellulose, and immunoblotted with a monoclonal antibody directed against either huPECAM-1 (upper panel) or phosphotyrosine (lower panels). Cells were first exposed to control media (Vanadate −) or to media containing sodium orthovanadate for 24 h (Vanadate +). The upper panels show uniform expression of PECAM-1 under these conditions. Note that the mutant forms of PECAM-1 have a slightly lower molecular weight. Under control conditions, little or no phosphotyrosine was detected in the full-length PECAM-1 (lane 1) or in either mutant (lanes 3 and 5). Treatment of cells with the tyrosine phosphatase inhibitor, sodium orthovanadate, however, induced very high levels of PECAM-1 tyrosine phosphorylation (lane 2). In contrast, L cells expressing PECAM-Δ11–16 did not exhibit tyrosine phosphorylation before (lane 3) or after (lane 4) treatment with sodium orthovanadate. However, orthovanadate treatment of cells expressing PECAM-9,10,14 resulted in the appearance of a strong band (lane 6) indicating that the tyrosine on exon 14 was phosphorylated under these conditions.
Tyrosine Phosphorylation of PECAM Converts Heterophilic to Homophilic Binding
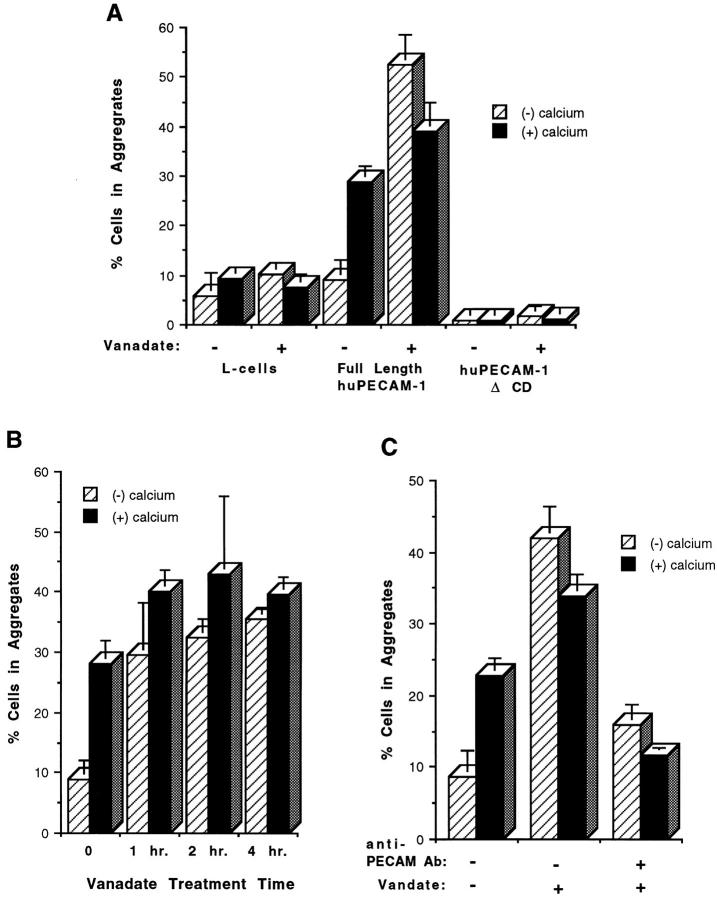
Having demonstrated that treatment of PECAM-expressing L cells with sodium orthovanadate led to high levels of tyrosine phosphorylation, the functional result of this change was analyzed by performing aggregation studies. Sham-transfected L cells, which normally aggregate at very low levels in the presence or absence of calcium were not induced to aggregate by the addition of sodium vanadate (Fig. 4 A). As described before, L cells expressing full-length huPECAM aggregated in a calcium-dependent, heterophilic fashion (Fig. 4 A). Exposure of these cells to orthovanadate, however, changed the aggregation pattern to one that was more robust and calcium independent. When mixed aggregation studies were performed, a pattern consistent with homophilic aggregation was observed (data not shown). Although our initial studies were performed after exposure of cells to vanadate for 24 h, the effect of vanadate was much more rapid. Fig. 4 B shows a time course experiment, where the full effects of orthovanadate were evident when the compound was added only during the aggregation assay (∼1 h of exposure).
Figure 4.
