Abstract
Oleamide is a sleep-inducing lipid originally isolated from the cerebrospinal fluid of sleep-deprived cats. Oleamide was found to potently and selectively inactivate gap junction–mediated communication between rat glial cells. In contrast, oleamide had no effect on mechanically stimulated calcium wave transmission in this same cell type. Other chemical compounds traditionally used as inhibitors of gap junctional communication, like heptanol and 18β-glycyrrhetinic acid, blocked not only gap junctional communication but also intercellular calcium signaling. Given the central role for intercellular small molecule and electrical signaling in central nervous system function, oleamide- induced inactivation of glial cell gap junction channels may serve to regulate communication between brain cells, and in doing so, may influence higher order neuronal events like sleep induction.
Studies on the molecular mechanisms for cellular interactions have traditionally been hindered by a deficiency of natural products that selectively target specific forms of intercellular communication. One primary mode for direct intercellular contact involves the cell-to-cell transmission of molecules through channels in a specialized cell surface membrane structure, the gap junction (Kumar and Gilula, 1996). Gap junctions allow the passive diffusion of molecules between cells with a selectivity based principally on size, allowing the exclusive movement of molecules smaller than 1,000 D. Such size-selective molecular communication is essential for many forms of multicellular function, including the regulation of events between cells during embryogenesis and the synchronization of cells in the myocardium (Dewey and Barr, 1962; Warner et al., 1984). Previously, we reported the structure determination of a novel, sleep-inducing lipid, 9(Z)-octadecenamide, or oleamide, originally isolated from the cerebrospinal fluid of sleep-deprived cats (Cravatt et al., 1995). In our continued efforts to identify and characterize cellular effects associated with oleamide, we now report that oleamide potently and selectively blocks gap junctional communication in rat glia without altering calcium wave transmission in these cells.
Materials and Methods
Cell Culture
Rat glial cells (Suter et al., 1987) obtained from Dr. Trosko's laboratory (Michigan State University, East Lansing, MI) were cultured in standard plastic tissue cultureware in Richter's Improved Minimal Essential Medium (Irvine Scientific, Santa Ana, CA), supplemented with 10% FCS and 50 μg/ml gentamicin sulfate, and incubated in a humidified atmosphere of 95% air/5% CO2 at 37°C. The cells were passaged by trypsinization and used at passages four through eight. BHK cells that were stably transfected with a β1 connexin cDNA (Kumar et al., 1995) were cultured in DME medium supplemented with 5% FCS and 50 μg/ml gentamicin sulfate and incubated in a humidified atmosphere of 95% air/5% CO2 at 37°C. To induce β1 connexin expression in the BHK cells, 100 μM zinc acetate was added to culture medium for 8–18 h when the cell culture was ∼90% confluent.
Gap Junction Dye Coupling Assays
Gap junctional communication in glial cell and BHK/β1 cell cultures was assayed by microinjection of 5% Lucifer yellow CH dye in 0.1 M LiCl solution and quantitated by determining the number of directly adjacent, neighboring cells that received dye (dye coupling). Micropipettes were loaded with the dye solution by backfilling. Cells were visualized using an inverted phase contrast/epifluorescent microscope (Carl Zeiss, Inc., Thornwood, NY) and impaled with dye-filled micropipettes using a microinjector (model 5246; Eppendorf Scientific, Inc., Madison, WI). 5 min after dye injection, the frequencies of dye transfer from microinjected, dye-loaded cells to directly adjacent cells (dye coupling) were determined using epifluorescent illumination. For each treatment condition, 10 cells were microinjected in each of three dishes. The percentages of dye-coupled, neighboring cells in each of three dishes were used to calculate the mean (±SD) of dye coupling percentages for each treatment condition. For scrape-loading experiments, Lucifer yellow CH (0.05% dye in PBS) was loaded intracellularly by cutting or scraping cells in the monolayer with a sharp knife. The dye solution was left in the dish for 90 s. The solution was then discarded, and the dish was subsequently washed with PBS. The cells were examined for dye transfer with an inverted epifluorescence microscope, and the degree of communication was assessed by determining the extent of Lucifer yellow transfer into contiguous cells.
Gap Junction Electrical Coupling Assay
Junctional conductance was measured using double whole-cell patch recording performed on pairs of rat glial cells as described (Miller et al., 1992) with a pipette solution of (nM): 160 Kaspartate, 10 EGTA, 2 CaC12, 4 ATP, 10 Hepes, pH 7.2. The external solution contained (mM): 160 NaCl, 4.5 KCl, 2 CaCl2 , 1 MgCl2, 10 Hepes, pH 7.4. Both cells were held at −40 mV, and pulses to −20 mV were alternately applied to each cell. Holding currents were subtracted in the records shown. Cells that were examined were generally in contact with other cells. The electrical conductance was calculated as the junctional current divided by 20 mV. All dye coupling and conductance studies were performed at room temperature.
Calcium Wave Images
Rat glial cells were loaded with 5 μM Fluo-3/AM (Calbiochem, La Jolla, CA) in Hank's balanced salt solution containing 25 mM Hepes buffer (HBSS/Hepes) for 1 h, at which point the loading buffer was exchanged for new HBSS/Hepes buffer. The cell cultures were then left at room temperature for at least 30 min. Mechanical stimulation of a single cell was performed as follows: a glass micropipette (tip diameter of ∼0.5 μm) was micromanipulated downward onto a single cell, causing a transient deformation of the cell membrane. The calcium image was then examined with an inverted fluorescence microscope and photographed with a digital fluorescence microscope (excitation = 506 nm, emission = 526 nm). The degree of calcium wave propagation was quantitated by counting at different time points the number of transmitting cells in one linear direction away from the stimulated cell. Junctional dye transfer rates were simultaneously examined by microinjection of Lucifer yellow CH in the same dishes; the methods for dye transfer assay were described in “Gap Junction Dye Coupling Assays.” For calcium wave and dye transfer studies, the drug was preincubated with the glial cells for 10 min, and the drug was left in the experimental solution throughout the examination. All experiments were performed at room temperature.
