Abstract
Drosophila melanogaster has proven to be a good model for understanding the physiology of ion channels. We identified two novel Drosophila DEG/ ENaC proteins, Pickpocket (PPK) and Ripped Pocket (RPK). Both appear to be ion channel subunits. Expression of RPK generated multimeric Na+ channels that were dominantly activated by a mutation associated with neurodegeneration. Amiloride and gadolinium, which block mechanosensation in vivo, inhibited RPK channels. Although PPK did not form channels on its own, it associated with and reduced the current generated by a related human brain Na+ channel. RPK transcripts were abundant in early stage embryos, suggesting a role in development. In contrast, PPK was found in sensory dendrites of a subset of peripheral neurons in late stage embryos and early larvae. In insects, such multiple dendritic neurons play key roles in touch sensation and proprioception and their morphology resembles human mechanosensory free nerve endings. These results suggest that PPK may be a channel subunit involved in mechanosensation.
T he DEG/ENaC1 superfamily includes proteins involved in mechanotransduction, proprioception, neurotransmission, and fluid and electrolyte homeostasis (11, 39, 51). Members of this superfamily are united by similarities in their amino acid sequences and in some cases by their function. The first identified protein and the largest number of family members are from Caenorhabditis elegans; these include MEC-4, MEC-10, DEG-1, UNC-105, UNC-8, and DEL-1 (10, 12, 15, 24, 33, 52). Elegant genetic studies in that organism have suggested a role for DEG/ ENaC proteins in mechanotransduction. MEC-4 and MEC-10 are expressed in mechanosensory neurons and are required for normal sensitivity to touch (12, 24). In addition, UNC-105 and UNC-8 are expressed in muscle and motor neurons, respectively, and are required for coordinated movement (33, 52). Three lines of evidence suggest that C. elegans family members may be ion channels. First, as discussed below, other family members have been shown to be ion channels. Second, residues in the transmembrane sequence of a related DEG/ ENaC channel, αENaC, can be substituted for residues in M2 of MEC-4 without loss of function in the worm and vice versa (23, 56). And third, specific mutations in several C. elegans family members cause a phenotype marked by neuronal swelling; this may suggest a loss of cell volume control, perhaps caused by unregulated opening of an ion channel (10, 12, 15, 24). Nevertheless, these C. elegans, proteins have not been shown experimentally to be channels either in vivo or in heterologous expression systems.
Subunits of the vertebrate epithelial Na+ channel (ENaC) subfamily form ion channels that mediate Na+ absorption across the apical membrane of epithelia (6, 8, 31, 35, 36, 54, 55). Studies of ENaC have served to define several functional properties of the family. First, ENaC generates Na+ currents that are reversibly blocked by the diuretic amiloride. Although Na+ selectivity is not a general feature, all DEG/ENaC proteins shown to be ion channels are amiloride-sensitive and conduct cations. Second, ENaC functions as a multimeric complex of three subunits, α-, β-, and γENaC. In Xenopus oocytes, expression of αENaC, but not β- or γENaC, generates a small amiloride-sensitive Na+ current (6). However, when β- and γENaC are coexpressed with αENaC, much larger Na+ currents are produced (8, 36). This finding illustrates a third feature of the family: some members can form ion channels when expressed alone, whereas others are ion channel subunits that do not function by themselves in oocytes. Fourth, biochemical studies of αENaC revealed a membrane topology consisting of two transmembrane domains (M1 and M2), cytoplasmic NH2 and COOH termini, and a large extracellular region containing cysteine-rich domains (7, 43, 45). Other family members are thought to have a similar molecular organization (30).
In addition to the C. elegans proteins, three other neuronal family members have been identified. FaNaCh is a neuronal channel activated by the neuropeptide FMRFamide (32). Brain Na+ channel 1 (BNC1, also named MDEG and BNaC1) is widely expressed in human brain. Although its physiologic function is unknown, its channel activity can be enhanced by mutation of a residue associated with neuronal swelling in C. elegans DEG/ENaC proteins (16, 42, 57). A channel very similar to BNC1, BNaC2 (also named ASIC), is expressed in brain and dorsal root ganglia, and is activated by extracellular protons (16, 58). These observations demonstrate a fifth feature of the family; in contrast to ENaC, neuronal DEG/ENaC channels may open only in the presence of specific stimuli or an activating mutation.
As a model system for investigating complex processes such as mechanosensation, Drosophila melanogaster offers several advantages. In addition to its value for genetic, molecular, and physiologic analysis, the morphology and development of several embryonic and larval structures have been characterized in detail. Therefore, we sought to identify new DEG/ENaC family members from Drosophila and to study their localization and function.
Materials and Methods
Cloning and Northern Analysis
DS06238, a P1 phage that contains a large genomic clone from the left arm of Drosophila chromosome 2 (20, 34), and LD03440 (21), an expressed sequence tag clone from Drosophila embryos, were cloned and partially sequenced by the Berkeley Drosophila Genome Project (BDGP). DS06238 is reported to lie in chromosomal region 35A1-4, near the alcohol dehydrogenase (Adh) gene (34). We confirmed this location by in situ hybridization to polytene chromosome squashes (not shown). Database searches were performed using the BLAST network server (National Center for Biotechnology Information). Partial pickpocket (ppk) cDNAs were cloned from adult Drosophila poly(A)+ RNA (Clontech, Palo Alto, CA), using 3′ and 5′ RACE PCR (GIBCO BRL, Gaithersburg, MD) and primers designed against candidate DEG/ENaC protein sequences in DS06238. A cDNA containing the entire ppk-coding region was constructed from partial PCR clones. The ppk sequence was confirmed by comparing it to the submitted sequence of DS06838, as well as the sequence of an independently obtained genomic clone of the region. A full-length ripped pocket (rpk) cDNA clone (expressed sequence tag clone LD03440) was kindly provided by BDGP; we sequenced this clone on both strands, and confirmed the location of its start codon using 5′ RACE PCR. PPK was named for its potential role in mechanosensation; RPK was named for its similarity to PPK and activation of its channel function by a specific mutation. Sequence comparisons were made using an alignment generated by CLUSTAL W (53). In some cases, slight adjustments were made manually. Northern blots were performed as previously described (42). Blots contained 2 μg of poly(A)+ RNA isolated from Drosophila embryos, larvae, or adults (Clontech). The ppk probe corresponded to the complete coding region; the rpk probe consisted of nucleotides 1–417 of the rpk cDNA.
Expression Constructs and Antibodies
The cDNA encoding PPK was constructed using the Muta-Gene phagemid in vitro mutagenesis kit (Bio-Rad Laboratories, Hercules, CA). In this construct, a NotI cloning site and a translational initiation sequence (9) were introduced immediately upstream of the ppk coding region in pCRII (Invitrogen, San Diego, CA). cDNAs encoding PPKmyc and RPK were generated by PCR using the ppk cDNA and LD03440, respectively, as templates. In PPKmyc the myc epitope (EQKLISEEDL) was inserted at the COOH terminus, and in RPK, the FLAG epitope (DYKDDDDK) was inserted at the COOH terminus. The cDNA encoding RPKA524V was generated by PCR using rpk cDNA as a template. In RPKA524V, a valine replaced the alanine at position 524 and the FLAG epitope was inserted at the COOH terminus. BNC1G430V and BNC1FLAG were constructed by single-stranded mutagenesis of BNC1 in pBluescript. In BNC1G430V, a valine replaced the glycine at position 430, and in BNC1FLAG, the FLAG epitope was inserted at the COOH terminus. The cDNAs encoding secreted alkaline phosphatase (SEAP) and cystic fibrosis transmembrane conductance regulator (CFTR) are described elsewhere (18, 50). All cDNA constructs were cloned into pMT3 (50) for expression.
