Abstract
Inhibitory components in myelin are largely responsible for the lack of regeneration in the mammalian CNS. Myelin-associated glycoprotein (MAG), a sialic acid binding protein and a component of myelin, is a potent inhibitor of neurite outgrowth from a variety of neurons both in vitro and in vivo. Here, we show that MAG's sialic acid binding site is distinct from its neurite inhibitory activity. Alone, sialic acid–dependent binding of MAG to neurons is insufficient to effect inhibition of axonal growth. Thus, while soluble MAG-Fc (MAG extracellular domain fused to Fc), a truncated form of MAG-Fc missing Ig-domains 4 and 5, MAG(d1-3)-Fc, and another sialic acid binding protein, sialoadhesin, each bind to neurons in a sialic acid– dependent manner, only full-length MAG-Fc inhibits neurite outgrowth. These results suggest that a second site must exist on MAG which elicits this response. Consistent with this model, mutation of arginine 118 (R118) in MAG to either alanine or aspartate abolishes its sialic acid–dependent binding. However, when expressed at the surface of either CHO or Schwann cells, R118-mutated MAG retains the ability to inhibit axonal outgrowth. Hence, MAG has two recognition sites for neurons, the sialic acid binding site at R118 and a distinct inhibition site which is absent from the first three Ig domains.
It is generally believed that inhibitory molecules in the mammalian central nervous system (CNS),1 which are particularly enriched in myelin, are largely responsible for the lack of axonal regeneration after injury to the brain or spinal cord (Johnson, 1993; Schwab et al., 1993; Keynes and Cook, 1995). A monoclonal antibody, termed IN-1, recognizes a component(s) in CNS myelin that is likely to play a major role in this inhibition (Caroni and Schwab, 1988a ,b; Schnell and Schwab, 1990; Bregman et al., 1995). The antigen(s) recognized by this antibody has yet to be described. Distinct from the inhibitory activity that is blocked by the IN-1 antibody, recent studies strongly suggest that myelin-associated glycoprotein (MAG), a well-characterized component of both CNS and peripheral nervous system (PNS) myelin, may be an important component of this inhibitory activity (Filbin 1995, 1996). We (Mukhopadhyay et al., 1994; DeBellard et al., 1996a ) and others (McKerracher et al., 1994) have recently shown MAG to be a potent inhibitor of axonal regrowth from a variety of postnatal neurons in vitro. The ability of MAG to induce growth cone collapse when presented to growing neurites in a multivalent form (Li et al., 1996) and the ability of a soluble form of MAG to inhibit axonal regeneration (Tang et al., 1997) provide strong evidence that MAG is indeed an inhibitory molecule and not merely a nonpermissive substrate.
The inhibitory properties of MAG vary depending on the age and the type of neuron. Thus, MAG inhibits outgrowth from adult dorsal root ganglion (DRG) neurons and from cerebellar, retinal, spinal, hippocampal, and superior cervical ganglion neurons of all postnatal ages (Mukhopadhyay et al., 1994; DeBellard et al., 1996a ) whereas it promotes axonal growth from neonatal DRG neurons (Johnson et al., 1989; Mukhopadhyay et al., 1994). We observed a 50–90% inhibition on MAG-expressing cells, depending on the type of neuron (Mukhopadhyay et al., 1994; DeBellard et al., 1996a ). McKerracher et al. (1994) compared neurite length from neurons cultured on CNS myelin as a substrate, to CNS myelin immunodepleted of MAG. Their parallel study demonstrated that MAG may account for 60% of the inhibitory properties of CNS myelin.
Studies with the MAG knockout mouse (MAG −/−) (Li et al., 1994; Montag et al., 1994) also suggest that MAG contributes to the inhibitory properties of myelin in vivo and in vitro. We found that neurites from a variety of neurons were about twice as long when grown on myelin from MAG −/− mice compared to MAG +/+ mice (DeBellard et al., 1996b ). Previous studies, however, reported inconsistent results with MAG −/− myelin using either the neuronal cell line NG108 or primary neurons (Bartsch et al., 1995; Li et al., 1996; Ng et al., 1996). In vivo the ability of MAG to inhibit regeneration has been clearly demonstrated in the PNS (Schafer et al., 1996). Normally after injury to the PNS, myelin is removed and Schwann cells downregulate expression of myelin proteins before axonal regeneration occurs (Fawcett and Keynes, 1990). In the mutant C57BL/Ola mouse, myelin is not rapidly removed, and regeneration takes place only very slowly, if at all (Brown et al., 1991, 1992). By itself, the C57BL/Ola mouse suggests a role for PNS myelin in inhibiting axonal regeneration, but in mice bred by crossing C57BL/Ola and MAG −/− mice, myelin is MAG deficient and regeneration is much more robust (Schafer et al., 1996). In the CNS, however, the extent to which regeneration in vivo is improved in the MAG −/− mouse compared to the MAG +/+ mouse is controversial; while one group reports both a significant improvement in the length regenerated and an increase in the number of regenerating axons (Li et al., 1996), another reports no change (Bartsch et al., 1995).
The identity of the neuronal molecule(s) that interacts with MAG is not known, but some light was recently shed on this question when MAG was shown to belong to a subgroup of the Ig super family, termed the sialoadhesins (Kelm et al., 1994). All members of the sialoadhesins have significant amino acid sequence similarity among their first four Ig-like domains, and all bind sialic acid. The five sialoadhesins identified so far are CD22, sialoadhesin, CD33, SMP (a protein very similar to MAG but found only in avian species), and MAG (Filbin 1995, 1996). Although all bind sialic acid, the specificity of binding may differ. For example, MAG prefers α 2,3-linked sialic acid attached to O-linked glycoconjugates, CD22 prefers α 2,6-linked sialic acid attached to N-linked glycoconjugates, while sialoadhesin recognizes α 2,3-linked sialic acid attached to either N- or O-linked glycoconjugates (Kelm et al., 1994). We have shown that whether it promotes (neonatal DRG neurons) or inhibits (cerebellar neurons) axonal regeneration, MAG binds to neurons in a sialic acid–dependent manner (Kelm et al., 1994; DeBellard et al., 1996a ). Although MAG binding to isolated gangliosides has been demonstrated (Yang et al., 1996), sialic acid–dependent binding to neurons is trypsin sensitive, indicating that MAG binds to a neuronal sialo-glycoprotein rather than to a sialo-glycolipid (DeBellard et al., 1996a ). More important, if neurons are desialylated before the neurite outgrowth assay, inhibition by soluble MAG is completely lost while inhibition by MAG expressed by CHO cells is only partially reversed (DeBellard et al., 1996a ; Tang et al., 1997). This strongly suggests that a neuronal sialo-glycoprotein mediates, directly or indirectly, the effects of MAG on axonal regeneration.
In this study, we further elucidate the role of the sialic acid–binding epitope of MAG on neurite outgrowth. Through site-directed mutagenesis, residues involved in the sialic acid–dependent binding of CD22 and sialoadhesin were recently mapped to the GFCC′C′′ face of the NH2-terminal domains, centered on Arg130 and Arg97, respectively (Vinson et al., 1996; van der Merwe et al., 1996). Alignment of the first Ig domain of MAG with CD22 and sialoadhesin reveals that this arginine is conserved in MAG at amino acid 118 (R118). Here, we show that if R118 in MAG is mutated to either an alanine (R118A) or an aspartic acid (R118D) the sialic acid–dependent binding of MAG, when either expressed by cells or in a soluble form, MAG-Fc, to cerebellar neurons is lost. Similarly, while MAG-Fc is a potent inhibitor of axonal regeneration (Tang et al., 1997) when mutated to either R118A- or R118D–MAG-Fc the capacity to inhibit regeneration is lost. Of note, however, when expressed by transfected CHO or Schwann cells, these R118-mutated forms of MAG are still potent inhibitors of axonal regeneration. Finally, although both sialoadhesin and a truncated form of MAG-Fc, consisting of the first three Ig-domains fused to Fc, each bind to neurons in a sialic acid–dependent manner, neither molecule has an effect on neurite outgrowth. Taken together, these results suggest that R118 plays an important role in the sialic acid–dependent binding of MAG to neurons. However, sialic acid binding by itself is insufficient to effect inhibition of axonal regeneration. Following from this they also indicate that there is a second site on MAG, a “neurite inhibition” site, that is distinct from the sialic acid–binding site and not present in the first three Ig-domains of MAG.