Immunoblotting
Plasma membrane fractions containing gap junctions were obtained after hypotonic alkali extraction of the glial cells. The extracted protein was dissolved in 2% SDS, and the total protein was determined using the Bio-Rad DC Protein Assay kit (Hercules, CA). 10 μg of protein was electrophoresed by 10% SDS-PAGE and subsequently blotted electrophoretically onto Immobilon-P membranes (Millipore Corp., Bedford, MA). Gap junction protein was detected using anti–α1 connexin polyclonal rabbit antibodies and the HRP/Chemiluminescence detection kit (Amersham Corp., Arlington Heights, IL) following the manufacturer's instructions.
Results
Gap Junction Dye Coupling and Electrical Coupling
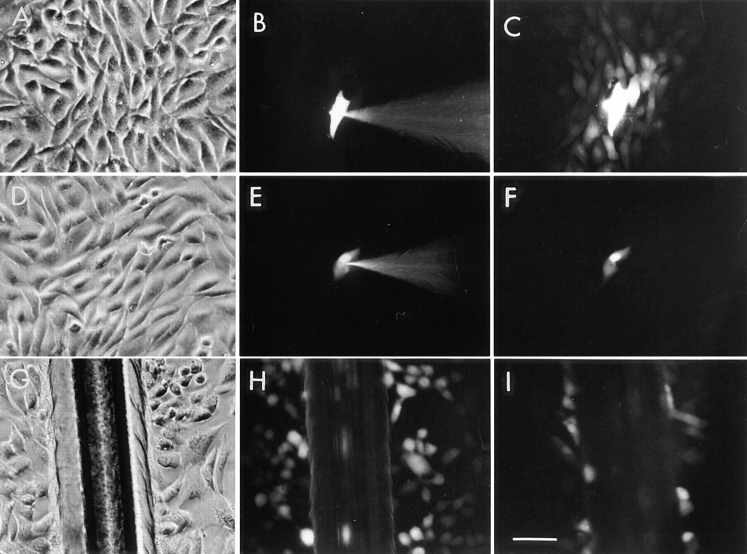
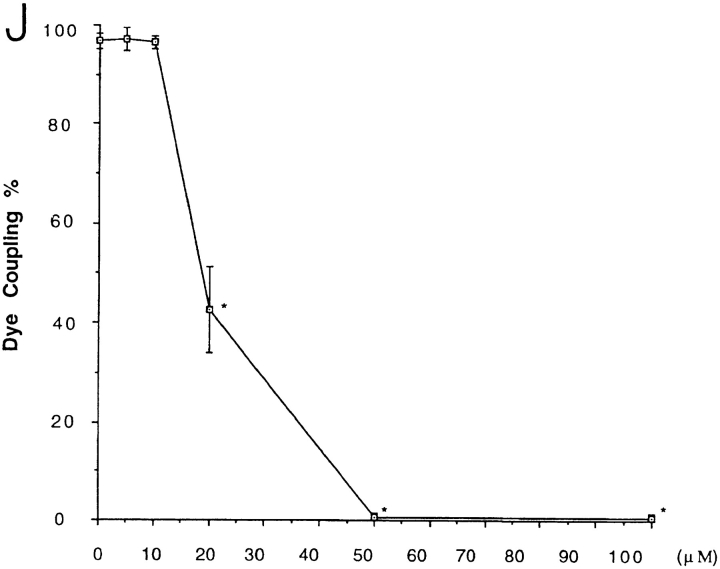
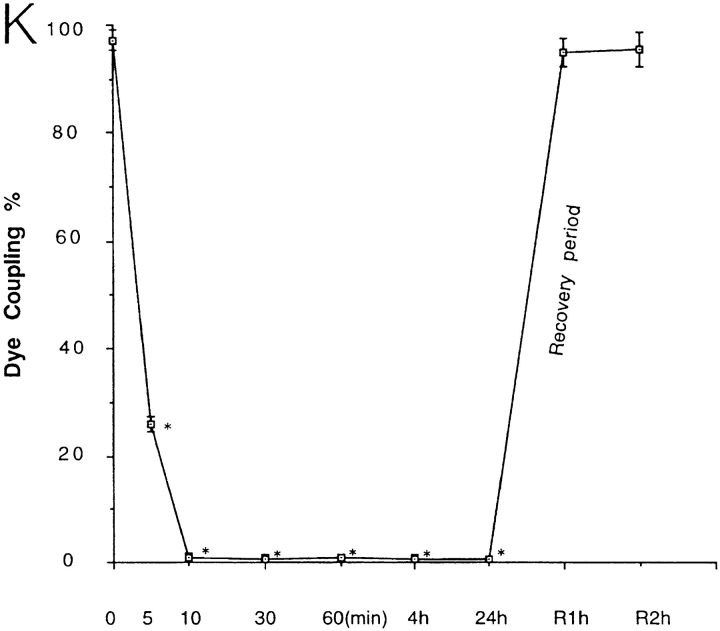
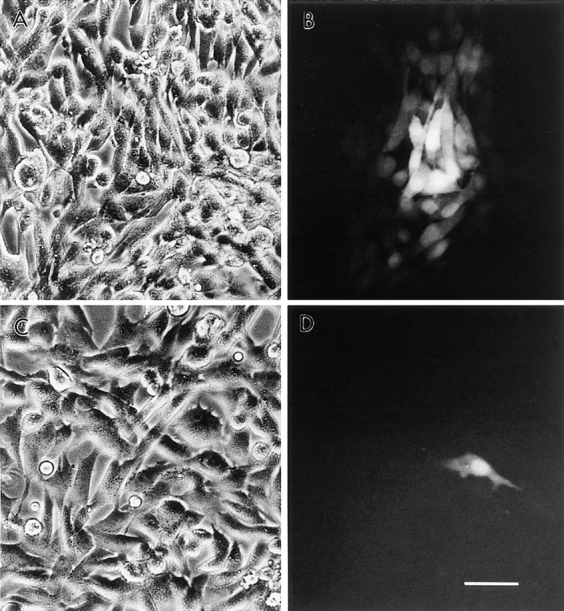
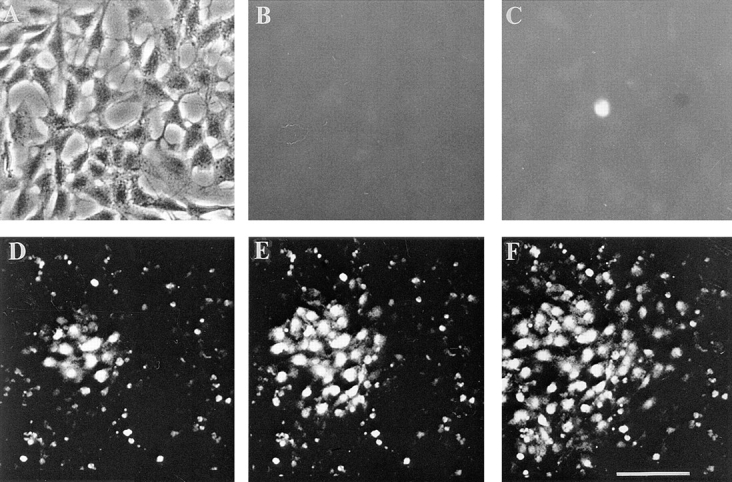
Gap junction–mediated intercellular communication in cultured rat glial cells (Suter et al., 1987) was evaluated by microinjection and scrape-loading (El-Fouly et al., 1987) of the fluorescent dye, Lucifer yellow. While under control conditions, microinjected glial cells demonstrated strong dye coupling as monitored by intercellular Lucifer yellow diffusion (Fig. 1, A–C). Pretreatment of these cells with 50 μM oleamide for 10 min completely blocked intercellular dye transfer (Fig. 1, D–F). Likewise, glial cells scrape-loaded with Lucifer yellow showed significant dye transfer that was fully abrogated by pretreatment with oleamide under the same experimental conditions used for microinjection (Fig. 1, G–I). Dose-response and time-course studies of oleamide-induced inhibition of transfer of microinjected dye were conducted (Fig. 1, J and K, respectively). Different concentrations of oleamide were examined for up to 4 h of treatment (Fig. 1 J); maximal inhibition occurred with a concentration of 50 μM. Consequently, we used this concentration to determine the time-dependent response. As shown in Fig. 1 K, at this concentration half-maximal inhibition occurred within 5 min, while complete inhibition was observed within 10 min. Oleamide's effects on gap junction permeability proved stable and completely reversible. Thus, whereas no restoration of dye coupling was observed in glial cells that were continually exposed to oleamide for up to 24 h, once oleamide was removed by changing the culture media, junctional communication recovered to control levels within 1–2 h (see Fig. 1 K, Recovery period). Similar inhibitory responses were observed also by using the scrape-loading dye method (data not shown).