The anti-FLAG monoclonal antibody, M2, was obtained from Kodak (New Haven, CT), and the anti-myc monoclonal antibody, 9E10, was a generous gift of Robert Deschenes (University of Iowa, Iowa City, IA). The monoclonal antibody 22C10 was generously provided by Seymour Benzer (California Institue of Technology, Pasadena, CA). Anti-PPK sheep antiserum was generated against amino acids 126-200 of PPK expressed as a GST fusion protein. The antisera was then affinity purified against the GST fusion protein covalently coupled to AminoLink coupling gel (Pierce Chemical Co., Rockford, IL). The antisera was not reactive with RPK or BNC1.
Immunoprecipitations and Western Blots
COS-7 cells were transfected by electroporation, as we have previously described (46). 1–3 d after transfection, cells were lysed in lysis buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris, pH 7.4, 50 μg/ml aprotinin, 10 μg/ml leupeptin, 50 μg/ml pepstatin A, 1 mM PMSF). For immunoprecipitations, cell lysates were incubated with anti-FLAG antibody (2 μg/ml) at 4°C for 2 h. Antigen–antibody complexes were precipitated with immobilized protein G (Pharmacia Biotech, Uppsula, Sweden), then washed extensively in lysis buffer containing 500 mM NaCl. In deglycosylation experiments, cell lysates or washed immunoprecipitates were treated with endoglycosidase H (Boehringer Mannheim, Indianapolis, IN) in 1% Triton X-100, 50 mM potassium phosphate buffer (pH 6.0) for 16 h at 37°C. Before SDS-PAGE, proteins were boiled for 5 min in sample buffer (4% SDS, 65 mM Tris [pH 6.8], 100 mM DTT, 20% glycerol, and 0.005% bromophenol blue). Proteins were separated on 8 or 10% polyacrylamide gels using SDS-PAGE, then transferred to a polyvinylidenedifluoride membrane (Millipore Corp., Bedford, MA). Western blots were incubated first with primary antibody (anti-FLAG, anti-myc, or anti-PPK antibody) and then with a horseradish peroxidase–coupled secondary antibody (Amersham Corp., Arlington Heights, IL) at a 1:10,000 dilution. Immunoreactive proteins were detected by enhanced chemiluminescence (Pierce Chemical Co.).
Immunocytochemistry and In Situ Hybridization
Staged Drosophila embryos were labeled using a modification of protocols previously described (27, 38). PPK protein distribution was detected using an affinity-purified, anti-PPK antibody at a 1:40 dilution in PBT (1× PBS, 0.5% bovine serum albumin, 0.2% Triton X-100) preabsorbed overnight at 4°C against 0–4 h Drosophila embryos immediately before use. For standard preparations, primary antibody was detected using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) and horseradish peroxidase immunochemistry following the manufacturer's recommendations. Embryos were mounted in 70% glycerol/1× PBS and photographed on a Nikon Optiphot microscope using Nomarski optics. For fluorescent preparations, anti-PPK antibody was detected by incubating for 1 h at room temperature with a 1:200 dilution of biotinylated anti– sheep antibody (Zymed Labs., Inc., San Francisco, CA), washing four times over 1 h with PBT, incubating for 1 h with 1:500 dilution of FITC-avidin in 1× PBS, 0.1% Triton X-100 and finally washing four times over 1 h with PBT. Embryos were mounted in 80% glycerol, 0.1 M Tris-HCl, pH 8.0, 4.6 mM PDA and imaged with a laser scanning confocal microscope (1024; Bio-Rad Laboratories, Hercules, CA). PNS neurons were labeled with the 22C10 antibody and visualized using a lissamine-conjugated anti– mouse antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). ppk or rpk transcript levels were assayed by whole mount in situ hybridization with digoxygenin (DIG) incorporated probes visualized with alkaline phosphatase conjugated anti-DIG antibody as previously described (40). Anti-sense, ppk-specific DIG-UTP RNA probes were generated by in vitro transcription of a 958-bp fragment of ppk cDNA encoding most of the extracellular region and M2 of the PPK protein. Anti-sense rpk probes were generated in a similar manner and contained the entire coding sequence.
Expression and Electrophysiological Analysis in Xenopus Oocytes
cDNA constructs were expressed in albino Xenopus laevis oocytes (Nasco, Fort Atkinson, WI) by nuclear injection of plasmid DNA. DNA was injected at concentrations ranging from 20 to 80 ng/μl. After injection, oocytes were incubated at 18°C in modified Barth's solution and then studied 1–2 d later. Whole cell currents were measured using the two-electrode voltage-clamp technique, as we have described previously (35). During recording, oocytes were bathed in frog Ringer's solution (116 mM NaCl or 116 mM KCl or 116 mM LiCl, 0.4 mM CaCl2, 1 mM MgCl2, 5 mM Hepes [pH 7.4]). Amiloride or gadolinium was added directly to the bath, and blocked current was determined by subtracting current after drug application from the basal current. The Na+/Li+ permeability ratio was calculated from the reversal potentials of amiloride-sensitive current in Na+ or Li+ Ringer's solution (22).
Results
Identification of Two Novel DEG/ENaC Proteins from Drosophila
To begin the study of DEG/ENaC proteins in Drosophila, we searched sequence databases and identified a Drosophila genomic clone, DS06238, that shares sequence homology with the conserved M2 of known DEG/ENaC genes. Then we used RACE PCR and primers containing the putative M2 sequence to amplify cDNA clones from adult Drosophila poly(A)+ RNA. With this approach, we cloned a cDNA encoding a novel DEG/ENaC protein that we have called PPK. Alignment of cDNA and genomic sequences revealed that the ppk coding region is composed of seven exons, and is contained within 2.3 kb of genomic DNA. Next, we searched for sequences related to PPK, and found an expressed sequence tag clone from Drosophila embryos, LD03440, that shares homology with the NH2-terminal portion of PPK. Further characterization of the sequence of LD03440 revealed that it also encodes a novel DEG/ENaC protein that we have called RPK.
The cDNAs encoding PPK and RPK predict proteins of 69 (606 amino acids) and 65 kD (562 amino acids), respectively. Hydrophobicity analysis suggests that PPK and RPK, like other DEG/ENaC proteins, possess two transmembrane domains (M1 and M2, Fig. 1 A). Both proteins contain all of the conserved sequences present in known DEG/ENaC proteins including conserved cysteines (Fig. 1 A, diamonds) in the predicted large extracellular region. PPK contains seven potential N-linked glycosylation sites within its predicted extracellular region, and RPK possesses two. Among known DEG/ENaC proteins, PPK and RPK most closely resemble each other, with 38% amino acid identity and 60% similarity. The next closest relative is the human neuronal ion channel BNC1 (15, 42, 57), which is 24% identical and 45% similar to PPK and 22% identical and 46% similar to RPK (Fig. 1, A and B). Within their predicted extracellular regions, PPK and RPK possess a unique domain not found in other family members (Fig. 1, A and B, hatched bars). This region is encoded largely by a single exon in the ppk gene. Phylogenetic analysis indicated that PPK and RPK may represent a new DEG/ENaC subfamily (Fig. 1 B).
Figure 1.