Materials and Methods
Mutation of R118 in MAG
The Chameleon double-stranded site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used to mutate the nucleotide sequence coding for R118 in L-MAG to either an alanine or an aspartate as described by the manufacturer. L-MAG in the antisense orientation in pBluescript (Stratagene, La Jolla, CA) was used for the initial annealing step. The mutations were confirmed by dideoxy sequencing. The R118D- and R118A-mutated forms of MAG were then subcloned into the pSJL plasmid for expression in CHO and Schwann cells. To obtain Fc chimeras of the mutated MAG proteins, a XhoI restriction fragment that cuts MAG cDNA at nucleotides 182 and at 1627, and that contains the mutated nucleotides, was cut and purified from the R118-mutated MAG cDNAs in pBluescript and subcloned into the pIG1 vector already harboring the extracellular domain of MAG.
Preparation of Fc Chimeras
The pIG1 vector containing the cDNA coding for the extracellular domain of wild-type sialoadhesin, wild-type MAG, mutated MAG, or MUC18 were prepared as described previously (Kelm et al., 1994). The plasmids were transfected into COS cells and the Fc-chimeric proteins purified from the media as described previously (Simmons, 1993; Kelm et al., 1994).
ELISA of MAG–Fc Chimeras
A 96-well ELISA was coated with anti–human Fc at 15 μg/ml in 0.1 M NaHPO4 for 3 h at 37°C. The plates were washed with PBS and blocked with 5% nonfat milk in PBS for 30 min at room temperature. The Fc chimeras were added at a concentration of 1 μg/ml and incubated overnight at 4°C. The MAG 513 monoclonal antibody was added at various concentrations (0.1–333 ng/ml) and incubated for 3 h at 37°C. The plates were washed and then blocked again with 3% normal goat serum in PBS for 30 min at room temperature. Peroxidase-conjugated sheep anti-mouse IgG diluted 1:500 (Sigma Chemcial Co., St. Louis, MO) was added and color was developed using O-phenylenediamine as a substrate. Absorbance was measured in an ELISA reader at 490 and 630 nm.
Expression of MAG and Sialoadhesin by Transfected CHO Cells
CHO cells deficient in the dhfr gene were transfected with the pSJL plasmid containing the L-MAG cDNA, R118D-MAG, R118A-MAG or, as a control, MAG in a 3′–5′ orientation as previously described (Mukhopadhyay et al., 1994). Expression of MAG protein was amplified and characterized by Western blotting and indirect immunofluorescence as previously described (Mukhopadhyay et al., 1994). Transfected cells were maintained in DME supplemented with 10% dialyzed FCS, proline (40 μg/liter), thymidine (0.73 mg/liter), and glycine (7.5 mg/liter) at 37°C in 5% CO2.
The cDNA for sialoadhesin (Crocker et al., 1994), both full-length sialoadhesin containing all 17 Ig-like domains or truncated sialoadhesin consisting of domains 1–6, in the pcDNA1 Amp vector (British Biotechnology, Oxford, UK) were cotransfected with pcDNA3 as a source of G418 resistance, into CHO cells. After selection in 200 μg/ml G418, colonies were pooled and live cells immunostained for sialoadhesin and subjected to FACS®. Cells expressing high levels of sialoadhesin were expanded and expression again assessed by Western blotting and by immunofluorescence of intact cells.
Expression of MAG by Transfected Schwann Cells
A spontaneously transformed Schwann cell line that does not express MAG was used (Porter et al., 1987; Owens et al., 1990). The cDNAs for MAG, R118D-MAG, or R118A-MAG in the pSJL plasmid were transfected into this Schwann cell line as previously described (Mukhopadhyay et al., 1994) and transfectants were selected in 200 μg/ml G418. Colonies were combined and live cells were stained for surface MAG with the 513 MAG monoclonal antibody. A second antibody, phycoprobe-conjugated anti–mouse IgG (1:100), was used and cells expressing high levels of MAG were separated by FACS®. These cells were expanded and expression of MAG characterized by Western blotting and by immunofluorescent staining as before (Mukhopadhyay et al., 1994).
Isolation of Neurons
Neurons were isolated as previously described (Doherty et al., 1990; Mukhopadhyay et al., 1994). Briefly, animals 1–7-d-old were used and the cerebella were removed. Tissue from one to two animals was combined and placed in 5 ml of 0.025% trypsin in PBS, triturated, and incubated for a further 10 min at 37°C. Trypsinization was stopped by addition of 5 ml DME containing 10% FCS, and cells were centrifuged at 1,000 rpm for 5 min. The cells were resuspended to a single-cell suspension in 2 ml of sato (Doherty et al., 1990), containing 2% FCS for neurite outgrowth on immobilized Fc chimeras or in 2 ml of sato (Doherty et al., 1990) without serum for neurite outgrowth on monolayers of CAM-expressing cells. Cells were counted with a Coulter counter (Coulter Electronics Ltd., Luton, England).
Desialylation of Neurons
A single-cell suspension of the cerebellar neurons was washed and resuspended in PBS, ∼2 × 106 cells were incubated with 50 mU of Vibrio cholerae sialidase (Calbiochem-Novabiochem, La Jolla, CA) in a final volume of 0.5 ml, for 2 h at 37°C. The neurons were washed with DME and resuspended in SATO containing 2% FCS for the neurite outgrowth experiments, or in PBS for the binding assay.
Binding of Fc Chimeras to Neurons
Fc-chimeric proteins were adsorbed for 3 h at 37°C onto wells of microtiter plates that had been coated for 2 h at 37°C with anti–human IgG at 15 μg/ml in 0.1 M bicarbonate buffer, pH 9.6. Before the binding assay, neurons were vitally labeled with the fluorescent dye, calcein AM (Molecular Probes, Inc., Eugene, OR), as previously described (DeBellard et al., 1996a). Where indicated, MAG 513 monoclonal antibody (Boehringer-Mannheim Biochemicals, Indianapolis, IN) was included in the assay at a concentration of 10 μg/ml and neurons were desialylated before being used. 100 μl of a suspension of vitally labeled neurons, containing 1–2 × 105 cells, was added to each well and allowed to incubate for 1 h at room temperature. The plates were washed three times with PBS applied to each well under gravity by allowing free flow from an elevated reservoir and the fluorescence measured in a FluorImager (Molecular Dynamics, Inc., Sunnyvale, CA).
The Fc Chimera Neurite Outgrowth Assay
The Fc chimera neurite outgrowth assay was carried out as described previously (Doherty et al., 1995; Tang et al., 1997). Briefly, L1-Fc (20–40 μg/ ml) was immobilized onto eight chamber slides (Lab-Tek, Naperville, IN) already coated with poly-l-lysine (16.6 μg/ml), and then anti–human IgG (15 μg/ml). Neurons and the Fc chimera at 25 μg/ml were added simultaneously to the immobilized L1-Fc at a concentration of 4 × 104 neurons per well and cultured for 16 h. Where indicated MAG 513 monoclonal antibody (25 μg/ml) was incubated with the MAG-Fc or neurons were desialylated with 50 mU V. cholerae sialidase and the sialidase was included during the culture period. Cultures were fixed, stained for GAP43, and neurite length was measured as described above.
Neurite Outgrowth on Transfected CHO and Schwann Cells
Confluent monolayers of CHO cells expressing wild-type MAG, wild-type sialoadhesin, mutated MAG or control cells were established over a 24-h period in individual chambers of an eight-well tissue culture slide (Lab-Tek). Cocultures were established as described previously (Doherty et al., 1990; Mukhopadhyay et al., 1994) by adding ∼5,000 cerebellar neurons to the CHO cell monolayers. Culture medium was SATO. Where indicated, 10 mU of V. cholerae sialidase were included throughout the coculture period. After 16–18 h, the cocultures were fixed for 30 min with 4% paraformaldehyde, and permeabilized with ice-cold methanol for 2 min. The cells were then blocked for 20 min with DME containing 10% FCS and incubated for 2 h with a rabbit polyclonal antibody against GAP43 (1: 4,000; from R. Curtis and G. Wilkins, Imperial College, London, UK). Cells were washed three times with PBS-BSA (2%), and then incubated for 30 min at room temperature with a biotinylated donkey anti–rabbit Ig (1:300; Amersham Corp., Arlington, Heights, IL), washed three times, and incubated with streptavidin-conjugated Texas red (1:300; Amersham Corp.) for 45 min. After three more washes, the slides were mounted in Permfluor (Baxter Healthcare Corp. Miami, FL) and viewed with a Zeiss fluorescent microscope (Carl Zeiss, Inc., Thornwood, NY). The length of the longest neurite for each GAP43-positive neuron was determined using the Uncor image analysis program.