Figure 1.
Oleamide inhibition of gap junction–mediated dye coupling between rat glial cells. Cultured rat glial cells (A and D, phase) were microinjected with Lucifer yellow (B and E) and showed efficient dye transfer to adjacent cells under (C) control conditions (0.1% ethanol present in culture media). (F) Treatment of glial cells with 50 μM oleamide for 10 min completely blocked dye transfer. Glial cells were also scrape-loaded (G, phase) with Lucifer yellow, and under control conditions dye was efficiently transferred from dye-loaded cells to adjacent cells (H), with no dye spread to adjacent cells when treated with oleamide (I). (J) Dose- response studies of oleamide-induced blockage of dye transfer were conducted by treating glial cells with various concentrations of oleamide (5–100 μM) for 4 h before injection of Lucifer yellow and monitoring of dye transfer. Approximately 50% inhibition of dye transfer was observed with 20 μM oleamide, and complete inhibition of dye transfer was observed with 50 μM oleamide. (K) Time course of oleamide-induced blockage of gap junction dye transfer was determined by treating glial cells with 50 μM oleamide for indicated times before injection of Lucifer yellow. Reversibility of oleamide's effect on gap junctions was established by removing oleamide-containing media from the culture dish, reculturing cells with fresh media without oleamide, and monitoring dye transfer over the subsequent hour. Complete recovery of dye transfer to control levels was observed within 1 to 2 h after removal of oleamide. R, recovery. *P < 0.01 versus control groups (Student's t test). Bar, 50 μm.
As an additional measure of Oleamide's effect on gap junction permeability, glial cell gap junctional conductance was examined in the presence of oleamide by using the double whole-cell recording technique (Neyton and Trautmann, 1985; Veenstra and DeHaan, 1986; Miller et al., 1992) (Fig. 2). Consistent with its effect on gap junction–mediated dye transfer, oleamide (50 μM) completely blocked junctional electrical coupling in glial cells. In experiments carried out on eight cell pairs, the mean of junctional conductance of control pairs was 13 ± 7 nS (n = 3), and the mean junctional conductance of cell pairs exposed to 50 μM oleamide was 0.5 ± 0.7 nS (n = 5).
Figure 2.