Sequence and alignment of PPK and RPK with other DEG/ENaC family members. (A) Amino acid sequence of PPK and RPK, and BNC1, their closest relative. Similar residues are indicated by shading. Transmembrane domains are indicated with solid bars. Diamonds indicate conserved cysteines. An asterisk indicates residue analogous to that which causes neurodegeneration in C. elegans when mutated. Hatched bars indicate residues of PPK encoded by the fourth exon of the ppk coding region. (B) The DEG/ENaC family. Sequence analysis indicates that the family can be divided into five subfamilies: C. elegans members (MEC-4, MEC-10, DEG-1, UNC-8, UNC-105, and DEL-1), mammalian ENaC subunits (α-, β-, and γENaC and δNaCh), mammalian neuronal channels (BNC1 and BNaC2), Drosophila PPK and RPK, and FaNaCh. Proteins are named as suggested in the first published report of their primary structure. DEG/ENaC proteins can also be classified by gross features of their extracellular regions. The schematic diagrams on the left represent a typical member from each subfamily (MEC-4, αENaC, PPK, BNC1, and FaNaCh), and show that members within each subfamily possess a characteristic extracellular domain not found in members of other subfamilies. Shading indicates regions containing sequences conserved in all DEG/ENaC proteins; checkered shading represents regions of conserved cysteines; black indicates the conserved transmembrane domains, M1 and M2; white indicates regions with poorly conserved sequences; hatched white bars indicates subfamily-specific extracellular domains. Note that within each subfamily there are some differences in length of the various domains.
Expression of PPK in a Subset of Peripheral Neurons
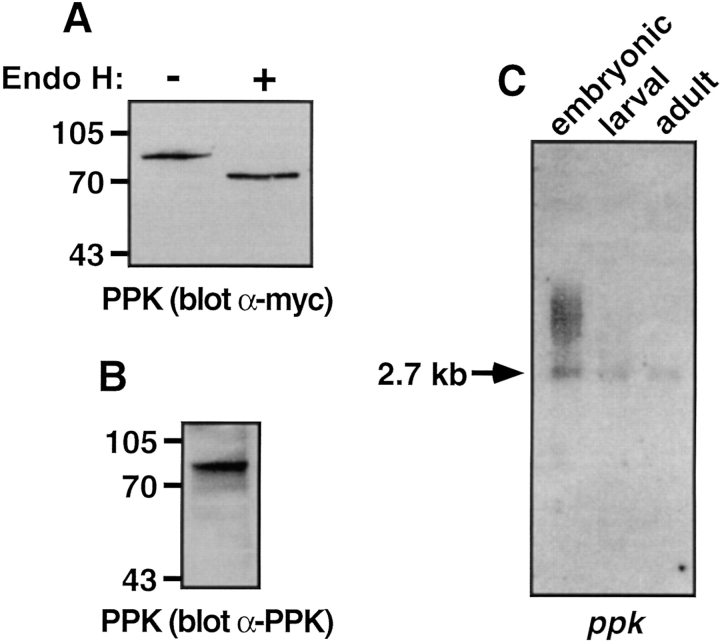
To confirm that ppk cDNA was capable of encoding protein in vivo, we transfected COS-7 cells with an epitope-tagged ppk construct, and examined expressed protein by Western blot. Expression of ppk cDNA yielded a 95-kD glycoprotein, that could be deglycosylated with endoglycosidase H (Fig. 2 A) or protein N-glycosidase (not shown). The deglycosylated form of PPK protein migrated at 69 kD, consistent with its predicted molecular mass. PPK could also be detected by Western blot using an antibody raised against a portion of its extracellular region (Fig. 2 B).
Figure 2.
Expression and Northern analysis of PPK. (A and B) COS-7 cells were transfected with cDNA encoding PPKmyc. Cell lysates were incubated overnight with or without endoglycosidase H, as indicated. Proteins were separated on 8% polyacrylamide gels, and detected by Western blot using either anti-myc antibody (A), or anti-PPK antibody (B). (C) Northern blot analysis of Drosophila embryonic, larval, and adult poly(A)+ RNA using a ppk probe. Exposure time was 36 h.
Northern analysis showed a 2.7-kb ppk transcript in embryonic, larval, and adult poly(A)+ RNA, as well as larger embryonic transcripts that may be alternatively processed (Fig. 2 C). The presence of ppk mRNA at all three stages suggested that PPK may have a functional role throughout the Drosophila life cycle.
To determine the tissue distribution of PPK, we labeled Drosophila embryos and early first instar larvae with either an antisense ppk probe (Fig. 3 A), or with anti-PPK antibody (Fig. 3 B). Using both methods, we detected PPK expression exclusively in a subset of peripheral neurons. Expression became apparent in late stage embryos (stage 17) and was present in early larvae. To confirm that the cells expressing PPK were peripheral neurons, we double labeled embryos with both anti-PPK antibody and monoclonal antibody 22C10 that recognizes peripheral neurons (60). Fig. 3 C shows abdominal peripheral neurons labeled with 22C10, and Fig. 3 D shows that cells expressing PPK (arrows) were also recognized by 22C10. The colocalization is shown at higher magnification for one neuron in Fig. 3, E–G.
Figure 3.
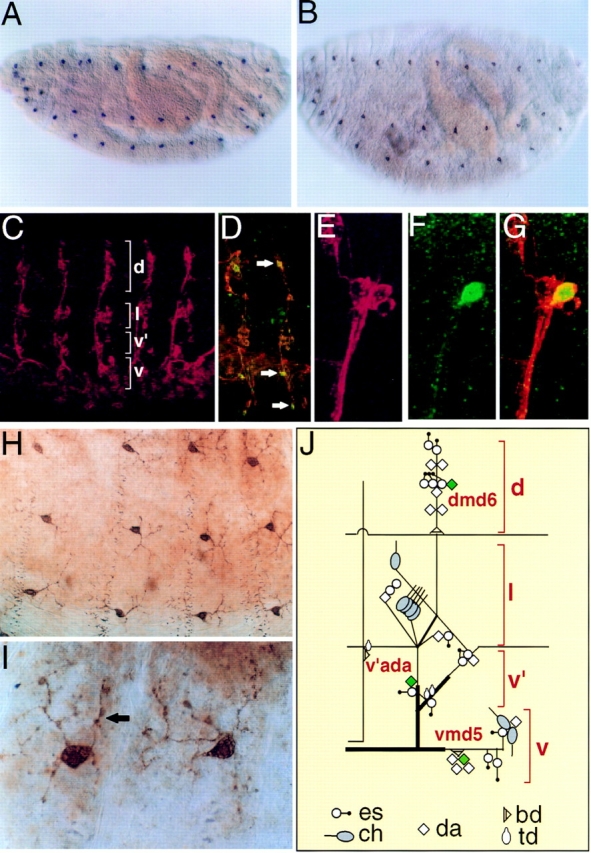
Embryonic expression pattern of PPK. (A and B) Late stage Drosophila embryos labeled with either an antisense ppk riboprobe (A, purple) or anti-PPK antibody (B, brown). (C) Peripheral neurons in five abdominal hemisegments labeled with antibody 22C10, which labels peripheral neurons. (D) Abdominal hemisegments labeled with both anti-PPK antibody (green) and 22C10 (orange). Neurons expressing PPK are stained by both antibodies and are yellow (arrows). (E and F) High magnification of dorsal cluster of abdominal peripheral neurons double labeled with 22C10 and anti-PPK antibody. Panels show 22C10 staining (E, red), anti-PPK staining (neuron dmd6; F, green), and colocalization (G, yellow). (H and I) High magnification views of PPK+ neurons labeled with anti-PPK antibody showing dendritic arborization and varicosities (I, arrow). (J) Schematic diagram of abdominal PNS neurons. PPK is expressed in one neuron (green) in each cluster of neurons. Diagram and nomenclature are according to Brewster and Bodmer (5).