Binding of Human Erythrocytes to CHO and Schwann Cells
Confluent monolayers of CHO or Schwann cells expressing wild-type MAG, mutated MAG, or control cells were established over a 24-h period in individual chambers of an eight-well tissue culture slide (Lab-Tek). Monolayers were treated for 1 h with 200 mU/ml V. cholerae at 37°C and washed twice. The medium was changed to DME and erythrocytes, either desialylated or not, were added. Unbound erythrocytes were washed off and cells were examined under a phase-contrast microscope and photographed.
Results
The Sialic Acid Binding Site on MAG Maps to R118 of the First Ig Domain
All members of the sialoadhesin family are sialic acid– binding proteins, although the specific sialic acid linkage recognized by each may differ (Kelm et al., 1994). In keeping with the characteristics of this family of molecules, we have shown that MAG binds to neurons in a sialic acid– dependent manner (Kelm et al., 1994; DeBellard et al., 1996a ). Recently, Arg97 in sialoadhesin and Arg130 in CD22 were shown to be critical for the sialic acid binding of each molecule (Vinson et al., 1996; van der Merwe et al., 1996). If the first Ig domain of MAG is aligned with the first Ig domain of sialoadhesin and CD22 (Fig. 1), then this arginine appears conserved in all three proteins. In MAG, the conserved amino acid corresponds to Arg118 (R118). To determine if R118 in MAG is involved in sialic acid– dependent binding to neurons, the nucleotides coding for this amino acid were mutated to code either for alanine (R118A), a nondisruptive substitution, or for aspartate (R118D), a disruptive substitution. The mutations were created in both a soluble, chimeric form of MAG, MAG-Fc, and in the full-length MAG cDNA.
Figure 1.
Alignment of the first Ig domains of sialoadhesin (Sn), rat CD22 (CD22) and MAG (MAG). Predicted β-strands are labeled as A, B, C, C′, C′′, D, E, and F. Amino acids involved in sialic acid binding of sialoadhesin and conserved in MAG are highlighted. The arrow refers to Arg118 in MAG.
Before carrying out the neuron binding or neurite outgrowth assays, we assessed the recognition of R118A- and R118D–MAG-Fc by a conformation-dependent MAG monoclonal antibody, the 513 MAG antibody (Poltorak et al., 1987). Fig. 2 a shows that MAG 513 monoclonal antibody binds to immobilized, unmutated-, R118A-, and R118D–MAG-Fc in a dose-dependent manner. Furthermore, and more importantly, the characteristics of binding of the antibody to both R118A- and R118D–MAG-Fc are indistinguishable from that of the unmutated MAG-Fc (Fig. 2 a). This demonstrates the affinity of the antibody for MAG is unchanged by these mutations suggesting that the conformation of MAG is maintained in R118A- and R118D–MAG-Fc.
Figure 2.
The effect of mutation of R118 in MAG-Fc on antibody recognition, binding to neurons and inhibition of axonal growth. (a) Binding of conformation-dependent MAG 513 monoclonal antibody to mutated MAG-Fc. The Fc chimeras were immobilized at a concentration of 1 μg/ml on an ELISA plate already coated with anti-Fc antibody. MAG 513 monoclonal antibody was added at various concentrations and bound antibody was detected by ELISA. MAG-Fc (▴), R118A–MAG-Fc (▵), R118D– MAG-Fc (▪), or MUC18-Fc (□). Results represent three experiments +/− SEM. (b) Binding of R118-mutated MAG to cerebellar neurons. Dissociated PND4 cerebellar neurons, vitally labeled with calcein AM, were allowed to bind to MAG-Fc (MAG), R118A– MAG-Fc (R118A), R118D–MAG-Fc (R118D), or MUC18-Fc (MUC) immobilized at a concentration of 10 μg/ml, on a 96-well dish. 200,000 neurons were added to each well and after incubation and washing, the number of cells bound was quantitated with a FluorImager. Results are from three experiments, each with 10 samples, and represent the mean ± SEM. (c) The effect of R118-mutated MAG-Fc on neurite outgrowth. Dissociated PND4 cerebellar neurons were incubated with MAG-Fc, R118A– MAG-Fc (R118A-Fc), R118D–MAG-Fc (R118D-Fc), or MUC18-Fc (MUC-Fc) at a concentration of 25 μg/ml before being plated onto a substrate of L1-Fc immobilized on wells coated with anti-Fc antibody. Neurons were cultured for 18 h, fixed and immunostained for GAP 43, and neurite length was measured. Results show the mean length of the longest neurite per cell (±SEM) for 100–200 individual neurons.
To determine if R118 is involved in neuronal adhesion a binding assay was performed with wild-type and the R118A- and R118D-mutated forms of MAG-Fc. For this assay, the Fc-chimeras were immobilized on a 96-well ELISA plate. Another five-domain Ig family member, MUC18, fused to Fc, was used as a control chimera. A single-cell suspension of neurons, vitally labeled with the fluorescent dye calcein AM, was allowed to bind to the immobilized Fc chimeric molecules. Using this assay previously, we showed that as the concentration of immobilized MAG-Fc increases, the number of neurons bound also increases until saturation is reached at about 5 μg/ml (DeBellard et al., 1996a). Confirming our earlier results, Fig. 2 b shows that at a saturating concentration of 10 μg/ml MAG-Fc, a significant number of neurons are specifically bound. MAG antibody blocks this binding (results not shown), and there is no binding to the control chimera, MUC18. In contrast, although the MAG conformation-dependent antibody, 513, binds the mutated forms of MAG, neither immobilized R118A- nor R118D–MAG-Fc, at saturating concentrations, will bind cerebellar neurons. Binding remains at background levels, indistinguishable from binding to MUC18-Fc (Fig. 2 b). Since antibody binding suggests that both mutated forms of MAG retain their native conformation, the abolition of neuronal binding by either a nondisruptive or a disruptive substitution at R118 indicates that this residue is critical for sialic acid– dependent binding of MAG.
Inhibition of Neurite Outgrowth by Soluble MAG Requires the R118 Binding Site
We have shown previously that when soluble MAG-Fc at a concentration of 25 μg/ml, is added to cerebellar neurons growing on a substrate of purified, immobilized L1-Fc (a neurite promoting molecule), there is ∼50% inhibition of neurite outgrowth while MUC18-Fc, at an equivalent concentration, has no effect (Tang et al., 1997). To determine if soluble R118A- or R118D–MAG-Fc also have an inhibitory effect on cerebellar neurons growing on immobilized L1-Fc, each mutated MAG chimera was added to the cultures at a concentration of 25 μg/ml. As before, unmutated MAG-Fc inhibited axonal outgrowth by ∼50% while MUC18-Fc had no effect, and neurite length was the same as without Fc chimera (Fig. 2 c). When either R118A- or R118D–MAG-Fc was added to the growing neurites at the same concentration, no effect was seen on neurite outgrowth. Neurite length was similar to that for neurons grown with MUC18-Fc or in the absence of Fc chimera (Fig. 2 c). Therefore, it appears that soluble MAG-Fc is entirely dependent on its ability to bind neurons in a sialic acid– dependent manner to inhibit axonal growth.
Sialic Acid–dependent Binding can Be Dissociated from Inhibition of Neurite Outgrowth
To determine if CD22 and sialoadhesin also bind to neurons, like MAG, in a sialic acid–dependent manner, we performed a binding assay with each of these sialoadhesins. Each of the sialoadhesin Fc chimeras was immobilized at a concentration of 10 μg/ml, on a 96-well ELISA plate and vitally labeled neurons were allowed to bind as described above. Of the three sialoadhesins, binding of neurons to MAG-Fc and sialoadhesin is significant and equivalent. Furthermore, sialoadhesin binding, as with MAG binding, is abolished by desialylation of the neurons prior to the assay (Fig. 3 a). There is no neuronal binding to CD22 (Fig. 3 a). These results are consistent with the fact that sialoadhesin, but not CD22, recognizes sialic acid in a linkage similar to the one recognized by MAG (NeuAc α 2,3 Gal in O-linked glycans). When a truncated form of MAG-Fc, consisting of the first three Ig-domains, MAG (d1-3)-Fc, was used as a substrate for binding, binding was equivalent to the binding of the full-length, unmutated MAG-Fc (Fig. 3 a). Furthermore, binding of MAG(d1-3)-Fc is also completely sialic acid–dependent (Fig. 3 a). This is not an unexpected finding as the sialic acid binding site on MAG, R118, is in the first Ig-domain (see above).