Aggregation of L cell transfectants expressing huPECAM-1 before and after sodium orthovanadate treatment. (A) Standard aggregation studies were performed with and without (1 mM) calcium and with and without 24 h of pretreatment with sodium orthovanadate in sham-transfected L cells, L cell transfectants expressing full-length huPECAM-1, and L cells expressing a mutant of PECAM-1 lacking the cytoplasmic domain (huPECAM-1ΔCD). Sham-transfected L cells and L cells expressing huPECAM-1ΔCD did not aggregate in the presence or absence of calcium. No aggregation was induced by exposure to vanadate. As expected, L cells expressing full-length huPECAM-1 demonstrated calcium-dependent aggregation under control conditions. Exposure of these cells to orthovanadate, however, changed the aggregation pattern to one that was more robust and calcium independent. (B) Time course. Standard aggregation studies were performed with and without calcium (1 mM) in L cell transfectants expressing full-length huPECAM-1. The cells were exposed to media containing sodium vanadate for various lengths of time. Augmentation of aggregation and conversion to calcium-independent aggregation occurred after as little as 1 h of exposure to vanadate. (C) Antibody inhibition studies. Standard aggregation studies were performed with and without calcium (1 mM) in L cell transfectants expressing full-length huPECAM-1 in the presence or absence of sodium orthovanadate and a polyclonal anti–huPECAM antibody. Exposure of cells to orthovanadate changed the aggregation pattern to one that was more robust and calcium independent. Both calcium- and calcium-independent aggregation was blocked to nearly baseline levels by the anti–PECAM-1 antibody. These data presented are from at least three experiments done in duplicate or triplicate and of at least two independent clones. Bars represent mean values. Error bars depict the standard error of the mean. All the transfectants described above expressed PECAM-1 at comparable levels by FACS® analysis (mean log fluorescence intensities of 100–120).
Additional controls were performed to document the specificity of this response. Cells expressing a mutant form of huPECAM-1 lacking the entire cytoplasmic domain (PECAM-ΔCD) have no ability to aggregate under normal conditions (DeLisser et al., 1994b ). Addition of orthovanadate did not induce aggregation (Fig 4 A). To further document that the change in aggregation behavior was PECAM dependent, the ability of an anti-PECAM polyclonal antibody that has previously been shown to inhibit PECAM-mediated aggregation (Albelda et al., 1991) was tested. As shown in Fig. 4 C, addition of this antibody to cells expressing full-length huPECAM-1 in the presence of othovanadate blocked both calcium-dependent and -independent aggregation nearly completely.
Similar results were also observed using L cells expressing the murine form of PECAM-1. As shown in Fig. 5, exposure of muPECAM-1–transfected L cells to orthovanadate for a period as short as 1 h converted calcium-dependent, heterophilic (Fig. 5, A and B) aggregation to calcium-independent, homophilic (Fig. 5, A and C) aggregation.
Figure 5.
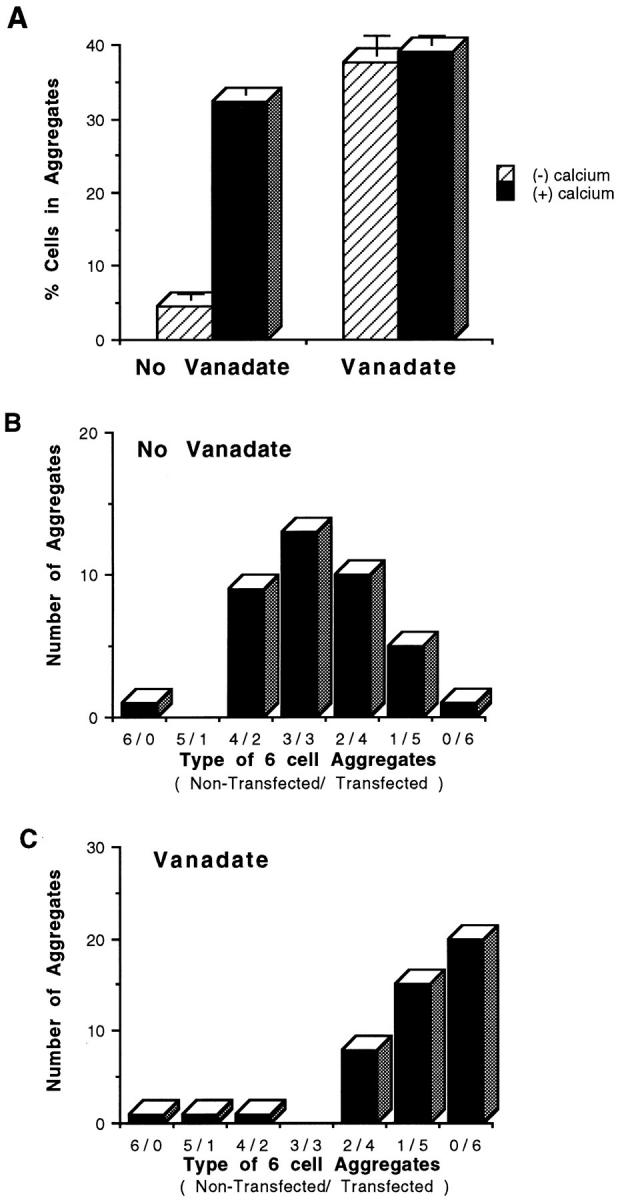
Aggregation of full-length muPECAM-1 L cell transfectants with and without exposure to sodium othovanadate. Standard and mixed aggregation studies were performed with and without (1 mM) calcium and with and without 1 h of pretreatment with sodium orthovanadate in L cell transfectants of full-length muPECAM-1. In standard aggregation studies, (A) L cells expressing full-length huPECAM-1 demonstrated calcium-dependent aggregation under control conditions. Identical to the huPECAM-1 cells, exposure to orthovanadate, however, changed the aggregation pattern to one that was more robust and calcium independent. Bars represent mean values from at least three experiments. Error bars depict the standard error of the mean. In mixed aggregation assays (with 1 mM calcium), the transfectants of muPECAM-1 without exposure to vanadate formed mixed aggregates (heterophilic interaction) (B), while the cells expressing muPECAM-1 after 1 h of exposure to sodium orthovanadate formed primarily self-aggregates (homophilic interaction) (C). These data are representative of at least three experiments.