The Drosophila peripheral nervous system (PNS) assumes a stereotypical pattern (Fig. 3, C–J) with three main types of neurons (3, 17, 26). External sensory (es) and chordotonal (ch) neurons innervate specific mechanosensory organs. These neurons each have a single uniterminal sensory dendrite. Multiple dendritic (md) neurons possess variable numbers of fine dendritic processes that lie beneath the epidermis (3). Higher magnification revealed PPK staining on the surface of the cell bodies and in multiple fine processes that extended from the cell (Fig. 3, H–I), indicating that PPK was expressed in md neurons. On the basis of their dendritic morphology, md neurons are classified into three types; da (dendrites that arborize), td (tracheal-associated dendrites), and bd (bipolar dendrites; reference 3). Moreover, like other PNS neurons, each md neuron occupies a well-defined and stereotypical position in the PNS. Based on their morphology, number and anatomical position, PPK+ neurons represented a subset of da neurons (Fig. 3 J). In the abdominal segments A1-A7, PPK was expressed in one of the six dmd neurons, in the v'ada neuron, and in one of the five vmd neurons (3). In the thoracic segments T1-T3, PPK was expressed in one of five dmd neurons and in one of five v'md neurons.
Since da neurons serve a variety of mechanosensory functions in insects (14, 37, 59), the neuronal expression pattern of PPK suggested that it might play a role in mechanosensation. Along the dendrites of PPK+ neurons, PPK staining was observed at a number of discrete varicosities, giving the dendrites a beaded appearance (Fig. 3, H–I). Interestingly, in the butterfly Pieris rapae crucivora, varicosities on the dendritic processes of da neurons are thought to be sites of mechanotransduction (47, 48). Thus, the presence of PPK in dendritic varicosities also suggested a mechanosensory function.
PPK Alters the Function of a Related DEG/ENaC Ion Channel
We tested the hypothesis that PPK might form an ion channel by expressing it in Xenopus oocytes and measuring whole cell current with the two-electrode voltage-clamp. Expression of PPK alone failed to generate basal currents larger than those of uninjected control oocytes (not shown). In this respect, PPK resembled several other DEG/ENaC proteins, such as MEC-4 and MEC-10 (unpublished data) and β- and γENaC subunits (8, 36), that do not produce current when expressed in Xenopus oocytes. These observations suggested that activation of PPK might require a specific, unknown ligand, or coexpression with other DEG/ ENaC proteins.
Although PPK did not produce currents when expressed alone, its structural similarity to DEG/ENaC channels suggested it was likely to participate in the formation of channels. To test this possibility, we coexpressed PPK with a closely related DEG/ENaC protein, BNC1. The rationale for this experiment was based on the observation that channels can be composed of multiple ENaC subunits, even though some of the subunits do not form channels by themselves (8). We used a BNC1 mutant, BNC1G430V, that contains an activating mutation and produces much larger whole cell currents than wild-type BNC1 (57). Expression of PPK with BNC1G430V generated amiloride-sensitive currents that were much smaller than currents in oocytes expressing BNC1G430V with control proteins (SEAP or CFTR; Fig. 4 A). An interaction between BNC1 and PPK was also detected biochemically; Fig. 4 B shows that PPK coimmunoprecipitated with BNC1. Our data do not tell us how association of PPK disrupts the function of BNC1; it could alter gating, conduction, or delivery to the cell surface. Nevertheless, the biochemical and functional association of PPK with BNC1 suggested that PPK may be an ion channel subunit that is dependent upon another DEG/ENaC protein for its channel function.
Figure 4.
Coexpression of PPK and BNC1. (A) Oocytes expressed BNC1G430V plus either SEAP, CFTR, or PPK. Current inhibited by a maximal dose of amiloride (100 μM) at −100 mV was measured. Amiloride-sensitive currents (Iamiloride) were normalized to average current from the SEAP control group on that day. For each group, n ⩾ 10 oocytes. Error bars represent SEM; asterisk indicates a significant difference from control current (P < 0.0001). In oocytes expressing BNC1G430V and SEAP, Iamiloride averaged 1912 ± 159 nA. (B) Coimmunoprecipitation of PPK with BNC1. COS-7 cells were transfected with cDNAs encoding BNC1FLAG and/or PPKmyc. Cell lysates were immunoprecipitated with anti-FLAG antibody. Precipitating protein was separated on a 10% polyacrylamide gel and detected by Western blot using the anti-myc antibody.
RPK Is Encoded by Maternal Messages in Drosophila Embryos
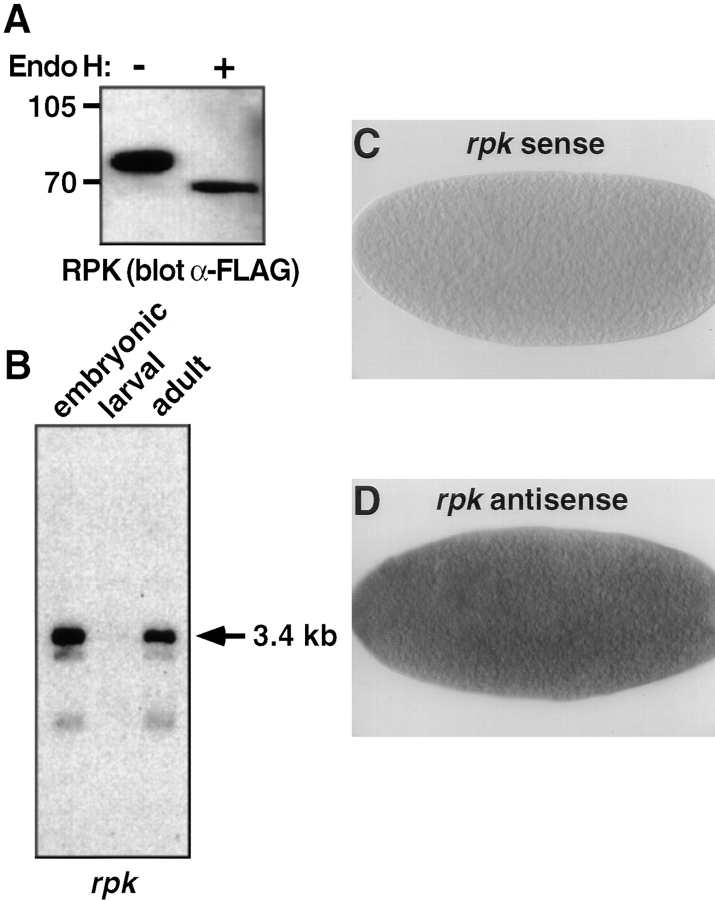
We also examined the molecular and functional characteristics of RPK. Expression of an epitope-tagged RPK construct in COS-7 cells generated a 73-kD glycoprotein that could be deglycosylated to its predicted molecular mass of 65 kD (Fig. 5 A). On Northern analysis, an rpk probe detected transcripts only in embryonic and adult RNA, where we observed a major 3.4-kb transcript, as well as two smaller less-abundant messages (Fig. 5 B). Thus, the expression patterns of RPK and PPK were different.