Figure 3.
Binding to and effect on neurite outgrowth from cerebellar neurons of sialoadhesin-Fc, CD22-Fc, and MAG(d1-3)-Fc. (a) Dissociated PND4 cerebellar neurons, vitally labeled with calcein AM, were allowed to bind to MAG-Fc, MAG(d1-3)-Fc, sialoadhesin (SN)-Fc, CD22-Fc, or MUC18-Fc immobilized at a concentration of 10 μg/ml, on a 96-well dish coated with anti-Fc antibody. 200,000 neurons, either pretreated with sialidase (stippled bars) or not (solid bars) were added to each well and after incubation and washing, the number of cells bound was quantitated with a FluorImager. Results are from three experiments, each with 10 samples and represent the mean ± SE. (b) Dissociated PND4 cerebellar neurons were incubated with MAG-Fc, sialoadhesin (SN)-Fc, MAG(d1-3)-Fc, or MUC18-Fc (MUC-Fc) at a concentration of 25 μg/ml before being plated onto a substrate of L1-Fc immobilized on wells coated with anti-Fc antibody. Neurons were cultured for 18 h, fixed and immunostained for GAP 43, and neurite length was measured. Results show the mean length of the longest neurite per cell (± SEM) for 100–200 individual neurons.
As both sialoadhesin and MAG(d1-3)-Fc bind to neurons in a sialic acid–dependent manner similar to MAG-Fc, we tested their effect on axonal regeneration. We compared neurite outgrowth from cerebellar neurons growing on a substrate of immobilized L1-Fc in the presence of 25 μg/ml of MAG-Fc, MAG(d1-3)-Fc, sialoadhesin-Fc, or as a control MUC18-Fc. As reported above, under these conditions, MAG-Fc inhibited neurite outgrowth by ∼50% while MUC18-Fc had no effect (Fig. 3 b). Surprisingly, although they each bind to neurons via sialic acid, in a linkage similar to MAG, neither sialoadhesin nor MAG (d1-3)-Fc had any effect on neurite outgrowth (Fig. 3 b). Neurite length was the same as when neurons were grown in the presence of the control MUC18-Fc or in the absence of Fc chimera. Therefore, although mutation of R118 in MAG-Fc indicates that sialic acid–dependent binding is required for MAG-Fc to inhibit axonal growth, the results with both sialoadhesin and MAG(d1-3)-Fc demonstrate that sialic acid binding alone is insufficient to effect inhibition of axonal regeneration.
Isolation of Transfected CHO and Schwann Cells Expressing High Levels of MAG
To assess the effect of mutation at R118 on inhibition of neurite outgrowth by full-length MAG expressed by cells, cDNAs coding for full-length, mutated MAG in the pSJL vector were transfected into CHO cells and into Schwann cells as previously described (Mukhopadhyay et al., 1994). For the CHO cells, after selection in G418, gene amplification by the methotrexate/dhfr strategy and single-cell cloning, a number of colonies were screened for expression of the mutated forms of MAG. Fig. 4 a shows immunostaining of a Western blot of the lysates from two such clones. Although each of these cell lines expresses slightly less MAG (per mg total protein) than the cells that express unmutated MAG (compare lanes c and d with lane b), the level of expression is within the range we previously showed to effectively inhibit regeneration (Mukhopadhyay et al., 1994). In addition, both mutated forms of MAG have a molecular weight of ∼100 kD, similar to that of wild-type MAG expressed either in CHO cells or by sciatic nerve. This indicates that both R118A- and R118D-MAG are glycosylated to the same extent as wild-type MAG. To ensure that mutated MAG is reaching the surface of these transfected CHO cells, live cells were immunostained with the MAG 513 monoclonal antibody. Fig. 4 b shows: first, the MAG 513 antibody recognizes mutated MAG when expressed by CHO cells, again demonstrating the conformational integrity of the mutated proteins; and second, MAG reaches the cell surface in cells expressing unmutated MAG, R118A-MAG, and R118D-MAG. Furthermore, the intensity of staining is comparable for all three cell lines, indicating that equivalent amounts of MAG are reaching the surface.
Figure 4.
Characterization of the expression of mutated forms of MAG by CHO cells. (a) Immunodetection of MAG (arrow) in lysates of control transfected CHO cells (lane a), MAG-expressing cells (lane b), R118A-MAG–expressing cells (lane c), R118D–MAG-expressing cells (lane d), and rat sciatic nerve (lane e). Each lane was loaded with 50 μg of protein except for lane e, which was loaded with 30 μg. Proteins were separated by polyacrylamide gel electrophoresis (8%), transferred to PVDF membrane, and immunostained for MAG with a monoclonal rat anti-MAG antibody (1:100), followed by alkaline phosphatase-conjugated, goat anti–mouse (1: 1,000). The substrate was 5-bromo-4-chloro-3-indolylphosphate, and nitroblue tetrazolium was the chromogen. Bars refer to molecular weight standards from top to bottom, 198, 120, 88, 70, and 56 kD. (b) Surface detection of mutated-MAG on transfected CHO cells by immunofluorescent staining. Live CHO cells expressing R118A-MAG (panel a) or R118D-MAG (panel b) were incubated with MAG 513 monoclonal antibody (5 μg/ml), and then fixed with 4% paraformaldehyde and incubated with phycoprobe-conjugated, goat anti-mouse IgG (1:50). (c) Binding of human red blood cells to transfected CHO cells. Confluent monolayers of MAG-expressing (A) and (B), R118A-MAG-expressing (C), and R118D-MAG–expressing (D) CHO cells were desialylated by incubation with sialidase. A single-cell suspension of human red blood cells was added to each monolayer and allowed to bind. For B, the red blood cells were desialylated before being added to the monolayer. Binding was assessed by appearance of rosettes of human red blood cells bound to the monolayers under phase microscopy.
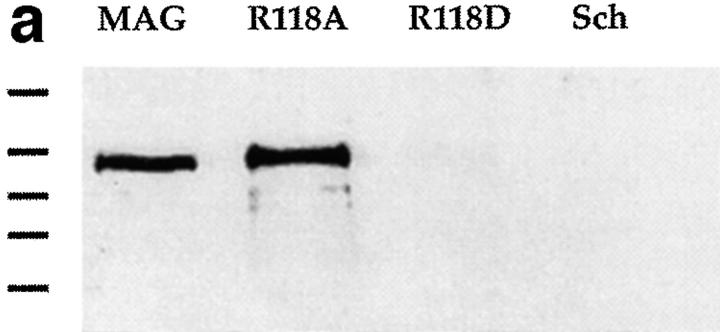
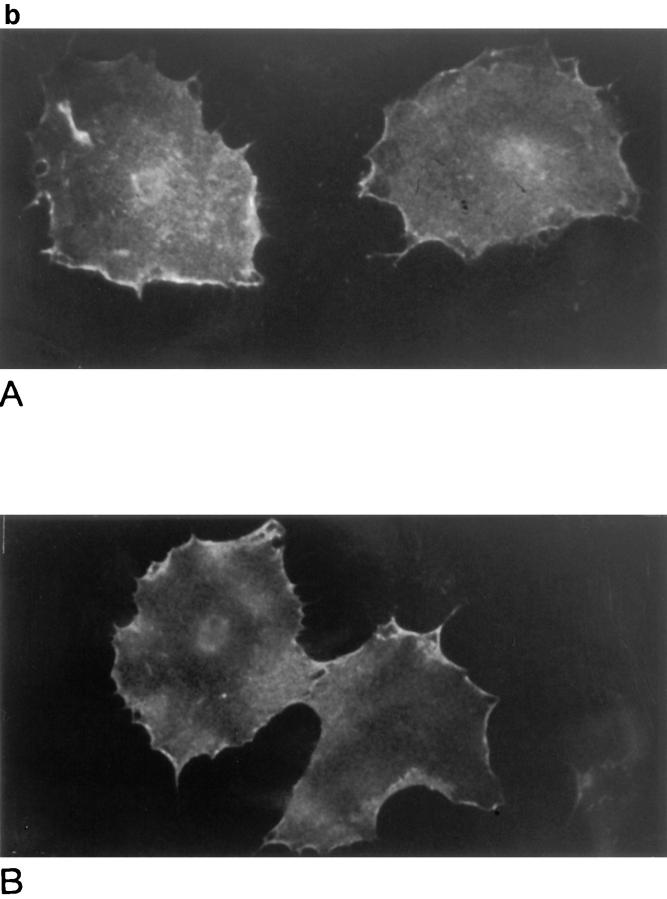
For the Schwann cells, a spontaneously transformed Schwann cell line, which does not express MAG and is permissive for axonal growth was used (Porter et al., 1987; Owens et al., 1990). We have shown previously that when these Schwann cells are induced to express wild-type MAG after transfection, neurite outgrowth is inhibited by ∼40% (Shen et al., 1996). Here, after transfection and selection in G418, resistant colonies were pooled, live cells were immunofluorescently stained using the 513 MAG monoclonal antibody and sorted by FACS® for cells expressing high levels of MAG. These cells were expanded, expression of MAG was characterized by Western blotting and immunofluorescence of intact cells, and these high expressors were used in the neurite outgrowth assay. It can be seen from the immunostained Western blot in Fig. 5 a that the Schwann cells expressing wild-type MAG and those expressing R118A-MAG are expressing approximately the same amount of MAG (per mg of total protein). Furthermore, both the wild type and the R118A-MAG expressed by these Schwann cells are ∼100 kD in size and therefore most likely glycosylated to the same extent as MAG expressed in vivo. However, no expression of MAG was detected in the cells transfected with the R118D-mutated MAG. This is surprising because a small proportion of these cells appeared to express high levels of R118D-MAG upon FACS® sorting. After expansion of the first FACS®-sorted R118D-MAG transfected cells, the cells were again FACS® sorted. Again a few of these cells appeared to express high levels of the R118D-MAG at the cell surface. However, when these Schwann cells were expanded and analyzed by Western blotting and immunostaining, again there was no detectable expression of R118D-MAG. These results suggest that the Schwann cells expressing high levels of this particular mutated form of MAG either died or stopped expressing the protein with passage. The latter possibility is most likely correct as there was no evidence of dying cells in the cultures. The reason why these Schwann cells stopped expressing R118D-MAG is not known as this protein would appear to be folded correctly based on the ability to bind the conformation-dependent MAG antibody, 513 (Figs. 2 a and 4 b). For the Schwann cells expressing wild-type MAG or R118A-MAG, Fig. 5 b shows that each of these proteins reaches the cell surface and is evenly distributed.