Thus, in cells expressing either human or murine PECAM-1, tyrosine phosphorylation of PECAM converts heterophilic to homophilic binding.
Tyrosine Phosphorylation of Exon 14 of PECAM-1 Converts Heterophilic to Homophilic Binding
The experiments described above establish that tyrosine phosphorylation of the cytoplasmic domain can change ligand specificity of mu- or huPECAM-1; however, they do not specifically implicate exon 14 as there are 5 tyrosines that are potential phosphorylation sites within the cytoplasmic domain (in exons 9, and 12–15). To determine the functional importance of phosphorylation of this tyrosine, we needed to study a form of PECAM-1 that (a) contained the tyrosine in exon 14, (b) aggregated in a heterophilic manner, and (c) did not include other regions of the cytoplasmic domain that contained tyrosine residues that could be phosphorylated after exposure to sodium vanadate.
To determine if phosphorylation of exon 14 alone could induce changes in ligand binding, we therefore used two previously described huPECAM-transfected L cell lines (Sun et al., 1996). huPECAM-1Δ11–16 contains the extracellular and transmembrane domains of huPECAM-1, plus a small portion of the cytoplasmic domain that contains exons 9 and 10. This construct thus contains only the tyrosine in exon 9. As described before, L cells transfected the huPECAM-1Δ11–16 aggregate in a homophilic manner. huPECAM-1(+)9,10,14 is identical to huPECAM-1Δ11–16 except that the cytoplasmic domain also contains all of exon 14 at its 3′ end. In contrast to huPECAM-1Δ11–16, L cells transfected with this construct aggregate in a heterophilic manner (Sun et al., 1996); therefore, the ability of vanadate treatment to convert this form of huPECAM-1 to homophilic binding would strongly implicate the tyrosine in exon 14 as being important.
L cells containing these two constructs were immunoprecipitated with anti–PECAM-1 antibody and then immunoblotted with antibodies against PECAM-1 or phosphotyrosine before and after treatment with vanadate. As shown in Fig. 3, the huPECAM-1Δ11–16 expressing cells showed no evidence of tyrosine phosphorylation either before or after orthovanadate (lanes 3 and 4), thus indicating that the tyrosine in exon 9 is not phosphorylated under these conditions. In contrast, the immunoblot of huPECAM-1 (+)9,10,14 after orthovanadate treatment (compare lane 5 with lane 6) shows a strong band, indicating that the tyrosine on exon 14 is phosphorylated under these conditions, although it is formally possible that the tyrosine on exon 9 was also phosphorylated (in the presence of exon 14).
To determine the functional significance of this phosphorylation, L cells transfected with huPECAM-1(+) 9,10,14 were tested in a mixed aggregation assay with and without orthovanadate. As shown in Fig. 6, addition of orthovanadate converted binding from calcium-dependent heterophilic aggregation to calcium-independent homophilic aggregation.
Figure 6.