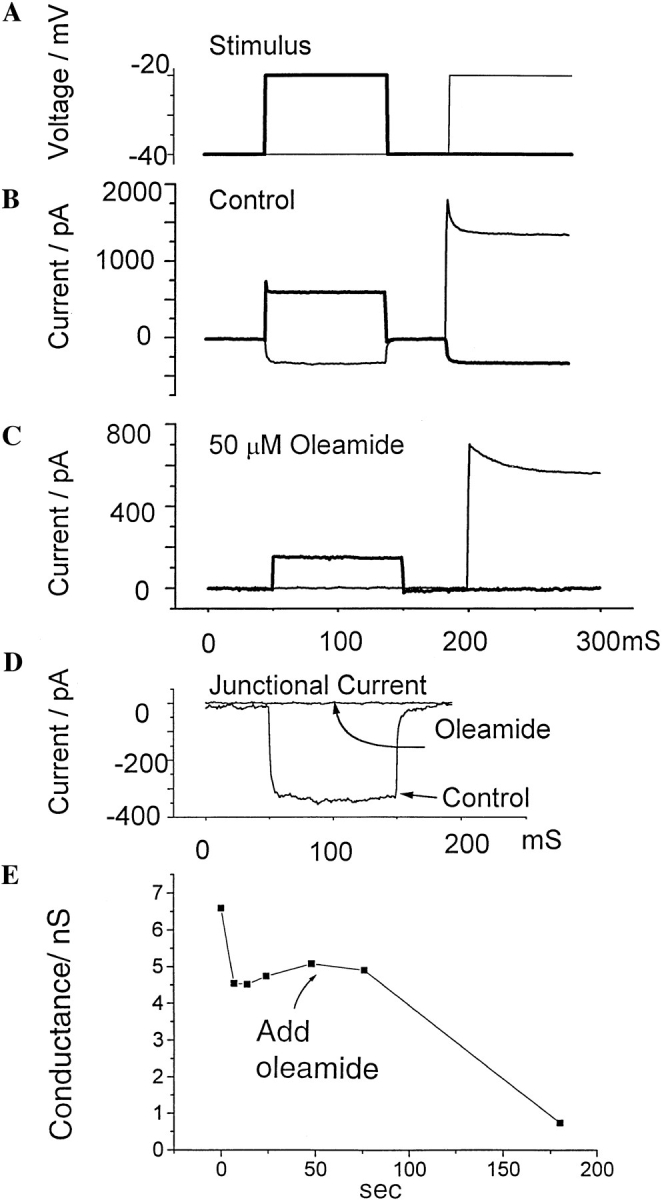
Double whole-cell patch recording combined with observation of dye spread was used to assess gap junctional conductance in cultured rat glial cells. (A) Time course of voltages applied to each cell. Cells were held at −40 mV, and first cell 1 and then cell 2 was stepped to −20 mV. The thick trace shows voltage applied to cell 1, and the thin trace shows the voltage applied to cell 2. (B and C) Response of cells to the voltage protocol shown in A. Thick traces show the currents in cell 1, and the thin traces show currents in cell 2. The upward current deflections show the sum of surface membrane and junctional current in the cell whose potential is changed from −40 to −20 mV. The upward deflections from 50 to 150 ms are currents in cell 1, and the deflections from 200 to 300 ms are currents in cell 2. Downward deflections consist solely of junctional currents recorded in the cell held at −40 mV. In B, large downward current deflections show a pair of control cells that were well coupled. Note that although the upward deflections elicited by pulsing first one cell, then the other, are different sizes, the downward current deflections, elicited by a 20-mV transjunctional voltage, are of equal size, demonstrating that a driving force of 20 mV produces a constant junctional current regardless of which cell is pulsed. In C, the lack of downward current deflections in response to a 20-mV pulse shows that a different pair of cells exposed to 50 μM oleamide was completely uncoupled. D compares junctional currents of control and experimental cell pairs during the first 150 ms of the experiments shown in B and C. Note that there is no detectable downward deflection in the trace from the cell pair exposed to oleamide. In this case, the experimental data set an upper limit on the residual junctional conductance of less than 50 pS. Thus, the junction is completely uncoupled. (E) Time course of recording trace of junctional electrical conductance, showing the rapid loss of electrical coupling after addition of 50 μM oleamide to the bathing solution.
Structure–Activity Relationship
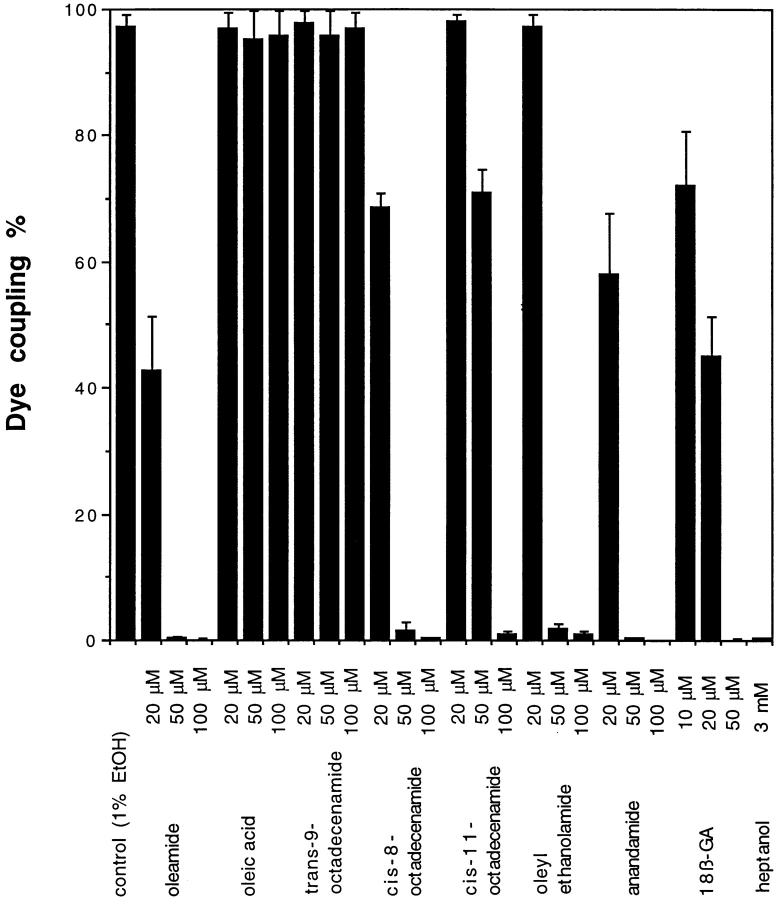
To evaluate oleamide's structure–activity relationship, several chemical analogues of oleamide were synthesized (Cravatt et al., 1996) and tested for their ability to block gap junction permeability (Fig. 3). In contrast to oleamide, oleic acid and trans-9-octadecenamide (trans-oleamide) showed no effect on glial cell dye coupling, even at higher doses. Interestingly, oleic acid has previously been demonstrated to inhibit gap junction communication in rat cardiac myocytes (Hirschi et al., 1993), which like rat glia, express the α1 connexin (Cx43). Thus, the selective response of the glial cells to oleamide is more likely a function of cell type rather than the primary structure of the α1 connexin. Other cis-monounsaturated fatty acid amides, in addition to oleamide, demonstrated varying degrees of inhibition. 50 μM cis-11-octadecenamide only slightly affected junctional coupling, but at 100 μM levels the compound completely blocked dye transfer. Oleyl ethanolamide and cis-8-octadecenamide were significant inhibitors of dye transfer at 50 μM levels but proved less potent than oleamide at lower doses. Thus, the key chemical features of oleamide that impart upon the compound its inhibitory properties appear to be the amide functionality and the cis-double bond, with discernible preference exhibited for a primary amide moiety and location of the degree of unsaturation at the Δ9 position along the alkyl chain. Oleamide's effect on glial cells was also compared with the activity of other established inhibitors of gap junction communication. Both 18β-glycyrrhetinic acid (18β-GA)1 (Davidson et al., 1996; Guan et al., 1996) and anandamide (Venance et al., 1995) blocked dye transfer at doses comparable to oleamide, while much higher concentrations of heptanol (Jalife et al., 1989; Mege et al., 1994) (3 mM) were required to inhibit junctional communication (Fig. 3).