Figure 5.
Expression, Northern blot analysis, and in situ hybridization of RPK. (A) RPK is a glycoprotein. COS-7 cells were transfected with cDNA encoding RPK. Cell lysates were incubated overnight with or without endoglycosidase H, as indicated. Proteins were separated on 8% polyacrylamide gels, and detected by Western blot using anti-FLAG antibody. (B) Northern blot analysis of Drosophila embryonic, larval and adult poly(A)+ RNA using an rpk probe. Exposure time was 12 h. The same blot was used for both ppk (Fig. 2 C) and rpk hybridizations. The presence of ppk message in larval RNA (Fig. 2 C) served as a positive control. The intensity of the rpk signal suggests that in embryos and adults rpk transcripts may be more abundant than ppk transcripts. (C and D) 0–3 h Drosophila embryos labeled with either a sense (C) or antisense (D) rpk riboprobe. rpk transcripts appear dark.
To determine the embryonic expression pattern of rpk transcripts, we performed in situ hybridization to whole mount embryos using an antisense rpk probe. In contrast to ppk transcripts, rpk transcripts were detected in early stage (0–3 h) embryos, but were not present in later stages of embryogenesis (Fig. 5, C and D). Furthermore, in early stage embryos, rpk transcripts were not localized to a specific embryonic region or cell type. In Drosophila embryos, zygotic transcription does not initiate until the third hour of development. Because rpk mRNA was detected in embryos before the initiation of zygotic transcription, this result suggested that embryonic rpk message is of maternal origin, and that RPK may play a role in early development.
RPK Is a Na+ Channel That Is Dominantly Activated by the Deg Mutation
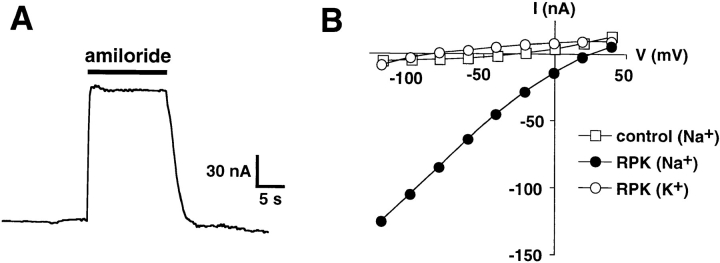
When expressed in Xenopus oocytes, RPK generated small whole cell Na+ currents that were reversibly blocked by amiloride (Fig. 6 A). RPK was impermeable to K+, as shown by the elimination of inward current when external Na+ was replaced with K+ (Fig. 6 B). Thus, in contrast to PPK, RPK formed functional ion channels by itself.
Figure 6.
Na+ channel currents in oocytes expressing RPK. (A) Current tracing from an oocyte expressing RPK. Oocyte was bathed in Na+ Ringers solution. Membrane voltage was clamped at −80 mV. Amiloride (100 μM) was present in the bath during time indicated by the solid bar. Time and current scales are shown. (B) Current–voltage relationships of amiloride-sensitive current from representative oocyte expressing either RPK or SEAP (control). Oocytes were bathed in Na+ or K+ Ringers, as indicated.
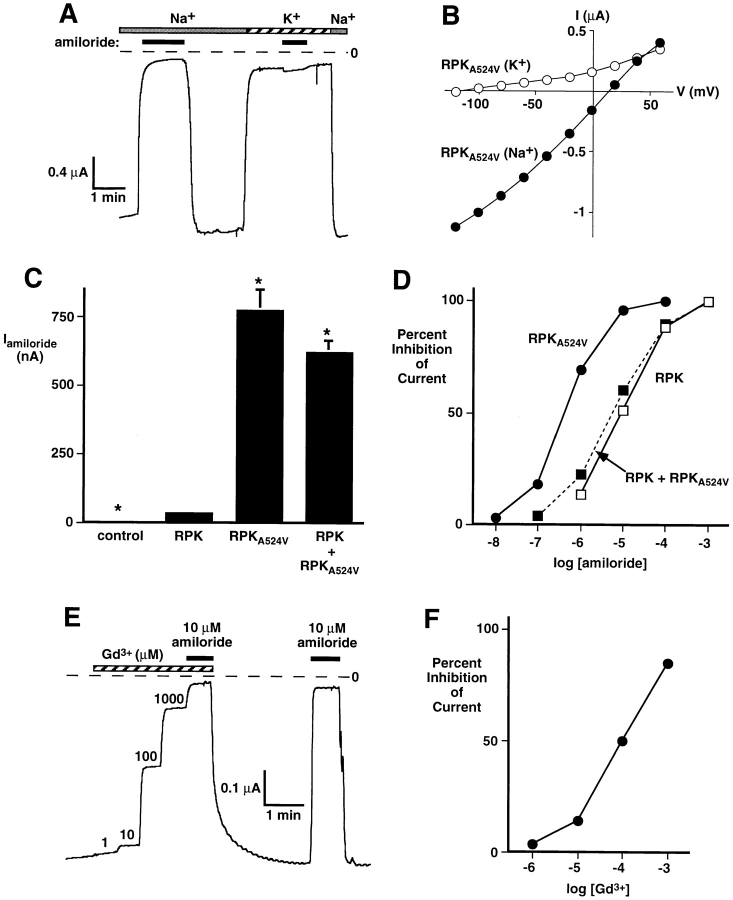
In several C. elegans degenerins, mutation of a specific residue near M2, the “Deg” mutation, causes a dominant form of neurodegeneration suggestive of constitutive ion channel activity (10, 12, 24). Similarly, BNC1 containing a Deg mutation (BNC1G430V) is activated, producing much larger currents in Xenopus oocytes (57). To learn whether RPK could also be activated by the Deg mutation, we incorporated a valine residue at the appropriate position (residue 524). Like wild-type RPK, RPKA524V generated Na+-selective currents that were reversibly inhibited by amiloride (Fig. 7, A and B). However, RPKA524V currents were 20–50 times larger than wild-type RPK currents (Fig. 7 C). This indicated that the Deg mutation activated RPK. RPKA524V was slightly more permeable to Li+ than Na+ (P Na/P Li = 0.89 ± 0.03, n = 5) but was impermeable to K+ (Fig. 7, A and B). RPKA524V was significantly more sensitive to amiloride than wild-type RPK (Fig. 7 D). Whereas 9.0 ± 0.1 μM amiloride blocked half of wild-type current, half-maximal inhibition of RPKA524V current required only 450 ± 50 nM amiloride. Interestingly, gadolinium, an inhibitor of mechanosensation and some stretch-activated channels (19), also reversibly inhibited RPKA524V current with half-maximal inhibition at 100 ± 5 μM (Fig. 7, E and F).
Figure 7.