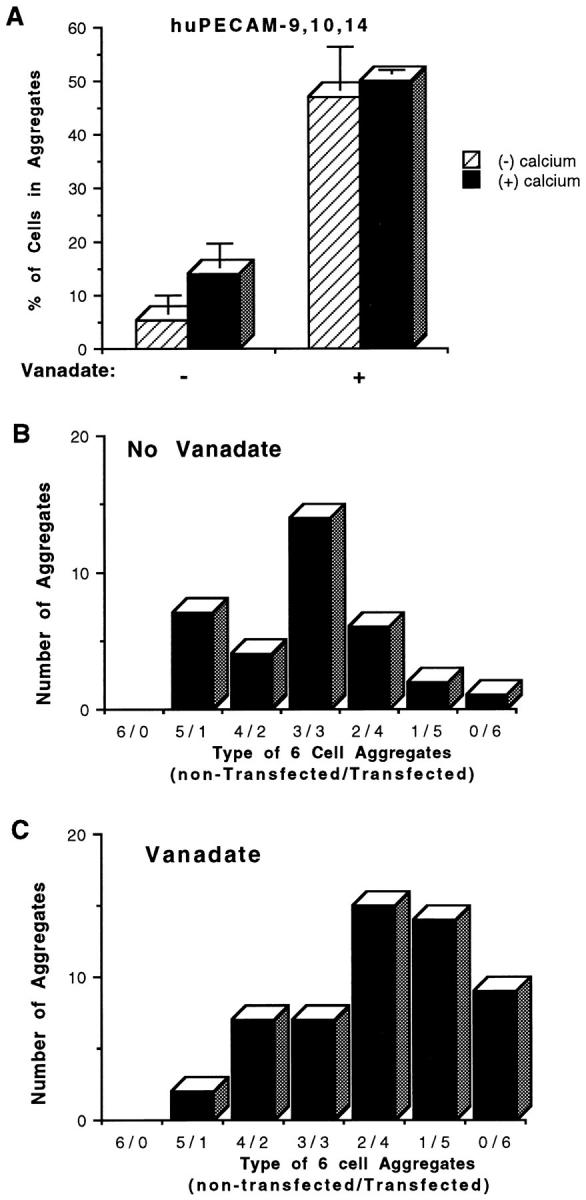
Aggregation of huPECAM-1–9,10,14 L cell transfectants with and without exposure to sodium orthovanadate. Standard and mixed aggregation studies were performed with and without (1 mM) calcium and with and without 24 h of pretreatment with sodium orthovanadate in L cell transfectants of huPECAM-1 lacking exons 11–16 with the addition of exon 14 (huPECAM-1–9,10,14). In standard aggregation studies (A), L cells expressing huPECAM-1–9,10,14 demonstrated calcium-dependent aggregation under control conditions. Exposure to orthovanadate, however, changed the aggregation pattern to one that was more robust and calcium-independent. Bars represent mean values from at least three experiments. Error bars depict the standard error of the mean. In mixed aggregation assays, the transfectants of huPECAM-1–9,10,14 without exposure to vanadate formed mixed aggregates (heterophilic interaction) (B), while the cells expressing huPECAM-1–9,10,14 after 1 h of exposure to sodium orthovanadate formed primarily self-aggregates (homophilic interaction) (C). These data are representative of at least three experiments.
Thus, phosphorylation of the tyrosine in exon 14 has the ability to convert heterophilic to homophilic binding.
Discussion
Previous studies using L cell aggregation as a model for PECAM-1–mediated adhesion have implicated a small region of the cytoplasmic domain, exon 14, as being central in regulating the ligand binding specificity of both mu- and huPECAM-1 (DeLisser et al., 1994b ; Yan et al., 1995; Sun et al., 1996). The purpose of this study was to isolate the precise region of this exon responsible for this activity and, by doing so, help elucidate the mechanism by which it regulated specificity. Since removal of all exon 14 converted heterophilic to homophilic binding, a series of deletion mutants were created in which smaller regions of exon 14 were removed. After transfection into L cells, they were tested for their ability to mediate aggregation. The results of these studies (Fig. 1) show that for heterophilic aggregation to occur, a conserved 5-amino acid region (VYSEI in the murine sequence or VYSEV in the human sequence) in the mid-portion of the exon must be present. Conversely, loss of this small region converted heterophilic to homophilic binding. Because this region contains a tyrosine in the context of a potential SH2 binding region, a final construct was generated in which the only mutation was the conservative change of this tyrosine into a phenylalanine. Unlike L cells expressing wild-type PECAM-1, L cells expressing this Y→ F mutant construct aggregated in a homophilic manner, implicating this tyrosine as critically important in the regulation of ligand binding. A similar mutation in the same tyrosine of huPECAM (Y686) has also recently been shown to affect migration rates in PECAM-1–transfected 3T3 cells (Lu et al., 1996).
The role of this tyrosine was further explored in a series of experiments examining the effects of enhanced phosphorylation of PECAM-1. This was accomplished by inhibiting tyrosine phosphatase activity by exposing cells to sodium orthovanadate using a protocol recently described by Lu et al. (1996). Similar to the findings of this group and others (Newman et al., 1992; Zhender et al., 1992), we found that at baseline, PECAM-1 showed only very low levels of tyrosine phosphorylation using a sensitive immunoprecipitation/immunoblotting technique. Inhibition of phosphatase activity by a short exposure to sodium orthovanadate, however, resulted in high levels of cytoplasmic tyrosine phosphorylation (Fig. 3). Interestingly, this enhanced phosphorylation of PECAM-1 led to a switch from heterophilic to homophilic aggregation. Although orthovanadate treatment of cells leads to global increases in tyrosine phosphorylation, specificity of this observation with regard to PECAM-1 was confirmed by (a) lack of effect of sodium orthovanadate in nontransfected L cells, (b) lack of effect in L cells transfected with forms of PECAM-1 lacking the cytoplasmic domain, and (c) the ability of an anti–PECAM-1 antibody to inhibit the phosphatase-induced homophilic aggregation. Although there are four tyrosines that can potentially undergo phosphorylation after treatment with orthovanadate, phosphorylation of the tyrosine on exon 14 was shown to be sufficient to induce conversion of heterophilic to homophilic aggregation in experiments using L cells expressing a construct containing exons 9, 10, and 14. Cells expressing this construct undergo heterophilic aggregation under control conditions (Sun et al., 1996). However, exposure of these cells to orthovanadate induced both tyrosine phosphorylation (Fig. 3) and conversion to homophilic aggregation (Fig. 6).