Figure 5.
Characterization of the expression of mutated forms of MAG by Schwann cells. (a) Immunodetection of MAG in lysates of wild-type MAG-transfected Schwann cells (MAG), R118A-MAG–transfected Schwann cells (R118A), R118D-MAG–transfected Schwann cells (R118D) and control transfected Schwann cells (Sch). Each lane was loaded with 20 μg of protein. Proteins were separated by polyacrylamide gel electrophoresis (8%), transferred to PVDF membrane, and immunostained for MAG with a monoclonal rat anti-MAG antibody (1:100), followed by alkaline phosphatase–conjugated, goat anti–mouse (1:1,000). Detection was by chemilluminescence. Bars refer to molecular weight standards from top to bottom, 198, 120, 88, 70, and 56 kD. (b) Detection of MAG on the surface of transfected Schwann cells by immunofluorescent staining. Live Schwann cells expressing wild-type MAG (A) or R118A-mutated MAG (B) were incubated with MAG 513 monoclonal antibody (5 μg/ml), then fixed with 4% paraformaldehyde and incubated with phycoprobe-conjugated, goat anti–mouse IgG (1:50). (c) Binding of human red blood cells to transfected Schwann cells. Confluent monolayers of MAG-expressing (A and B) and R118A-mutated MAG-expressing (C) Schwann cells were desialylated by incubation with sialidase. A single-cell suspension of human red blood cells was added to each monolayer and allowed to bind. For B, the red blood cells were desialylated before being added to the monolayer. Binding was assessed by appearance of rosettes of human red blood cells bound to the monolayers under phase microscopy.
Mutated MAG Expressed by CHO or Schwann Cells Fails to Mediate Sialic Acid–dependent Adhesion but Retains Its Ability to Inhibit Neurite Outgrowth
Before carrying out the neurite outgrowth assay, we first wanted to ensure that R118-mutated MAG expressed by either CHO cells or Schwann cells had, like R118-mutated MAG-Fc, lost the ability to bind sialic acid. To do this we monitored the binding of human erythrocytes to these cells, as the majority of cell–cell interactions of human erythrocytes is sialic acid dependent. Previously we reported that MAG-expressing COS cells bind human erythrocytes in a sialic acid–dependent manner (Kelm et al., 1994). However, high levels of binding of human erythrocytes to MAG-expressing CHO cells and MAG-expressing Schwann cells was only detected if the CHO or Schwann cells were desialylated before the binding assay (Figs. 4 c and 5 c).This finding implies that a large portion of MAG expressed by CHO or Schwann cells is engaged in sialic acid–dependent binding in a cis manner (i.e., with sialoglycoconjugates on the surface of these cells). Alternatively, the lack of binding before desialylation could be the result of a charge effect of the CHO–sialic acid residues. There was no binding of human erythrocytes to either CHO or Schwann cells not expressing MAG, regardless of whether they were desialylated or not. Similarly, neither R118A- nor R118D-MAG–expressing CHO, nor R118A-expressing Schwann cells supported human erythrocyte binding, regardless of whether they were desialylated or not. These results indicate that the sialic acid– dependent binding of MAG expressed by CHO or Schwann cells is lost, as in MAG-Fc, by mutation of R118 (Figs. 4 c and 5 c).
To test the effect of mutated MAG expressed by CHO and Schwann cells on neurite outgrowth, isolated cerebellar neurons were grown overnight on CHO cells expressing R118A- or R118D-MAG, or Schwann cells expressing R118A-MAG. Neurite length was compared to outgrowth from similar neurons grown on cells expressing unmutated MAG and on control transfected cells not expressing MAG. As we reported previously (Mukhopadhyay et al., 1994; DeBellard et al., 1996a ), axonal outgrowth from cerebellar neurons was inhibited by ∼70% when grown on MAG-expressing CHO cells and by ∼40% when grown on MAG-expressing Schwann cells, compared to control transfected cells not expressing MAG (Fig. 6 a and b). Importantly, a similar degree of neurite outgrowth inhibition still occurred when neurons were cultured on CHO or Schwann cells expressing the mutated MAG. These results indicate that even though mutation at R118 abolishes the ability of MAG to engage in measurable sialic acid–dependent binding, the capacity to inhibit neurite outgrowth is unaffected when mutated MAG is expressed in CHO or Schwann cells.
Figure 6.
The effect of R118-mutated-MAG expressed by CHO or Schwann cells on neurite outgrowth from cerebellar neurons. Dissociated PND4 cerebellar neurons were cultured for 18 h on confluent monolayers of (a) unmutated MAG-expressing, (MAG), R118A-MAG–expressing (R118A), R118D-MAG–expressing (R118D), or control (C) CHO cells or (b) MAG-expressing (MAG), R118A-MAG–expressing (R118A), or control Schwann cells not expressing MAG (Sch). Cocultures were fixed and immunostained for GAP 43, and neurite length was measured. Results show the mean length of the longest neurite per cell (± SEM) for 100–200 individual neurons.
Finally, we wanted to assess if sialoadhesin expressed by CHO cells had any effect. Neurite outgrowth on CHO cells expressing an abundance of sialoadhesin, either a truncated form of the molecule consisting of Ig domains 1–6 or the entire molecule, consisting of 17 Ig domains (characterized by Western blot and indirect immunofluorescence, results not shown) was compared to neurite outgrowth on MAG-expressing and control CHO cells. As before, neurite outgrowth was inhibited by ∼70% on the MAG-expressing CHO cells. However, when the same neurons are grown on CHO cells expressing sialoadhesin, (either d1-6 or d1-17) there is no difference in neurite length compared to outgrowth on control cells (Fig. 7). Axonal outgrowth, therefore, is not inhibited on sialoadhesin-expressing CHO cells. These results demonstrate that the binding of sialoadhesin expressed by CHO cells, like sialoadhesin-Fc, is insufficient to bring about inhibition. MAG, therefore, must carry a specific site responsible for inhibition of neurite outgrowth, separate from its sialic acid–binding epitope.
Figure 7.
The effect of sialoadhesin expressed by CHO cells on neurite outgrowth. Dissociated PND4 cerebellar neurons were cultured for 18 h on confluent monolayers of MAG-expressing (MAG), sialoadhesin-expressing (SN) or control CHO cells (C). Cocultures were fixed and immunostained for GAP43 and neurite length was measured. Results are the mean length of the longest neurite per cell (± SEM) for 100–200 individual neurons.