Activation of RPK by the Deg mutation. (A) Current tracing from an oocyte expressing RPKA524V. Oocyte was bathed in Na+ or K+ Ringers solution, as indicated by top bars. Membrane voltage was clamped at −80 mV. Amiloride (100 μM) was present in the bath during time indicated by solid bars. Time and current scales are shown; dashed line indicates zero current. (B) Current–voltage relationships of amiloride-sensitive current in oocytes expressing RPKA524V and bathed in Na+ or K+ Ringers, as indicated. (C) Amount of amiloride-sensitive current in oocytes expressing either RPK, RPKA524V, or a 1:1 ratio of RPK and RPKA524V. Control oocytes were injected with SEAP. Current inhibited by a maximal dose of amiloride (1 mM) at −60 mV was measured. Data are average ± SEM from at least eight oocytes. Asterisks indicate a significant difference from RPK current (P < 0.01). Currents in oocytes expressing RPKA524V or both RPK and RPKA524V were not significantly different (P > 0.1). (D) Dose–response curves showing effect of amiloride on oocytes expressing RPK, RPKA524V, or a 1:1 ratio of RPK and RPKA524V at −60 mV. Data are average ± SEM from at least 8 oocytes; error bars are hidden by symbols. (E) Current tracing from an oocyte expressing RPKA524V. Oocyte was bathed in Na+ Ringers solution and membrane voltage was clamped to −60 mV. During time indicated by the hatched bar, Gd3+ (at concentrations ranging from 1 μM to 1 mM) was present in bath solution. Solid bars indicate 10 μM amiloride. (F) Dose–response curve showing effect of Gd3+ on oocytes expressing RPKA524V at −60 mV. Data are average ± SEM from eight oocytes; error bars are hidden by symbols.
Individual DEG/ENaC proteins are subunits that form homo- or heteromultimeric ion channels. Because DEG/ ENaC proteins with Deg mutations produce a genetically dominant phenotype in C. elegans, it is thought that the Deg mutation in one or a few subunits might activate the channel complex, producing larger currents. We tested the hypothesis that the Deg mutation is dominant at the molecular level by asking if channels composed of both wild-type RPK and RPKA524V would generate small or large Na+ currents. Coexpression of RPK and RPKA524V generated large Na+ currents that were similar in size to those generated by RPKA524V alone (Fig. 7 C). However, the amiloride sensitivity of the current (half-maximal inhibition = 5.5 ± 0.3 μM), was similar to that generated by wild-type RPK alone (Fig. 7 D). These observations indicated that the increase in current amplitude depended on RPKA524V, and the low amiloride sensitivity depended on wild-type RPK. Thus, the data suggested that at least two subunits combine to produce multimeric channels, that the A524V mutation dominantly activated the channel, and that Ala524 dominantly determined amiloride sensitivity. Gadolinium also inhibited wild-type RPK current, and gadolinium sensitivity was not significantly altered by the presence of wild-type RPK in a complex with RPKA524V (not shown). Coexpression of PPK with RPK or RPKA524V did not significantly alter the amount, ionic selectivity, or amiloride sensitivity of RPK or RPKA524V current (not shown). Thus, it appeared that PPK and RPK are not subunits of the same ion channel but likely have distinct physiological roles.
Discussion
PPK and RPK are novel Drosophila members of the DEG/ENaC protein superfamily (39). The two proteins share several features. First, their primary sequence is similar and they appear to represent a new subfamily. A feature that differentiates PPK and RPK from other family members is a unique domain in the large extracellular region (Fig. 1 B). In PPK this domain is mostly encoded by one exon, perhaps suggesting its evolutionary origin. Much of the variability, as well as most of the conservation, between different DEG/ENaC proteins and DEG/ENaC protein subfamilies occurs in the extracellular region. The location of inserts within the extracellular region allows a grouping of the subfamilies (Fig. 1 B). However, we do not know the functional significance of the extracellular region. This region may form a ligand-binding site in FaNaCh and BNaC2 (32, 58). In addition, genetic studies with C. elegans DEG/ENaC proteins suggest an interaction with proteins in the extracellular matrix and perhaps a role in channel gating (13, 15, 33). Thus, it is possible that the unique extracellular sequences in PPK and RPK confer unique interactions and possibly regulation on these channels.
A second similarity is that both PPK and RPK appear to be ion channel subunits. The data is most direct for RPK which produced channels when expressed in Xenopus oocytes. In this regard, RPK is similar to αENaC, δNaCh, and BNCl, which generate small amiloride-sensitive Na+ currents when expressed alone. Several observations suggest that PPK is also a channel subunit: the sequence of PPK is similar to that of RPK; PPK associated with the related channel BNC1 as assessed by coimmunoprecipitation; and when coexpressed, PPK reduced current generated by BNC1.
Despite their similarities, PPK and RPK have significant differences. For example, the DEG mutation activated RPK, but not PPK (not shown). Another striking difference was that PPK, but not RPK, was expressed in the PNS of embryos and larvae. In addition, RPK transcripts were probably maternally derived, whereas PPK was expressed late in embryogenesis and in larvae. These data, plus the finding that coexpressing PPK with RPK did not alter RPK currents, indicate that the two subunits are not part of the same channel complex.
The expression pattern and function of RPK suggest it is a Na+ channel involved in early development. Amiloride-sensitive Na+ channels in mammalian embryos play an important role in fluid transport across the trophectoderm and in formation of the blastocyst (2). Like RPK, these Na+ channels have a low sensitivity to amiloride (Ki = 12 μM; 44).
The effect of blockers on RPK is also interesting. First, in contrast to other channels in the family, RPK is relatively insensitive to amiloride. This raises the possibility that some native DEG/ENaC channels may not be highly sensitive to amiloride. Alternatively, RPK may associate with other subunits in vivo to form channels with different amiloride sensitivity. Second, the Deg mutation increased amiloride sensitivity. An alanine at position 524 was dominant in conferring low sensitivity to amiloride and a valine at position 524 was dominant in activating the channel. Thus, the residue at the position of the Deg mutation influenced both channel activation and amiloride sensitivity. Third, although all DEG/ENaC proteins known to function as channels are blocked by amiloride, RPK is the first DEG/ENaC channel shown to be blocked by gadolinium. Interestingly, the doses of gadolinium required for inhibition of RPKA524V were similar to those needed for block of mechanosensitive channels in rat supraoptic neurons (41). Nevertheless, both amiloride and gadolinium can have targets other than DEG/ENaC proteins and stretch-activated channels. Thus, sensitivity to these agents by itself does not necessarily imply a role for RPK in mechanosensation.
PPK was expressed in a subset of the da type of md neurons and, to our knowledge, is the first described protein expressed exclusively in md neurons. da neurons are distinguished by their dendritic network that extends beyond segmental boundaries, arborizes extensively, and ramifies beneath the epidermis (3, 14, 26, 59). The dendrites from one da neuron often overlap considerably with dendrites from other da neurons, and the full complement of da dendrites gives the appearance of a “spiderweb” that covers the embryo. PPK expression was detectable only late in da neuron differentiation, suggesting that PPK is not involved in neuronal development, but may play a role in sensory function.
md neurons are found in most, if not all, insect species, and the structure and function of md neurons have been extensively characterized in several species (14, 26, 37, 59). In adult insects, md neurons are touch or stretch receptors that monitor a wide range of mechanical stimuli, including muscle tension, gut motility, and limb and wing position. Interestingly, most mechanosensation in humans occurs via free nerve endings that are morphologically similar to insect md neurons (1, 14). In several ways, the da neurons of Drosophila embryos and larvae closely resemble the larval subepidermal da neurons of the blowfly, Phormia regina. In Phormia, each larval abdominal hemisegment possesses 15 da neurons that are located directly beneath the epidermal cell layer and send out a meshwork of dendrites that terminate on the epidermis (14). Ultrastructural studies of these dendrites show that they penetrate the basement membrane and directly contact the basolateral surface of epidermal cells. Furthermore, since mechanical stimuli increase the frequency of neuronal firing, the subepidermal da neurons of Phormia larvae are clearly mechanoreceptive, and are positioned to sense both touch and internal mechanical forces (14). Thus, the cellular location of PPK suggests a mechanosensory function. Along the dendrites of PPK-stained da neurons, PPK was observed in varicosities. In stretch-sensitive da neurons of the butterfly, Pieris rapae crucivora, similar dendritic varicosities are sites of epithelial contact and are thought to be sites of mechanotransduction (48). Of note, Kernan et al. (29) have produced Drosophila mutants with mechanosensory defects. However, the neurons affected by those mutations innervate bristles and thus are probably not da neurons and do not express PPK.