Figure 3.
Structure–activity studies of oleamide-induced blockage of gap junction dye transfer in cultured glial cells were conducted to evaluate which chemical features of oleamide were required for its inhibitory effect. Oleic acid and trans-9-octadecenamide had no effect on dye transfer, while of the remaining cis-monounsaturated fatty acid amides, oleamide proved to be the most potent inhibitor. Following oleamide in order of potency were: cis-8-octadecenamide > oleyl ethanolamide > cis-11-octadecenamide. Oleamide was also compared with other gap junction inhibitors, anandamide, 18β-GA, and heptanol.
Oleamide Effects on β1 Connexin–transfected BHK Cells
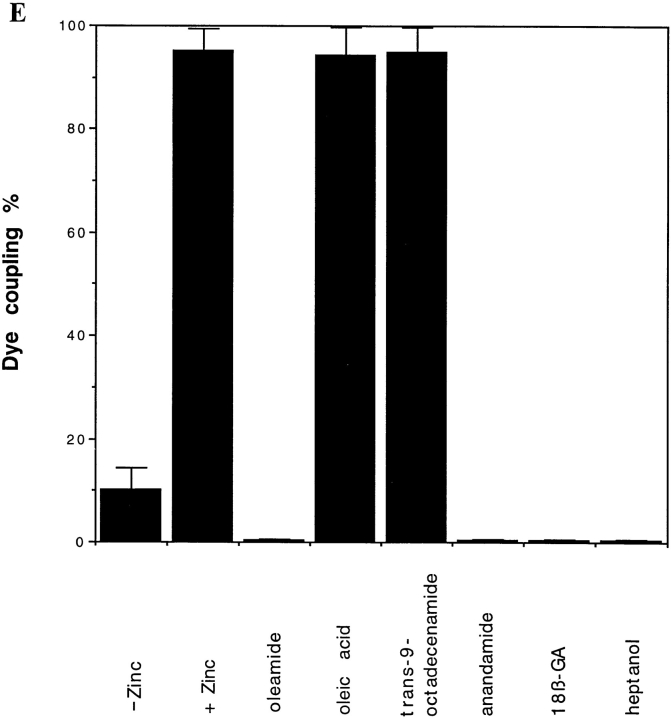
To evaluate whether oleamide's effect on α1-containing gap junctions was specific to the α1 junctional type, we determined dye transfer properties in BHK cells that were transfected with β1 connexin (Cx32) to produce β1-containing gap junctions. For this analysis, we applied the same experimental conditions that were used for the rat glial cell experiments. 50 μM oleamide was found to rapidly and completely block dye transfer (Fig. 4 D) between BHK/β1 cells, while oleic acid and trans-9-octadecenamide showed no effect (Fig. 4 E). Other inhibitory compounds, like anandamide, 18β-GA, and heptanol, had inhibitory effects on dye transfer between the BHK/β1 cells that were very similar to those observed in the rat glial cells (compare Figs. 4 E and 3).
Figure 4.
Oleamide effect on gap junction dye transfer between BHK/β1 cells. In the presence of zinc induction, BHK/β1 cells (A and C, phase) were microinjected with Lucifer yellow and showed efficient dye transfer to adjacent cells in the control condition (B) (with 0.1% ethanol in culture media) and no dye transfer between cells that were treated with oleamide (D) (50 μM oleamide for 10 min). (E) Comparison of dye transfer rates between BHK/β1 cells under different treatment conditions. BHK/ β1 cells without zinc induction had a low incidence (10 ± 4%) of dye transfer. After adding zinc to the culture medium for 8–18 h, dye transfer was significantly increased (95 ± 5%). 50 μM oleamide completely blocked dye transfer (0 ± 0%) in the zinc- induced sample. Anandamide (50 μM), 18β-GA (50 μM), and heptanol (3 mM) showed similar inhibition, while oleic acid (50 μM) and trans-9-octadecenamide (50 μM) had no effect on dye transfer (E). All determinations were made after 4 h of treatment. Bar, 50 μm.