Discussion
Myelin-associated glycoprotein can either promote or inhibit axonal regeneration, depending on the age and type of neuron (Johnson et al., 1989; Mukhopadhyay et al., 1994; McKerracher et al., 1994; DeBellard et al., 1996a ). In addition, we have shown that regardless of whether neurite outgrowth is promoted or inhibited, MAG binds to all neurons tested in a sialic acid–dependent manner (Kelm et al., 1994; DeBellard et al., 1996a ). As a sialic acid–binding protein and a member of the Ig superfamily, MAG has been included in the Ig subfamily, the sialoadhesins (Kelm et al., 1994). In this study we show that the sialic acid– dependent binding of MAG to neurons is abolished by mutating R118 in the first Ig domain. It is highly unlikely that the loss of binding by mutation of R118 results from a loss of native conformation because both a nondisruptive and a disruptive amino acid substitution at R118 have the same effect. Although a nondisruptive substitution is not predicted to alter conformation, the amino acid involved directly in binding can be expected to be highly conserved; therefore, even a nondisruptive substitution is predicted to abolish or diminish function of such an amino acid. In addition, the MAG 513 monoclonal antibody still recognizes mutated R118A- and R118D-MAG with the same affinity as it recognizes unmutated MAG. Since the epitope on MAG recognized by 513 is conformation dependent (Poltorak et al., 1987), it is very unlikely that MAG is misfolded as a result of the mutations introduced at R118. Together these results strongly suggest that R118 in the first Ig like domain is critical for the sialic acid–dependent binding of MAG.
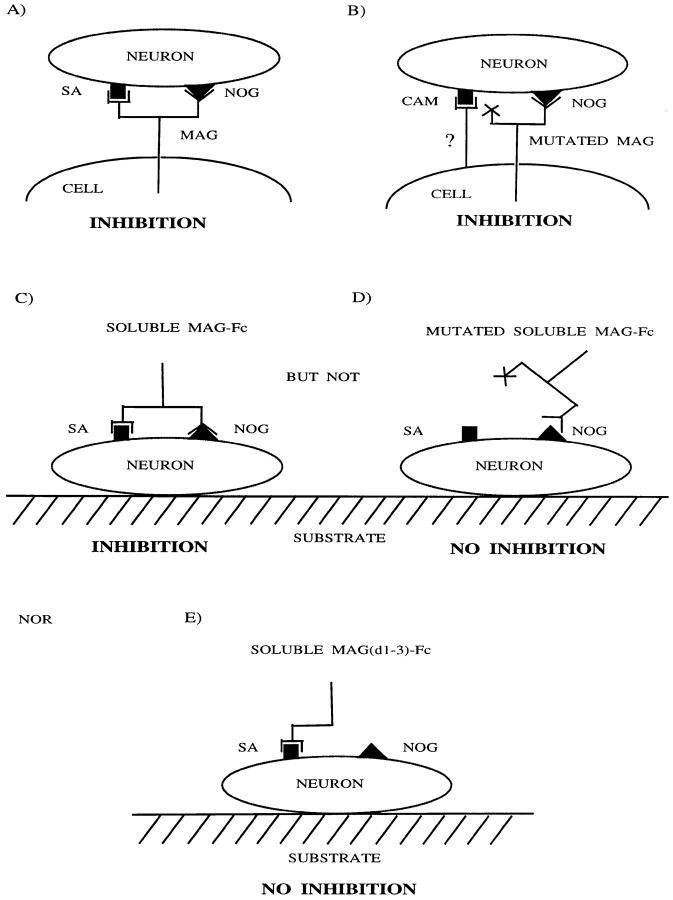
Previously, we showed that soluble MAG-Fc is completely dependent on sialic acid binding to inhibit neurite outgrowth (Tang et al., 1997) while only a portion of the inhibition exerted by MAG expressed by CHO cells is sialic acid–dependent (DeBellard et al., 1996). Consistent with these observations mutation of R118 abolishes completely the ability of MAG-Fc to inhibit neurite outgrowth. On the other hand, R118-mutated MAG expressed by either CHO or Schwann cells inhibits axonal regeneration to the same extent as wild-type MAG, even though we showed that R118-mutated forms of MAG expressed by these cells have lost their sialic acid binding capabilities. In contrast, two proteins, sialoadhesin and a truncated form of MAG, MAG(d1-3)-Fc, that each bind neurons in a sialic acid–dependent manner and each recognize the same sialic acid linkage as MAG (Kelm et al., 1994), do not inhibit axonal growth. Together, these results indicate that sialic acid binding to neurons is, in itself, insufficient to induce inhibition. Therefore, the intact extracellular domain of MAG must carry an additional degree of specificity that dictates inhibition. It follows that there must be two recognition sites on MAG for neurons, one that binds one or more sialoglycoproteins, and another that is the neurite inhibition effecting site. As shown schematically in Fig. 8, when R118-mutated MAG is expressed in CHO cells or Schwann cells, the absence of the sialic acid binding site can be compensated for by another, as yet unidentified, adhesion molecule(s) on these cells; this adhesion molecule allows the interaction of the MAG inhibition site with neurons. In contrast, soluble MAG-Fc is dependent exclusively on its own sialic acid–dependent binding to allow the neurite inhibition site to engage the neuron. If the sialic acid–dependent binding is abolished in soluble MAG-Fc, inhibition of neurite outgrowth by MAG-Fc is lost (Fig. 8). Furthermore, because MAG(d1-3)-Fc can bind neurons in a sialic acid–dependent manner but does not inhibit axonal growth, this truncated form of MAG carries only one of these sites, the sialic acid binding site. The site responsible for inhibition must be carried entirely by domains 4 and 5 or require these domains, together with domains 1–3, to effect inhibition (Fig. 8). An alternative interpretation of these results is that, to bring about inhibition, full-length MAG-Fc recognizes a sialoglycoconjugate distinct from those recognized by MAG(d1-3)-Fc, and sialoadhesin. This is unlikely as full-length MAG-Fc, MAG (d1-3)-Fc, and sialoadhesin-Fc all recognize sialic acid residues in an α 2,3-linkage attached to O-linked glycoconjugates (Kelm et al., 1994).
Figure 8.
The sialic acid binding site on MAG (R118) is critical for inhibition of axonal regeneration by soluble MAG-Fc but not MAG expressed by CHO cells. There are two recognition sites on MAG, a sialic acid binding site (SA; square symbol) and a neurite outgrowth inhibition site (NOG; triangular symbol). When MAG is expressed by CHO cells, both sites engage the neuron and neurite outgrowth is inhibited (A). When MAG mutated at its sialic acid binding site is expressed in CHO cells, another cell adhesion molecule (CAM) on the CHO cell surface engages the neuron along with the neurite outgrowth inhibition site and neurite outgrowth is still inhibited (B). When soluble MAG-Fc is added to neurons, both the sialic acid and the inhibition sites engage the neuron and neurite outgrowth is inhibited (C). However, when MAG-Fc mutated at the sialic acid binding site is added to neurons, it cannot bind to neurons and consequently the inhibition site cannot engage and there is no inhibition of neurite outgrowth (D). In contrast, when MAG(d1-3)-Fc is added to neurons in binds via its sialic acid binding site but does not inhibit axonal growth because the inhibition site is absent.
The idea of two distinct, neuronal recognition sites on MAG is supported by a number of other observations. First, high levels of sialic acid–dependent binding of human erythroctyes to either MAG-expressing CHO or Schwann cells can only be measured if the CHO or Schwann cells are desialylated before the binding assay. This could result from MAG expressed by these cells engaging in cis interactions and not being available for trans sialic acid binding with opposing neurons. However, both MAG-expressing CHO and Schwann cells inhibit neurite outgrowth without being desialylated before the assay. Therefore, high levels of MAG-sialic acid–dependent binding to neurons is not required for inhibition by MAG when expressed by cells. Second, the binding requirements of MAG 513 monoclonal antibody further support the suggestion of distinct sites on MAG. The epitope recognized by this monoclonal antibody is conformation dependent (Poltorak et al., 1987). However, this monoclonal antibody effectively blocks sialic acid–dependent binding of MAG to neurons but still recognizes R118A- and R118D-MAG. Therefore the epitope for the 513 monoclonal antibody must be very close to, but not coincident with R118, the sialic acid binding site and the antibody must block sialic acid–dependent binding by steric hindrance. In keeping with this observation, the 513 monoclonal antibody reverses inhibition of neurite outgrowth by soluble MAG-Fc, which is dependent on MAG-sialic acid–dependent binding (Tang et al., 1997), but it has no effect on inhibition of neurite outgrowth by MAG expressed by CHO cells (Mukhopadhyay et al., 1994).