Might PPK be a mechanosensor? Our evidence suggesting that PPK may be an ion channel subunit is consistent with theoretical and experimental data that mechanosensors are ion channels (25, 28, 49). Although studies with blockers are not definitive, the notion is consistent with the ability of amiloride and gadolinium to block the related RPK channel at doses similar to those needed to block mechanosensation in vivo (19). Finally, its restricted localization to a subset of da neurons suggests that PPK is in the appropriate place to play an important role in mechanosensation.
Acknowledgments
We thank Ellen Tarr, Dan Bucher, Christopher Welsh, Luke Setter, and Theresa Mayhew for excellent assistance. We thank the Berkeley Drosophila Genome Project for the clone LD03440, Seymour Benzer for monoclonal antibody 22C10, and Robert Deschenes for the anti-myc antibody. We are grateful to Peter Snyder and Chun-Fang Wu for many helpful discussions. P1 phage DS06238 was generated by the Berkeley Drosophila Genome Project. Confocal imaging was performed at the University of Iowa Central Microscopy Research Facility and special thanks are due to Tom Moninger for technical advice. We also thank the DNA Core of the Diabetes and Endocrine Research Center (DK25295).
This work was supported by the Howard Hughes Medical Institute and by grants from the National Institutes of Health (NIH; NS28743) and the American Cancer Society to W.A. Johnson. C.M. Adams is a Predoctoral Trainee supported by NIH/National Institute of Aging grant T32 AG 00214; D.G. Motto is an Associate of the Howard Hughes Medical Institute (HHMI); and M.J. Welsh is an Investigator of the HHMI.
Abbreviations used in this paper
- BNC1
brain Na+ channel
- CFTR
cystic fibrosis transmembrane conductance regulator
- da
dendrites that arborize
- DIG
digoxygenin
- ENaC
epithelial Na+ channel
- es
external sensory
- md
multiple dendritic
- PNS
peripheral nervous system
- PPK
pickpocket
- RPK
ripped pocket
- SEAP
secreted alkaline phosphatase
References
- 1.Akoev, G.N., B.V. Krylov, and N.P. Alekseev. 1988. Mechanoreceptors: Their Functional Organization. Springer-Verlag, Berlin. 1–189.
- 3.Bodmer R, Jan YN. Morphological differentiation of the embryonic peripheral neurons in Drosophila. . Roux's Arch Dev Biol. 1987;196:69–77. doi: 10.1007/BF00402027. [DOI] [PubMed] [Google Scholar]
- 4.Bodmer R, Carretto R, Jan Y-N. Neurogenesis of the peripheral nervous system in Drosophilaembryos: DNA replication patterns and cell lineages. Neuron. 1989;3:21–32. doi: 10.1016/0896-6273(89)90112-8. [DOI] [PubMed] [Google Scholar]
- 5.Brewster R, Bodmer R. Origin and specification of type II sensory neurons in Drosophilia. . Development. 1995;121:2923–2936. doi: 10.1242/dev.121.9.2923. [DOI] [PubMed] [Google Scholar]
- 6.Canessa CM, Horisberger J-D, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- 7.Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol. 1994;267:C1682–C1690. doi: 10.1152/ajpcell.1994.267.6.C1682. [DOI] [PubMed] [Google Scholar]
- 8.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 9.Cavener DR. Comparison of the consensus sequence flanking translational start sites in Drosophilaand vertebrates. Nucleic Acids Res. 1987;15:1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalfie M, Wolinsky E. The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. . Nature. 1990;345:410–416. doi: 10.1038/345410a0. [DOI] [PubMed] [Google Scholar]
- 11.Corey DP, García-Añoveros J. Mechanosensation and the DEG/ENaC ion channels. Science. 1996;273:323–324. doi: 10.1126/science.273.5273.323. [DOI] [PubMed] [Google Scholar]
- 12.Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegansgenes that can mutate to induce neuronal degeneration. Nature. 1991;349:588–593. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- 13.Du H, Gu G, William CM, Chalfie M. Extracellular proteins needed for C. elegansmechanosensation. Neuron. 1996;16:183–194. doi: 10.1016/s0896-6273(00)80035-5. [DOI] [PubMed] [Google Scholar]
- 14.Finlayson, L.H. 1976. Abdominal and thoracic receptors in insects, centipedes and scorpions. In Structure and Function of Proprioceptors in the Invertebrates. P.J. Mill, editor. John Wiley & Sons, New York. 153–211.
- 15.Garcia-Anoveros J, Ma C, Chalfie M. Regulation of caenorhabditis elegans degenerin proteins by a putative extracellular domain. Curr Biol. 1995;5:441–448. doi: 10.1016/s0960-9822(95)00085-6. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci USA. 1997;94:1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghysen A, Dambly-Chaudiére C, Aceves E, Jan L-Y, Jan Y-N. Sensory neurons and peripheral pathways in Drosophilaembryos. Roux's Arch Dev Biol. 1986;195:281–289. doi: 10.1007/BF00376060. [DOI] [PubMed] [Google Scholar]
- 18.Gregory RJ, Cheng SH, Rich DP, Marshall J, Paul S, Hehir K, Ostedgaard L, Klinger KW, Welsh MJ, Smith AE. Expression and characterization of the cystic fibrosis transmembrane conductance regulator. Nature. 1990;347:382–386. doi: 10.1038/347382a0. [DOI] [PubMed] [Google Scholar]
- 19.Hamill OP, McBride DW., Jr The Pharmacology of mechanogated membrane ion channels. Pharmacol Rev. 1996;48:231–252. [PubMed] [Google Scholar]
- 20.Hartl DL, Nurminsky DI, Jones RW, Lozovskaya ER. Genome structure and evolution in Drosophila: applications of the framework P1 map. Proc Natl Acad Sci USA. 1994;91:6824–6829. doi: 10.1073/pnas.91.15.6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey, D., L. Hong, M. Evans-Holm, J. Pendleton, C. Su, P. Brokstein, S. Lewis, and G.M. Rubin. 1997. Berkeley Drosophila Genome Project/ Howard Hughes Medical Institute EST Project.
- 22.Hille, B. 1992. Ionic Channels in Excitable Membranes. Sinauer Associates, Inc., Sunderland, MA. 341–345.
- 23.Hong K, Driscoll M. A transmembrane domain of the putative channel subunit MEC-4 influences mechanotransduction and neurodegeneration in C. elegans. . Nature. 1994;367:470–473. doi: 10.1038/367470a0. [DOI] [PubMed] [Google Scholar]
- 24.Huang M, Chalfie M. Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. . Nature. 1994;367:467–470. doi: 10.1038/367467a0. [DOI] [PubMed] [Google Scholar]
- 25.Hudspeth AJ. How's the ear's works works. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- 26.Jan, Y.-N., and L.-Y. Jan. 1993. The Peripheral Nervous System. In The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Plainview, NY. 1207–1244.