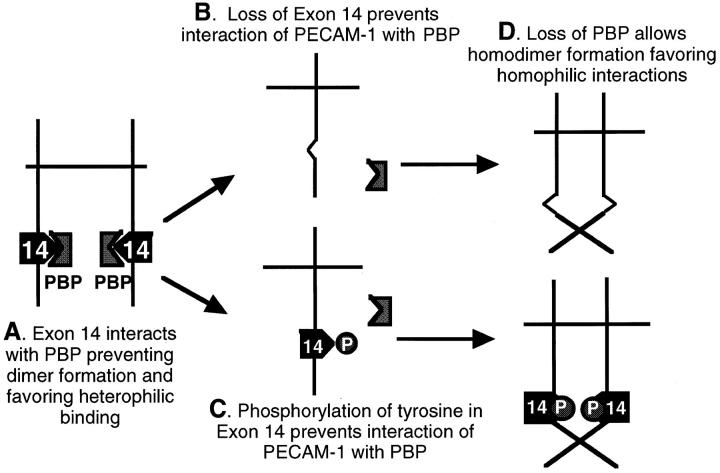
Our data thus indicate either loss of the tyrosine in exon 14 (by deletion or mutation) or phosphorylation of this tyrosine (by inhibition of phosphatase activity) results in a change in ligand specificity from heterophilic to homophilic binding. At first glance, these data appear difficult to reconcile; however, the unifying feature in both conditions is a loss of the normal nonphosphorylated tyrosine residue, suggesting that this amino acid is critical for a potential protein–protein interaction. Although a great deal of attention has been paid to understanding the mechanisms of the binding of cytoplasmic proteins (i.e., SH2 and SH3 domain-containing proteins) to phosphorylated tyrosine residues (i.e., Songyang et al., 1993), there is at least one other example of protein interactions that depend on the presence of nonphosphorylated molecules and, in fact, are inhibited by tyrosine phosphorylation. Galcheva-Gargova et al. (1996) have recently described a zinc finger protein termed ZPR1 that binds only to the cytoplasmic tyrosine kinase domain of the epidermal growth factor receptor (EGFR) when the receptor is in its nonactivated, nonphosphorylated state. Treatment of cells with ligand (EGF) induced tyrosine phosphorylation of the EGFR and caused decreased binding of ZPR1. We speculate that a similar type of interaction may be occurring with PECAM-1 and thus hypothesize (Fig. 7) that under baseline conditions, when full-length PECAM-1 is in its nonphosphorylated state, it interacts with a cytoplasmic protein (a theoretical PECAM binding protein [PBP]) that binds to a region of the cytoplasmic domain at or near the tyrosine in exon 14 (Fig. 7 A). This binding appears to keep PECAM-1 in a form that favors heterophilic binding. Potential mechanisms for this effect could be induction of a conformation change that is transmitted to the extracellular domain or, possibly, as shown in Fig. 7, maintenance of PECAM-1 in a monomeric, nondimerized form, a conformation that we believe favors heterophilic binding (Sun et al., 1996). We postulate that either loss of the tyrosine in exon 14 due to deletion/mutation (Fig. 7 B) or phosphorylation of this tyrosine (Fig. 7 C) inhibits the interaction of PECAM-1 with this PBP that then allows either a conformational change or favors the formation of homodimers that promote homophilic binding (Fig. 7 D). It is also possible, as has been recently speculated by Newman (1997), that PECAM-1–induced heterophilic aggregation is mediated by a second molecule. In our schema, loss of a PBP bound to the tyrosine in exon 14 that transmitted a signal to this other L cell adhesion protein could also lead to a loss of PECAM-1–induced heterophilic aggregation. A search for such a PECAM-1 binding protein is currently underway in our laboratory.
Figure 7.
Proposed mechanism for the role of exon 14 in PECAM-1–dependent adhesion. (A) Under baseline conditions, when full-length PECAM-1 is in its nonphosphorylated state, it interacts with a cytoplasmic protein (a PBP, PECAM binding protein) that binds to a region of the cytoplasmic domain at or near the tyrosine in exon 14. This interaction appears to keep PECAM-1 in a form that favors heterophilic binding. Potential mechanisms for this effect could be induction of a conformation change that is transmitted to the extracellular domain, or possibly maintenance of PECAM-1 in a monomeric, nondimerized form, a conformation that we believe favors heterophilic binding. Either loss of the tyrosine in exon 14 due to deletion/mutation (B) or phosphorylation of this tyrosine (C) inhibits the interaction of PECAM-1 with this PBP that then allows either a conformational change or favors the formation of homodimers that promote homophilic binding (D).