Gap Junctional Intercellular Communication and the Calcium Wave
Since several previous studies have indicated that calcium waves in brain cell populations can propagate in a gap junction–dependent manner (Charles et al., 1992; Enkvist and McCarthy, 1992; Finkbeiner, 1992), we next evaluated oleamide's effect on calcium wave transmission among glial cells. Using the intracellular calcium indicator, Fluo-3 (Cornell-Bell et al., 1990), to monitor changes in intracellular calcium levels within the glial cell population, we found that 50 μM oleamide had no impact on mechanically induced calcium wave propagation (Fig. 5, D–F, and Table I). Intrigued by oleamide's contrasting effects on gap junction communication and calcium wave transmission, we compared oleamide's properties to other gap junction inhibitors. 18β-GA (40 μM) and heptanol (3 mM) showed no discrimination in their inhibitory activity, completely blocking both dye transfer and calcium wave propagation in glial cells (Fig. 5 C and Table I). In contrast, we found that anandamide, an amidated lipid-like oleamide, resembled oleamide in activity, selectively inhibiting gap junction communication (dye transfer and electrical coupling) without affecting calcium wave transmission (Table I).
Figure 5.
Effect of oleamide and 18β-GA on calcium wave propagation in rat glial cells. Rat glia (phase, A) were loaded with the calcium indicator dye, Fluo-3, and changes in the intracellular free calcium concentration ([Ca2+]i) were documented as changes in the intensity of fluorescent dye both before (B) and after (C–F) mechanical stimulation. Fluorescence changes were monitored with an inverted fluorescence microscope. In the presence of 40 μM 18β-GA (incubated with glia for 10 min before mechanical stimulation), mechanical stimulation resulted in an increase in [Ca2+]i in the stimulated cell, but this [Ca2+]i change did not propagate to any other cells (C). In contrast, in rat glia treated with 50 μM oleamide (preincubation from 10 min to 4 h) mechanical stimulation produced an increase in [Ca2+]i that was rapidly propagated to cells at long distances in an indistinguishable manner from control cell populations (D–F, time course of wave propagation; D, 2 s; E, 4 s; F, 8 s). For a quantitative comparison of calcium wave propagation in cells treated by different agents and control cell populations, see Table I. Bar, 30 μm.
Table I.
Effects of Different Agents on Calcium Wave Propagation and Gap Junctional Communication
| Treatment | Number of cells participating in wave (in one direction) | Junction dye coupling (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 s | 4 s | 8 s | 16 s | |||||||
| Control | 1 ± 0 | 3 ± 1 | 7 ± 1 | 11 ± 1 | 98 ± 2 | |||||
| Oleamide (50 μM) | 1 ± 0 | 2 ± 1 | 7 ± 1 | 11 ± 2 | 0 ± 0* | |||||
| Anandamide (50 μM) | 1 ± 0 | 2 ± 1 | 7 ± 1 | 11 ± 1 | 0 ± 0* | |||||
| 18β-GA (40 μM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0* | 0 ± 0* | |||||
| Heptanol (3 mM) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0* | 0 ± 0* | |||||
| Suramin (200 μM) | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0* | 98 ± 1 | |||||
| Oleamide + suramin | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0* | 0 ± 0* | |||||
The number of cells participating in calcium wave propagation was quantified by counting the number of responding cells in one direction from the stimulated cell. The time points indicated above were analyzed after the initiation of calcium signaling; the responding cell numbers are expressed as mean ± SD. Gap junction dye transfer was expressed as dye coupling percentage (mean ± SD), which is described in Materials and Methods. Each treatment was performed in three dishes, and 10 individual fields were examined in each dish, n = 30.
P < 0.01 versus control group (student's t test).
In mammary gland cells (Enomoto et al., 1992), mast cells (Osipchuk and Cahalan, 1992), and insulin-secreting cells (Cao et al., 1997) calcium waves have been shown to propagate by a process dependent on extracellular release of ATP from the stimulated cell. To examine whether glial cells also transmitted ATP-dependent calcium waves, the glia were treated with the P2-purinergic receptor antagonist, suramin (Osipchuk and Cahalan, 1992; Hansen et al., 1993), and subsequently tested for calcium wave transmission. As shown in Table I, suramin (200 μM) blocked glial calcium wave transmission without affecting gap junction communication in these cells. Co-treatment of the glia with suramin and oleamide produced the combined phenotype of blocked calcium wave transmission and blocked gap junction communication, further supporting the notion that the two pathways for these interglial interactions are distinct and separate.
Changes of α1 Connexin Phosphorylation
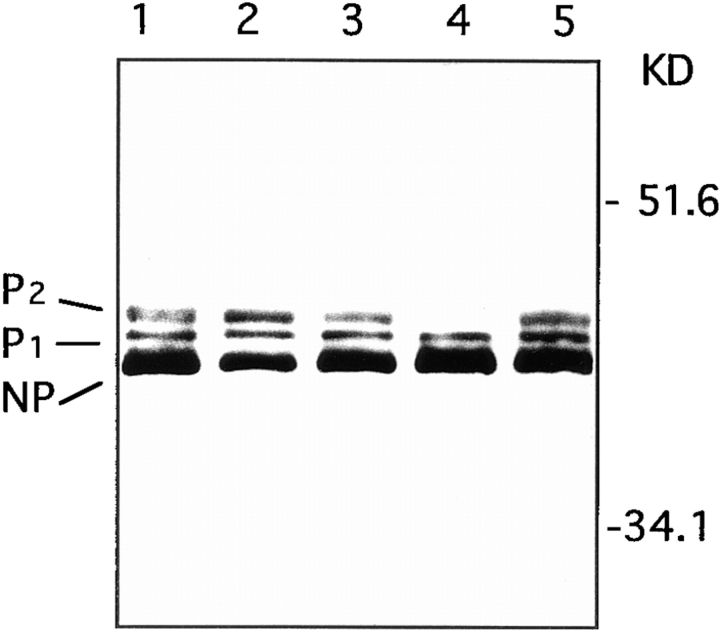
In an effort towards defining the molecular mechanism of oleamide's action on gap junctions, we examined the phosphorylation profile of the α1 connexin in glial cells upon treatment with oleamide. The α1 connexin has previously been shown by Western blotting to exist in three distinguishable isoforms (Musil et al., 1990; Guan et al., 1995): nonphosphorylated, NP (∼42 kD) and two phosphorylated isoforms, P1 (∼44 kD), and P2 (∼46 kD). Upon exposure to oleamide (50 μM), glial cells demonstrated a dramatic loss of the P2 isoform with no discernible change in the levels of P1 and NP (Fig. 6, lane 4). The effect of oleamide on the α1 phosphorylation profile proved reversible, as removal of oleamide from the glial cell culture media was associated with a restoration of P2 to control levels (Fig. 6, lane 5). No change in the α1 phosphorylation profile was detected in glial cells exposed to oleic acid or trans-9-octadecenamide (Fig. 6, lanes 2 and 3), two oleamide analogues that did not inhibit gap junction communication.