For both MAG-Fc and MAG expressed by CHO and Schwann cells, we have shown that their sialic acid binding capabilities are lost by mutation of R118. Although we suggest here that the sialic acid binding of MAG is not required to inhibit neurite outgrowth when expressed by CHO or Schwann cells, we previously reported that inhibition by MAG expressed by CHO cells was partially reversed by desialylation of the neurons (DeBellard et al., 1996a ). A similar small, but significant reversal in inhibition of neurite outgrowth by R118-mutated MAG upon neuronal desialylation was also observed (results not shown). There are two possible explanations for this apparent discrepancy in findings. First, because this improvement in outgrowth is small (rarely more than 25%) it may result from removal of a steric effect brought about by the charge on sialic acid. A small effect on neurite outgrowth resulting from changes in charge may not be apparent in the more robust neurite outgrowth observed on the control cells. Second, although sialic acid–dependent binding of R118-mutated MAG could not be measured for either MAG-Fc or MAG expressed by CHO or Schwann cells, it is possible that sialic acid–dependent binding is not completely abolished by this mutation. That is to say, mutation of R118 may decrease the affinity of MAG binding to sialic acid to a level that cannot be measured in the binding assays available. On the other hand, when expressed by CHO or Schwann cells, mutated, R118-mutated MAG may still be able to engage the neuron in low-affinity sialic acid–dependent binding, which is sufficient to influence neurite outgrowth. However, the inability of MAG(d1-3) to inhibit axonal outgrowth despite binding to neurons in a sialic acid–dependent manner argues that even if sialic acid binding of MAG is required, alone it is insufficient to inhibit axonal growth.
Arginine 118 in MAG was chosen because mutation of an arginine conserved at the same location in two other members of the sialoadhesins, CD22 (R130) and sialoadhesin (R97), have been shown to abolish sialic acid–dependent binding (Vinson et al., 1996; van der Merwe et al., 1996). In those studies a number of point mutations were introduced at different sites in the first Ig domain of each molecule. The mutated proteins were assessed for sialic acid–binding and, using a panel of conformation-dependent monoclonal antibodies, for misfolding. For sialoadhesin, disruptive substitutions at six residues abolished sialic acid–dependent binding with little effect on protein folding. In a model of Ig domain 1 of sialoadhesin, based on the crystal structure of CD8, these amino acids in the G, F, and C β-strands form a discrete cluster on one β-sheet, surrounded by residues that do not affect binding upon mutation. When a conservative amino acid change was introduced at these various sites, only substitution at R97 led to a complete loss of binding (Vinson et al., 1996). Therefore, it was concluded that these six amino acids, with R97 as a key residue, are critical for sialic acid–dependent binding of sialoadhesin. Similar analysis concluded that R130 in CD22 is a key amino acid in sialic acid–dependent binding (van der Merwe et al., 1996). Therefore, it appears that an arginine, conserved in the first Ig domain of at least three sialoadhesin family members, is imperative for the successful binding of these molecules to sialic acid.
It is of interest that R118 in MAG is the first residue in an Arg-Gly-Asp (RGD) motif, a motif known to be recognized by some integrins (Ruoslahti and Pierschbacher, 1987). However, studies with antibodies to RGD-containing peptides and with the peptides themselves, indicate that MAG does not use this sequence in its interactions (Pedraza et al., 1990; Sadoul et al., 1990). Antibodies to a 20-mer peptide corresponding to a MAG–amino acid sequence spanning the RGD sequence does not bind to native MAG but recognizes denatured MAG. This implies that the RGD sequence is not exposed at the surface of the molecule and consequently is not available for interaction (Pedraza et al., 1990). Consistent with this observation, a synthetic MAG peptide containing this RGD sequence failed to block binding to axons by MAG incorporated into liposomes (Sadoul et al., 1990). From the studies with sialoadhesin, the six amino acids that comprise the sialic acid binding site are not consecutive but are found on β-strands G, F, and C (Vinson et al., 1996). Three of these amino acids, including R118, are conserved in MAG (Fig. 1). It is probable, therefore, that only R118 of the RGD sequence of MAG is involved in binding and that this binding is distinct from integrin-mediated binding.
What role does the sialic acid–binding epitope of MAG play in the inhibition of neurite outgrowth? The simplest interpretation of our results is that MAG requires this site for neuronal binding, which then permits an interaction of the inhibition epitope. This is certainly true for soluble MAG-Fc but not so apparent for MAG expressed by CHO cells where, taking all the information together, inhibition is somewhat independent of MAG's sialic acid binding. This would seem fitting since sialic acid residues attached in an O-linkage to glycoconjugates are ubiquitous in the nervous system (Varki and Marth, 1995) and evolutionary selection of a second site for inhibition on MAG would allow greater specificity to be exerted. The sialic acid binding of MAG may promote, but may not be essential for, the interaction of the inhibition site. It is not known if the two components recognized by the sialic acid–binding site and the neurite outgrowth inhibition site of MAG are carried by a single neuronal protein or closely associated proteins. This point can only be resolved when the component(s) with which MAG interacts on neurons has been identified.
A two-site model has also been proposed for the interaction of P-selectin with its ligand PSGL-1 (Pouyani and Seed, 1995; Sako et al., 1995). P-selectin interacts with sialylated, fucosylated O-linked glycans attached to a variety of ligands (Varki, 1994). For interaction with the PSGL-1 ligand, however, P-selectin also requires a sulfated tyrosine for high affinity binding (Pouyani and Seed, 1995; Sako et al., 1995). Consequently, two binding sites are proposed for the interaction of P-selectin with PSGL-1, one that is carbohydrate-dependent and one that is amino acid (sulfated)–dependent. In contrast, another family member, E-selectin, also binds to PSGL-1 but only with low affinity and only via the carbohydrate epitope (Asa et al., 1995); E-selectin does not carry the sulfated-tyrosine–recognition epitope.
In conclusion, we provide evidence that R118 plays an important role in the sialic acid binding of MAG but that sialic acid binding alone is insufficient to induce inhibition of neurite outgrowth. We propose a model whereby MAG recognizes neurons via two sites, a sialic acid binding site and a neurite outgrowth inhibition site. A model based on two separate sites on MAG leads to the possibility that a mutated form of MAG can eventually be engineered to bind the neuronal receptor but not inhibit neurite outgrowth. Such a form of MAG would act as an antagonist of inhibition and could be used to reverse inhibition of axonal outgrowth by MAG in vivo and be of possible therapeutic value after injury to the CNS.
Acknowledgments
We would like to thank R. Persell for critically reading this manuscript.
This work was supported by a grant from the National Multiple Sclerosis Society, and a core facility Research Centers in Minority Institutions, National Institutes of Health grant. M.T. Filbin is the recipient of an Established Investigator Award from the American Heart Association, New York Chapter.