It is intriguing to note that a very similar tyrosine-containing sequence (IYSEVKK) is also present in the cytoplasmic domain of biliary glycoprotein (BGP/CD66a), an immunoglobulin superfamily molecule related to carcinoembryonic antigen found on granulocytes and other cells (Rojas et al., 1990; Afar et al., 1992). Biliary glycoprotein can also be alternatively spliced, and vanadate treatment leads to increased phosphorylation (Afar et al., 1992). Interestingly, phosphorylation of the analogous tyrosine-containing region leads to binding of the protein tyrosine phosphatase SHP-1 (Beauchemin et al., 1997). The functional consequences of these events on the cell adhesion properties of BGP, which can participate in homotypic and heterotypic binding (Oikawa et al., 1992), have not been described.
It is becoming clear that the tyrosine phosphorylation status of PECAM-1 may be a critical factor in regulating the function of the molecule. Although initial studies of PECAM-1 found evidence of serine and threonine phosphorylation without tyrosine phosphorylation (Newman et al., 1992; Zehnder et al., 1992), a number of recent studies have begun to define conditions where tyrosine phosphorylation has been detected. Lu et al. (1996) found that PECAM-1 was tyrosine phosphorylated in cells after trypsinization and suggested that β1 integrin engagement might induce PECAM-1 dephosphorylation. More recently, this group has found that the tyrosine phosphorylation status of Tyr686 changes during vasculogenesis in the murine conceptus (Pinter et al., 1997) and that phosphorylation of this tyrosine could be induced by cSrc (Lu et al., 1997). Tyrosine phosphorylation of PECAM-1 has also been observed in endothelial cells after mechanical stimulation (Osawa et al., 1997) and after aggregation of the high affinity IgE receptor in a basophilic cell line (Sagawa et al., 1997). Tyrosine phosphorylation of platelet PECAM-1 has also been observed (Modderman et al., 1994; Newman, 1997).
In addition to our evidence that the phosphorylation status of Y686 in PECAM-1 regulates ligand specificity, tyrosine phosphorylation is also likely to be important in other functions of PECAM-1. Recent work by Newman's group (Jackson et al., 1997), suggests that phosphorylation of the tyrosines in exons 13 and 14 provide a docking site for the protein tyrosine phosphatase, SHP-2. SHP-2 may then function as an active phosphatase or serve as a docking protein. Although PECAM-1 is not tyrosine phosphorylated under the conditions of our aggregation assay (data not shown) and thus not expected to interact with SHP-2, we did specifically look for evidence of SHP-2/PECAM-1 association by coimmunoprecipitation/immunoblot analysis before, during, and after aggregation. Despite seeing SHP-2 association with PECAM-1 after vanadate treatment, we found no evidence of PECAM-1–tyrosine phosphorylation during aggregation and, as expected, no association of SHP-2 with PECAM-1 during the aggregation process (data not shown). Thus, while the interaction of SHP-2 and PECAM-1 likely plays a role in “outside-in” signaling, we found no evidence to suggest that this association is directly involved in determining ligand specificity.
In summary, we have identified a unique mechanism for control of ligand specificity in an important cell adhesion molecule. By processes such as alternative splicing or regulation of the balance between tyrosine phosphorylation/ dephosphorylation, vascular cells could determine whether PECAM-1 functions as a heterophilic or homophilic adhesion molecule. Both of these mechanisms appear to be operative during cardiovascular development (Baldwin et al., 1994; Pinter et al., 1997). Defining other conditions under which these changes occur will be important in understanding the biology of PECAM-1 in transmigration, angiogenesis, inflammation, and other processes in which this molecule might play an important role.
Acknowledgments
The authors thank M. Daise for her technical assistance and Dr. C. Buck for his helpful comments.
This work was supported by grants from the Robert Wood Johnson Foundation Minority Faculty Development Program and National Institutes of Health grants HL-46311 and HL-03382. Much of this work was completed while S.M. Albelda was an Established Investigator of the American Heart Association.
Abbreviations used in this paper
- huPECAM-1
human platelet/endothelial cell adhesion molecule
- muPECAM-1
murine platelet/endothelial cell adhesion molecule
- PBP
PECAM binding protein
- PECAM-1
platelet/endothelial cell adhesion molecule
Footnotes
Address all correspondence to Steven M. Albelda, 809 Maloney Bldg., University of Pennsylvania Medical Center, 3600 Spruce St., Philadelphia, PA 19140-4283. Tel: (215) 662-3307. Fax: (215) 349-5172.