Figure 6.
Western blot analysis of the α1 connexin protein from glia treated with oleamide and its inactive structural analogues. Lane 1 contains a sample of control cells treated with 0.1% ethanol for 4 h. Lanes 2–4 contain samples of cells treated for 4 h with 50 μM oleic acid, 50 μM trans-9-octadecenamide, and 50 μM oleamide, respectively. The loss of P2 α1 in glia treated with oleamide was reversible, as P2 was present at control levels after removal of oleamide from the culture dish and culturing the cells in normal culture media for 4 h (lane 5). The nonphosphorylated (NP) and phosphorylated (P1 and P2) α1 connexin isoforms and molecular mass standards are indicated on the right of the blot.
Discussion
The sleep-inducing lipid, oleamide, exhibits the special capacity to block gap junction communication in glial cells as monitored by dye transfer and electrical conductance without inhibiting intercellular calcium wave transmission in these same cells. Additionally, oleamide induces a dramatic change in the phosphorylation profile of the α1 connexin protein, the principle component of the glial cell gap junction channels. The loss of both gap junction permeability and α1 connexin P2 in the presence of oleamide is consistent with previous work indicating that the P2 connexin isoform is associated with the formation of functional gap junction plaques (Musil et al., 1990; Musil and Goodenough, 1991; Guan et al., 1995). However, the precise causal relationship between oleamide-induced gap junction blockage and the loss of the connexin-phosphorylated P2 isoform remains uncertain, and this must be examined in more detail to determine if there is a specific association between the two events.
The observation that calcium waves can propagate in glial cells when gap junction communication pathways have been eliminated implies that calcium waves in these cells need not, as previously suggested (Charles et al., 1992; Enkvist and McCarthy, 1992; Finkbeiner, 1992), be exclusively dependent on gap junctional communication. The precise relationship between these observations and previously reported studies on gap junction–associated calcium waves remains to be clarified. However, our observation that suramin, a P2-purinergic receptor antagonist, blocked calcium wave transmission in glial cells without affecting gap junction communication suggests that these cells may transmit intercellular calcium signals by an ATP-dependent mechanism akin to those previously reported for mammary gland cells (Enomoto et al., 1992), mast cells (Osipchuk and Cahalan, 1992), liver epithelial cells (Frame and deFeijter, 1997), insulin-secreting cells (Cao et al., 1997), neuroepitheliomas (Palmer et al., 1996), and astrocytes (Hassinger et al., 1996). Interestingly, the realization that intercellular calcium waves in glial cells can persist without functional gap junctions may help to explain the presence of calcium waves in certain tissues like the retina, where thus far gap junctional pathways have not been definitively described among all cell types that participate in transmission of the calcium wave (Feller et al., 1996).
In the course of studying oleamide's effect on gap junction communication, we also accumulated evidence that previously identified gap junction inhibitors, such as 18β-GA and heptanol, are not selective in their inhibitory activity on gap junction channels but rather appear to act as more general nonspecific perturbants of the plasma membrane and its corresponding functions. Since medium chain alcohols (Jalife et al., 1989; Mege et al., 1994) and glycerrhetinic acid derivatives (Davidson et al., 1996; Guan et al., 1996) are often used as tools for specifically studying the gap junctions, we would suggest that, in the future, their biological effects be evaluated in the context of the entire cell. Otherwise, the role of gap junctions in complex cellular phenomena like calcium wave transmission may remain obscure.
Although it is not possible yet to determine the precise mechanism that oleamide uses to exert its effect on gap junction channels, the results from this initial analysis indicate that oleamide will block gap junction channels that contain different connexins (α1 and β1 connexin). Based on these observations, it is reasonable to consider the possibility that oleamide exerts its action on some generalized structural property of the connexin oligomers or channels in the lipid bilayer. Such a mechanism of action would not be dependent on the integrity of the carboxy-terminal domain or other diverse primary sequence properties that exist between the members of the connexin multigene family.
To try to determine if oleamide treatments affect other cell biological processes in addition to gap junction channels, we examined several other cellular systems and membrane activities: the in vitro differentiation of chick embryo myoblasts (Guan, X., and N.B. Gilula, unpublished data); the differentiation of mouse C2C12 myoblasts (Ledbetter, M.L., unpublished data); the differentiation of mouse F9 teratocarcinoma cells (Guan, X., and N.B. Gilula, unpublished data); the maintenance of steady-state potassium levels (Ledbetter, M.L., unpublished data); and the potential toxic effects on rat glial and rat liver WB-F344 cells (Guan, X., and N.B. Gilula, unpublished data). In all of these studies, no significant effects were observed. Many of these cellular systems or processes were examined under conditions where the cells were exposed to oleamide with doses as high as 150 μM with treatment as long as 3 d. Thus, it is quite unlikely that oleamide exerts general and nonspecific effects on a number of normal cellular processes. Although it is not possible to rule out additional targets for the action of oleamide based on the limited studies thus far, it is noteworthy that its observed effects on gap junction channel permeability are remarkably specific.