- 27.Johnson WA. Characterization of neuron-specific transcription factors in Drosophila melanogaster. . Methods Neurosci. 1992;9:362–380. [Google Scholar]
- 28.Kernan M, Zuker C. Genetic approaches to mechanosensory transduction. Curr Opin Neurobiol. 1995;5:443–448. doi: 10.1016/0959-4388(95)80003-4. [DOI] [PubMed] [Google Scholar]
- 29.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. . Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 30.Lai C-C, Hong K, Kinnell M, Chalfie M, Driscoll M. Sequence and transmembrane topology of MEC-4, an ion channel subunit required for mechanotransduction in Caenorhabditis elegans. . J Cell Biol. 1996;133:1071–1081. doi: 10.1083/jcb.133.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegansdegenerins. FEBS Lett. 1993;318:95–99. doi: 10.1016/0014-5793(93)81336-x. [DOI] [PubMed] [Google Scholar]
- 32.Lingueglia E, Champigny G, Lazdunski M, Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature. 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Schrank B, Waterston RH. Interaction between a putative mechanosensory membrane channel and a collagen. Science. 1996;273:361–364. doi: 10.1126/science.273.5273.361. [DOI] [PubMed] [Google Scholar]
- 34.Martin, C.H., M.M. Bondoc, A. Chiang, T. Cloutier, C.A. Davis, C.L. Ericsson, M.A. Jaklevic, R.J. Kim, M.T. Lee, M. Li, et al. 1994. Sequencing of the alcohol dehydrogenase (ADH) region of Drosophila melanogaster. Berkeley Drosophila Genome Project.
- 35.McDonald FJ, Snyder PM, McCray PB, Jr, Welsh MJ. Cloning, expression, and tissue distribution of a human amiloride-sensitive Na+channel. Am J Physiol. 1994;266:L728–L734. doi: 10.1152/ajplung.1994.266.6.L728. [DOI] [PubMed] [Google Scholar]
- 36.McDonald FJ, Price MP, Snyder PM, Welsh MJ. Cloning and expression of the beta- and gamma-subunits of the human epithelial sodium channel. Am J Physiol. 1995;268:C1157–C1163. doi: 10.1152/ajpcell.1995.268.5.C1157. [DOI] [PubMed] [Google Scholar]
- 37.McIver, S.B. 1985. Mechanoreception. In Comprehensive Insect Physiology, Biochemistry, and Pharmacology. Volume 6, Nervous System: Sensory. G.A. Kerkut and L.I. Gilbert, editors. Pergamon Press, New York. 71–132.
- 38.Mitchison TJ, Sedat J. Localization of antigenic determinants in whole Drosophilaembryos. Dev Biol. 1983;99:261–264. doi: 10.1016/0012-1606(83)90275-0. [DOI] [PubMed] [Google Scholar]
- 39.North RA. Families of ion channels with two hydrophobic segments. Curr Opin Cell Biol. 1996;8:474–483. doi: 10.1016/s0955-0674(96)80023-8. [DOI] [PubMed] [Google Scholar]
- 40.O'Neill JW, Bier E. Double-label in situ hybridization using biotin and digoxigenin-tagged RNA probes. Biotechniques. 1994;17:874–875. [PubMed] [Google Scholar]
- 41.Oliet SHR, Bourque CW. Gadolinium uncouples mechanical detection and osmoreceptor potential in supraoptic neurons. Neuron. 1996;16:175–181. doi: 10.1016/s0896-6273(00)80034-3. [DOI] [PubMed] [Google Scholar]
- 42.Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+channel. J Biol Chem. 1996;271:7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- 43.Renard S, Lingueglia E, Voilley N, Lazdunski M, Barbry P. Biochemical analysis of the membrane topology of the amiloride-sensitive Na+channel. J Biol Chem. 1994;269:12981–12986. [PubMed] [Google Scholar]
- 44.Robinson DH, Bubien JK, Smith PR, Benos DJ. Epithelial sodium conductance in rabbit preimplantation trophectodermal cells. Dev Biol. 1991;147:313–321. doi: 10.1016/0012-1606(91)90289-f. [DOI] [PubMed] [Google Scholar]
- 45.Snyder PM, McDonald FJ, Stokes JB, Welsh MJ. Membrane topology of the amiloride-sensitive epithelial sodium channel. J Biol Chem. 1994;269:24379–24383. [PubMed] [Google Scholar]
- 46.Snyder PM, Price MP, McDonald FJ, Adams CM, Volk KA, Zeiher BG, Stokes JB, Welsh MJ. Mechanism by which Liddle's syndrome mutations increase activity of a human epithelial Na+channel. Cell. 1995;83:969–978. doi: 10.1016/0092-8674(95)90212-0. [DOI] [PubMed] [Google Scholar]
- 47.Sugawara T. Stretch reception in the bursa copulatrix of the butterfly, Pieris rapae crucivora, and its role in behaviour. J Comp Physiol. 1979;130:191–199. [Google Scholar]
- 48.Sugawara T. Fine structure of the stretch receptor of the bursa copulatrix of the butterfly, Pieris rapae crucivora. . Cell Tissue Res. 1981;217:23–36. doi: 10.1007/BF00233822. [DOI] [PubMed] [Google Scholar]
- 49.Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscLalone. Nature. 1994;368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 50.Swick AG, Janicot M, Cheneval-Kastelic T, McLenithan JC, Lane MD. Promoter-cDNA-directed heterologous protein expression in Xenopus laevisoocytes. Proc Natl Acad Sci USA. 1992;89:1812–1816. doi: 10.1073/pnas.89.5.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tavernarakis N, Driscoll M. Molecular modeling of mechanotransduction in the nematode Caenorhabditis elegans. . Annu Rev Physiol. 1997;59:659–689. doi: 10.1146/annurev.physiol.59.1.659. [DOI] [PubMed] [Google Scholar]
- 52.Tavernarakis N, Shreffler W, Wang S, Driscoll M. unc-8, a DEG/ENaC family member, encodes a subunit of a candidate mechanically gated channel that modulates C. eleganslocomotion. Neuron. 1997;18:107–119. doi: 10.1016/s0896-6273(01)80050-7. [DOI] [PubMed] [Google Scholar]
- 53.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivty of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voilley N, Lingueglia E, Champigny G, Mattei MG, Waldmann R, Lazdunski M, Barbry P. The lung amiloride-sensitive Na+channel: biophysical properties, pharmacology, ontogenesis, and molecular cloning. Proc Natl Acad Sci USA. 1994;91:247–251. doi: 10.1073/pnas.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+channel. J Biol Chem. 1995;270:27411–27414. doi: 10.1074/jbc.270.46.27411. [DOI] [PubMed] [Google Scholar]
- 56.Waldmann R, Champigny G, Lazdunski M. Functional degenerin-containing chimeras identify residues essential for amiloride-sensitive Na+channel function. J Biol Chem. 1995;270:11735–11737. doi: 10.1074/jbc.270.20.11735. [DOI] [PubMed] [Google Scholar]
- 57.Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans . J Biol Chem. 1996;271:10433–10436. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- 58.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 59.Wright, B.R. 1976. Structure and Function of Proprioceptors in the Invertebrates. Chapman & Hall, London. 323 pp.
- 60.Zipursky SL, Venkatesh TR, Telow DB, Benzer S. Neuronal development in the Drosophiliaretina: monoclonal antibodies as molecular probes. Cell. 1984;36:15–26. doi: 10.1016/0092-8674(84)90069-2. [DOI] [PubMed] [Google Scholar]