References
- Afar D, Stanners CP, Bell JC. Tyrosine phosphorylation of biliary glycoprotein, a cell adhesion molecule related to carcinoembryonic antigen. Biochim Biophys Acta. 1992;1134:46–52. doi: 10.1016/0167-4889(92)90026-8. [DOI] [PubMed] [Google Scholar]
- Albelda SM, Muller WA, Buck CA, Newman P. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HS, Shen HM, Yan H, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, Buck CA. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development (Camb) 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- Beauchemin N, Kunath T, Robitaille J, Chow B, Turbide C, Daniels E, Veillette A. Association of biliary glycoprotein with protein tryosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene. 1997;14:783–790. doi: 10.1038/sj.onc.1200888. [DOI] [PubMed] [Google Scholar]
- Berman ME, Muller WA. Ligation of platelet/endothelial cell adhesion molecule 1 [PECAM-1/CD31] on monocytes and neutrophils increases binding capacity of leukocyte CR3 [CD11b/CD18] J Immunol. 1995;154:299–307. [PubMed] [Google Scholar]
- Bogen S, Pak J, Garifallou M, Deng X, Muller WA. Monoclonal antibody to murine PECAM-1 (CD31) blocks acute inflammation in vivo. J Exp Med. 1994;179:1059–1064. doi: 10.1084/jem.179.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Doyonnas R, Newton JP, Blystone SD, Brown EJ, Watt SM, Simmons DL. Identification of αvβ3 as a heterotypic ligand for CD31/PECAM-1. J Cell Sci. 1996;109:437–445. doi: 10.1242/jcs.109.2.437. [DOI] [PubMed] [Google Scholar]
- DeLisser HM, Yan H-C, Newman PJ, Muller WA, Buck CA, Albelda SM. Platelet/endothelial cell adhesion molecule-1 (CD31)-mediated cellular aggregation involves cell surface glycosaminoglycans. J Biol Chem. 1993;268:16037–16046. [PubMed] [Google Scholar]
- DeLisser HM, Newman PJ, Albelda SM. Molecular and functional aspects of PECAM-1/CD31. Immunol Today. 1994a;15:490–494. doi: 10.1016/0167-5699(94)90195-3. [DOI] [PubMed] [Google Scholar]
- DeLisser HM, Chilkotowsky J, Yan H-C, Daise ML, Buck CA, Albelda SM. Deletions in the cytoplasmic domain of platelet-endothelial cell adhesion molecule-1 (PECAM-1, CD31) result in changes in ligand binding properties. J Cell Biol. 1994b;124:195–203. doi: 10.1083/jcb.124.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser, H.M., M. Christofidou-Solomidou, R.M. Strieter, M.D. Burdick, C.S. Robinson, R.S. Wexler, J.S. Kerr, C. Garlanda, J. Merwin, J.A. Madri, and S.M. Albelda. 1997. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am. J. Pathol. In press. [PMC free article] [PubMed]
- Galcheva-Gargova Z, Konstantinov KN, Wu IH, Klier FG, Barett T, Davis RJ. Science (Wash DC) 1996;272:1797–1802. doi: 10.1126/science.272.5269.1797. [DOI] [PubMed] [Google Scholar]
- Goldberger A, Middleton KA, Oliver JA, Paddock C, Yan H-C, DeLisser HM, Albelda SM, Newman PJ. Biosynthesis and processing of the cell adhesion molecule PECAM-1 includes production of a soluble form. J Biol Chem. 1994;269:17183–17191. [PubMed] [Google Scholar]
- Horton RM, Cai Z, Ho SN, Pease LR. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- Jackson DE, Ward CM, Wang R, Newman PJ. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. 1997. J Biol Chem. 1997;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- Kirschbaum NE, Gumina RJ, Newman PJ. Organization of the gene for human platelet/endothelial cell adhesion molecule-1 (PECAM-1) reveals alternatively spliced isoforms and functionally complex cytoplasmic domain. Blood. 1994;84:4028–4037. [PubMed] [Google Scholar]
- Leavesley DI, Oliver JM, Swart BW, Berndt MC, Haylock DN, Simmons PJ. Signals from platelet/endothelial cell adhesion molecule enhance the adhesive activity of the very late antigen-4 integrin of human CD34+hematopoietic progenitor cells. J Immunol. 1994;153:4673–4683. [PubMed] [Google Scholar]
- Lu TT, Yang LG, Madri JA. Integrin engagement mediates tyrosine dephosphorylation on platelet-endothelial cell adhesion molecule-1. Proc Natl Acad Sci USA. 1996;93:11808–11813. doi: 10.1073/pnas.93.21.11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TT, Barreuther M, Davis S, Madri JA. Platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) is phosphorylatable by C-SRC, binds SRC-SH2 domain and exhibits itam-like properties. J Biol Chem. 1997;272:14442–14446. doi: 10.1074/jbc.272.22.14442. [DOI] [PubMed] [Google Scholar]
- Matsumura T, Wolff K, Petzelbauer P. Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. J Immunol. 1997;158:3408–3416. [PubMed] [Google Scholar]