Abbreviations used in this paper
- CNS
central nervous system
- DRG
dorsal root ganglion
- MAG
myelin-associated glycoprotein
Footnotes
Address all correspondence to Marie T. Filbin, Department of Biological Sciences, Hunter College of City University of New York, 695 Park Avenue, New York, NY 10021. Tel.: (212) 772-5270. Fax: (212) 772-5227. e-mail: filbin@genectr.hunter.cuny.edu
References
- Asa D, Raycroft L, Ma L, Aeed PA, Kaytes PS, Elhammer AP, Geng JG. The P-selectin glycoprotein ligand functions as a common human leukocyte ligand for P- and E-selectins. J Biol Chem. 1995;270:11662–11670. doi: 10.1074/jbc.270.19.11662. [DOI] [PubMed] [Google Scholar]
- Bartsch L, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M. Lack of evidence that the myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. doi: 10.1016/0896-6273(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunke-Bagden E, Schnell L, Dal HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature (Lond) 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perry VH, Lunn ER, Gordon S, Heumann R. Macrophage dependence of peripheral sensory nerve regeneration: possible involvement of nerve growth. Neuron. 1991;6:359–370. doi: 10.1016/0896-6273(91)90245-u. [DOI] [PubMed] [Google Scholar]
- Brown MC, Lunn ER, Perry VH. Consequences of slow Wallerian degeneration for regenerating motor and sensory axons. J Neurobiol. 1992;23:521–536. doi: 10.1002/neu.480230507. [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988a;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988b;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker PR, Mucklow S, Bouckson V, McWilliam A, Willis AC, Gordon S, Milon G, Kelm S, Bradfield P. Sialoadhesin, a macrophage sialic acid binding receptor for haemopoieic cells with 17 immunoglobulin-like domains. EMBO (Eur Mol Biol Organ) J. 1994;13:4490–4503. doi: 10.1002/j.1460-2075.1994.tb06771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBellard ME, Tang S, Mukhopadhyay G, Shen Y, Filbin MT. Myelin-associated glycoprotein inhibits axonal regeneration from a variety of neurons via interaction with a sialoglycoprotein. Mol Cell Neurosci. 1996a;7:89–101. doi: 10.1006/mcne.1996.0007. [DOI] [PubMed] [Google Scholar]
- DeBellard ME, Tang T, Roder J, Filbin MT. Inhibition of axonal regeneration by myelin involves a neuronal sialoglycoprotein. Soc Neurosci Abs. 1996b;130:5. [Google Scholar]
- Doherty P, Fruns M, Seaton P, Dickson G, Barton CH, Sears TA, Walsh FA. A threshold effect of the major isoforms of N CAM on neurite outgrowth. Nature (Lond) 1990;343:464–466. doi: 10.1038/343464a0. [DOI] [PubMed] [Google Scholar]
- Doherty P, Williams E, Walsh FS. A soluble chimeric form of the L1 glycoprotein stimulates neurite outgrowth. Neuron. 1995;14:57–66. doi: 10.1016/0896-6273(95)90240-6. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated glycoprotein: a role in myelination and in the inhibition of axonal regeneration? . Curr Opin Neurobiol. 1995;5:588–595. doi: 10.1016/0959-4388(95)80063-8. [DOI] [PubMed] [Google Scholar]
- Filbin MT. The muddle with MAG. Mol Cell Neurosci. 1996;8:84–92. doi: 10.1006/mcne.1996.0047. [DOI] [PubMed] [Google Scholar]
- Johnson AR. Contact inhibition in the failure of mammalian CNS axonal regeneration. Bioessays. 1993;15:807–813. doi: 10.1002/bies.950151206. [DOI] [PubMed] [Google Scholar]
- Johnson PW, Abramow-Newerly W, Seilheimer B, Sadoul R, Tropak MB, Arquint M, Dunn RJ, Schachner M, Roder JC. Recombinant myelin associated glycoprotein confers neural adhesion and neurite outgrowth function. Neuron. 1989;3:377–385. doi: 10.1016/0896-6273(89)90262-6. [DOI] [PubMed] [Google Scholar]
- Kelm S, Pelz A, Schauer R, Filbin MT, Tang S, de Bellard M-E, Schnaar RL, Mahoney JA, Hartnell A, Bradfield P, Crocker PR. Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid–dependent adhesion molecules of the immunoglobulin superfamily. Curr Biol. 1994;4:965–972. doi: 10.1016/s0960-9822(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Keynes RJ, Cook GMW. Repulsive and inhibitory signals. Curr Opin Neurobiol. 1995;5:75–82. doi: 10.1016/0959-4388(95)80090-5. [DOI] [PubMed] [Google Scholar]
- Li C, Tropak MB, Gerlai R, Clapoff S, Abramow-Newerly W, Trapp B, Peterson A, Roder J. Myelination in the absence of myelin-associated glycoprotein. Nature (Lond) 1994;369:747–750. doi: 10.1038/369747a0. [DOI] [PubMed] [Google Scholar]
- Li M, Shibata A, Li C, Braun PE, McKerracher L, Roder J, Kater SB, David S. Myelin-associated glycoprotein inhibits neurite/axon growth and causes growth cone collapse. J Neurosci Res. 1996;46:404–414. doi: 10.1002/(SICI)1097-4547(19961115)46:4<404::AID-JNR2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite outgrowth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Montag D, Giese KP, Bartsch U, Martini R, Lang Y, Bluthmann H, Karthigasan J, Kirschner DA, Wintergerst ES, Nave KA, et al. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron. 1994;13:229–246. doi: 10.1016/0896-6273(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Ng WP, Cartel N, Li C, Roder J, Lozano A. Myelin from MAG-deficient mice is a strong inhibitor of neurite outgrowth. Neuroreport. 1996;7:861–864. doi: 10.1097/00001756-199603220-00005. [DOI] [PubMed] [Google Scholar]
- Owens GC, Boyd CJ, Bunge RP, Salzer JL. Expression of recombinant myelin-associated glycoprotein in primary Schwann cells promotes the initial investment of axons by Schwann cells. J Cell Biol. 1990;111:1171–1182. doi: 10.1083/jcb.111.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza L, Owens GC, Green LA, Salzer JL. The myelin-associated glycoproteins: membrane diposition, evidence for a novel disulfide linkage between immunoglobulin-like domains, and posttranslational palmitylation. J Cell Biol. 1990;111:2651–2661. doi: 10.1083/jcb.111.6.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak M, Sadoul R, Keilhauer G, Landa C, Fahrig T, Schachner M. Myelin-associated glycoprotein, a member of the L2/HNK-1 family of neural cell adhesion molecules, is involved in neuron-oligodendrocyte and oligodendrocyte interaction. J Cell Biol. 1987;105:1897–1899. doi: 10.1083/jcb.105.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SL, Glaser L, Bunge RP. Release of autocrine growth factor by primary and immortalized Schwann cell. Proc Natl Acad Sci USA. 1987;83:7768–7771. doi: 10.1073/pnas.84.21.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyani T, Seed B. PSGL-1 recognition of P-selectin is controlled by a tyrosine sulfation consensus at the PSGL-1 amino terminus. Cell. 1995;83:333–343. doi: 10.1016/0092-8674(95)90174-4. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science (Wash DC) 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sadoul R, Fahring T, Bartig U, Schachner M. Binding properties of liposomes containing the myelin-associated glycoprotein MAG to neural cell cultures. J Neurosci Res. 1990;25:1–13. doi: 10.1002/jnr.490250102. [DOI] [PubMed] [Google Scholar]
- Sako D, Comess KM, Barone KM, Camphausen RT, Cumming DA, Shaw GD. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell. 1995;83:323–332. doi: 10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- Schafer M, Fruttiger M, Montag D, Schachner M, Martini R. Disruption of the gene for the myelin-associated glycoprotein improves axonal regrowth in C57BL/Wld mice. Neuron. 1996;16:1107–1113. doi: 10.1016/s0896-6273(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature (Lond) 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Kapfhammer JP, Bandtlow CE. Inhibitors of neurite growth. Annu Rev Neurosci. 1993;16:565–596. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- Shen Y, Walsh FS, Filbin MT. MAG expressed by Schwann cells inhibits axonal growth and branching. Soc Neursci Abstr. 1996;130:6. [Google Scholar]
- Simmons, D.L. 1993. Cloning cell surface molecules by transient expression in mammalian cells. In Cellular Interactions in Development - a Practical Approach. D.A. Hartley, IRL Press, Oxford. 93–128.
- Tang, S., R.W. Woodhall, Y.J. Shen, M.E. DeBellard, J.L. Saffell, P. Doherty, F.S. Walsh, and M.T. Filbin. 1997. Soluble myelin-associated glycoprotein (MAG) found in vivo inhibits axonal regeneration. Mol. Cell. Neurosci. In press. [DOI] [PubMed]
- van der Merwe A, Crocker PR, Vinson M, Barclay AN, Schauer R, Kelm S. Localization of the putative sialic acid–binding site on the immunoglobulin superfamily cell-surface molecule CD22. J Biol Chem. 1996;271:9273–9280. [PubMed] [Google Scholar]
- Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Marth J. Oligosaccharides in vertebrate development. Semin Dev Biol. 1995;6:27–36. [Google Scholar]
- Vinson M, van der Merwe A, Kelm S, May A, Jones EY, Crocker PR. Characterization of the sialic acid binding site in sialoadhesin by site-directed mutagenesis. J Biol Chem. 1996;271:9267–9280. doi: 10.1074/jbc.271.16.9267. [DOI] [PubMed] [Google Scholar]
- Yang LJ, Zeller CB, Shaper NL, Kiso M, Hasegawa A, Shapiro RE, Schnaar RL. Gangliosides are neuronal ligands for myelin-asociated glycoprotein. Proc Natl Acad Sci USA. 1996;93:814–818. doi: 10.1073/pnas.93.2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]