- Modderman PW, Von Dem Borne AEGK, Sonnenberg A. Tyrosine phosphorylation of P-selectin in intact platelets and in a disulfide-linked complex with immunoprecipitated pp60. Biochem J. 1994;299:613–621. doi: 10.1042/bj2990613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Berman ME, Newman PJ, DeLisser HM, Albelda SM. A heterophilic adhesion mechanism for platelet/endothelial cell adhesion molecule 1 (CD31) J Exp Med. 1992;175:1401–1404. doi: 10.1084/jem.175.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ. Cell Adhesion in vascular biology. The biology of PECAM-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ, Berndt MC, Gorski J, White GC, III, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science (Wash DC) 1990;247:1219–1222. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- Newman PJ, Hillery CA, Albrecht R, Parise LV, Berndt MD, Mazurov AV, Dunlop LC, Zhang J, Rittenhouse SE. Activation-dependent changes in human platelet PECAM-1: phosphorylation, cytoskeletal association, and surface membrane redistribution. J Cell Biol. 1992;119:239–246. doi: 10.1083/jcb.119.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa S, Motomu K, Matsuoka Y, Goro K, Hiroshi N. Homotypic and heterotypic Ca2+-independent cell adhesion activities of bilary glycoprotein, a member of the carcinoembryonic antigen family expressed on CHO cell surfaces. Biochem Biophys Res Commun. 1992;186:881–887. doi: 10.1016/0006-291x(92)90828-9. [DOI] [PubMed] [Google Scholar]
- Osawa M, Masuda M, Harada N, Bruno-Lopes R, Fujiwara K. Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur J Cell Biol. 1997;72:229–237. [PubMed] [Google Scholar]
- Piali L, Albelda SM, Baldwin HS, Hammel P, Gisler RH, Imhof BA. Murine platelet endothelial cell adhesion molecule (PECAM-1)/ CD312 modulates β2 integrins on lymphokine-activated killer cells. Eur J Immunol. 1993;23:2464–2471. doi: 10.1002/eji.1830231013. [DOI] [PubMed] [Google Scholar]
- Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for αvβ3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter E, Barreuther M, Lu T, Imhof BA, Madri JA. Platelet-endothelial cell adhesion molecule-1 (PECAM-1/CD31) tyrosine phosphorylation state changes during vasculogenesis in the murine conceptus. Am J Pathol. 1997;150:1523–1529. [PMC free article] [PubMed] [Google Scholar]
- Prager E, Sunder-Plassmann R, Hansmann C, Koch C, Holter W, Knapp W, Stocklinger H. Interaction of CD31 with a heterophilic counterreceptor involved in downregulation of human T cell responses. J Exp Med. 1996;184:41–50. doi: 10.1084/jem.184.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Fuks A, Stammers CP. Biliary glycoprotein, a member of the immunoglobulin supergene family, function in vitro as Ca2+-dependent intercellular adhesion molecule. Cell Growth Differ. 1990;1:527–533. [PubMed] [Google Scholar]
- Sagawa K, Swain W, Zhang J, Unsworth E, Siraganian RP. Aggregation of the high affinity IgE receptor results in the tyrosine phosphorylation of the surface adhesion protein PECAM-1 (CD-31) J Biol Chemistry. 1997;272:13412–13418. doi: 10.1074/jbc.272.20.13412. [DOI] [PubMed] [Google Scholar]
- Sieber F, Roseman S. Quantitative analysis of intercellular adhesive specificity in freshly explanted and cultured cells. J Cell Biol. 1981;90:55–62. doi: 10.1083/jcb.90.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Rafnofsky S, Lechleider RJ, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Sun J, Williams JH, Yan HC, Albelda SM, DeLisser HM. PECAM-1 homophilic adhesion is mediated by Ig-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J Biol Chem. 1996;271:18561–18570. doi: 10.1074/jbc.271.31.18561. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Albelda SM, Horgan KJ, van Seventer GA, Shimizu Y, Newman W, Hallam J, Newman PJ, Buck CA, Shaw S. CD31 expressed on distinctive T cell subsets is a preferential amplifier of β1integrin-mediated adhesion. J Exp Med. 1992;176:245–253. doi: 10.1084/jem.176.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaporciyan AA, DeLisser HM, Yan HC, Mendiguren II, Thom SR, Jones ML, Ward PA, Albelda SM. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science (Wash DC) 1993;262:1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- Watt SM, Williamson J, Genevier H, Fawcett J, Simmons DL, Hatzfeld A, Nesbitt SA, Coombe DR. The heparin binding PECAM-1 adhesion molecule is expressed by CD34+hematopoietic precursor cells with early myeloid and B-lymphoid cell phenotypes. Blood. 1993;82:2649–2663. [PubMed] [Google Scholar]
- Yan HC, Baldwin HS, Sun J, Buck CA, Albelda SM, DeLisser HM. Alternative splicing of a specific cytoplasmic exon alters the binding characteristics of murine platelet/endothelial cell adhesion molecule-1 (PECAM-1) J Biol Chem. 1995;270:23672–23680. doi: 10.1074/jbc.270.40.23672. [DOI] [PubMed] [Google Scholar]
- Zehnder JL, Hirai K, Shatski M, McGregor JL, Levitt LJ, Leung LLK. The cell adhesion molecule CD31 is phosphorylated after cell activation. J Biol Chem. 1992;267:5243–5249. [PubMed] [Google Scholar]