In this context, oleamide and related molecules such as anandamide should prove to be very useful reagents, serving as more specific probes for determining the function of gap junction channels in vivo than the relatively nonspecific reagents that have been previously applied, such as heptanol and glycerrhetinic acid. Furthermore, although oleamide can block gap junction channels that are composed of different connexins, there appears to be a cell-specific property in determining the effect of oleamide on gap junction channels. For example, in a preliminary analysis we have observed that the gap junctional communication property between mammalian myocardial cells is not as sensitive to the inhibitory action of oleamide as are the gap junction channels in other mammalian cell types (Guan, X., and N.B. Gilula, unpublished observations). The finding of a different sensitivity for different cell types is consistent with previous reports of other related molecules, such as arachidonimide and oleic acid. These chemicals have a different effect on junctional communication in myocardial cells and in vascular smooth muscle cells (Fluri et al., 1990; Hirschi et al., 1993). Hence, such cell-specific responses to bioactive lipids, such as oleamide, may be extremely beneficial for protecting the myocardium from the effects of such molecules in vivo.
Finally, by blocking gap junction permeability in glial cells, oleamide may be expected to exert intricate modulatory effects on brain function and physiology, preserving certain glial forms—and perhaps also glial–neuronal (Charles, 1994; Nedergaard, 1994; Parpura et al., 1994) forms—of cell–cell interaction, like calcium wave transmission, in the absence of the chemical and electrical forms of intercellular contact mediated by gap junctions. The precise mechanism by which oleamide exerts its profound effect on gap junction channels is unknown. However, in addition to the potential direct interaction with the assembled gap junction or its associated proteins, a most intriguing possibility is that oleamide functions by perturbing the lipid environment of membrane proteins and organelles (Gill and Lawrence, 1976), thus representing a new class of biologically active lipids that act as fluidity transmitters.
Acknowledgments
We thank J.E. Trosko for providing the rat glial cells used for this study and K.M. Hahn (The Scripps Research Institute) for his generous assistance in recording the calcium images.
Footnotes
1. Abbreviation used in this paper: 18β-GA, 18β-glycyrrhetinic acid.
B.F. Cravatt was supported by a Predoctoral Fellowship from the National Science Foundation. This work was supported by the National Institutes of Health and the Lucille P. Markey Charitable Trust.
Address all correspondence to Norton B. Gilula, Department of Cell Biology, The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037. Tel.: (619) 784-9770. Fax: (619) 784-2345. E-mail: nbg@scripps.edu
References
- Cao D, Lin G, Westphale EM, Beyer EC, Steinberg TH. Mechanisms for the coordination of intercellular calcium signaling in insulin- secreting cells. J Cell Sci. 1997;110:497–504. doi: 10.1242/jcs.110.4.497. [DOI] [PubMed] [Google Scholar]
- Charles AC. Glia-neuron intercellular calcium signaling. Dev Neurosci. 1994;16:196–206. doi: 10.1159/000112107. [DOI] [PubMed] [Google Scholar]
- Charles AC, Naus CC, Zhu D, Kidder GM, Dirksen ER, Sanderson MJ. Intercellular calcium signaling via gap junctions in glioma cells. J Cell Biol. 1992;118:195–201. doi: 10.1083/jcb.118.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA. Chemical characterization of a family of brain lipids that induce sleep. Science. 1995;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Lerner RA, Boger DL. Structure determination of an endogenous sleep-inducing lipid, cis-9-octadecenamide (oleamide): a synthetic approach to the chemical analysis of trace quantities of a natural product. J Am Chem Soc. 1996;118:580–590. [Google Scholar]
- Davidson JS, Baumgarten IM, Barely EH. Reversible inhibition of intercellular junctional communication by glycyrrhetinic acid. Biochem Biophys Res Commun. 1996;134:29–36. doi: 10.1016/0006-291x(86)90522-x. [DOI] [PubMed] [Google Scholar]
- Dewey MM, Barr L. Intercellular connection between smooth muscle cells: the nexus. Science. 1962;137:670–672. doi: 10.1126/science.137.3531.670-a. [DOI] [PubMed] [Google Scholar]
- El-Fouly MH, Trosko JE, Chang C. Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168:422–430. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- Enkvist MOK, McCarthy KD. Activation of protein kinase C blocks astroglial gap junction communication and inhibits the spread of calcium waves. J Neurochem. 1992;59:519–526. doi: 10.1111/j.1471-4159.1992.tb09401.x. [DOI] [PubMed] [Google Scholar]
- Enomoto K, Furuya K, Yamagishi S, Maeno T. Mechanically induced electrical and intracellular calcium responses in normal and cancerous mammary cells. Cell Calcium. 1992;13:501–511. doi: 10.1016/0143-4160(92)90018-n. [DOI] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblen FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. Calcium waves in astrocytes—filling in the gaps. Neuron. 1992;8:1101–1108. doi: 10.1016/0896-6273(92)90131-v. [DOI] [PubMed] [Google Scholar]
- Fluri GS, Rudisuli A, Willi M, Rohr S, Weingart R. Effects of arachidonic acid on the gap junctions of neonatal rat heart cells. Pflugers Arch. 1990;417:149–156. doi: 10.1007/BF00370692. [DOI] [PubMed] [Google Scholar]
- Frame MK, deFeijter AW. Propagation of mechanically induced intercellular calcium waves via gap junctions and ATP receptors in rat liver epithelial cells. Exp Cell Res. 1997;230:197–207. doi: 10.1006/excr.1996.3409. [DOI] [PubMed] [Google Scholar]
- Gill, E.W., and D.K. Lawrence. 1976. The physiocochemical mode of action of tetrahydrocannabinol on cell membranes. In The Pharmacology of Marihuana. M.C. Braude and S. Szara, editors. Raven Press, New York. 147–155.
- Guan XJ, Bonney WJ, Ruch RJ. Changes in gap junction permeability, gap junction number, and connexin43 expression in lindane-treated rat liver epithelial cells. Toxicol Appl Pharmacol. 1995;130:79–86. doi: 10.1006/taap.1995.1011. [DOI] [PubMed] [Google Scholar]
- Guan XJ, Wilson S, Schlender KK, Ruch RJ. Gap junction disassembly and connexin43 dephosphorylation induced by 18-Glycyrrhetinic acid. Mol Carcinog. 1996;16:157–164. doi: 10.1002/(SICI)1098-2744(199607)16:3<157::AID-MC6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Hansen M, Boitano S, Dirksen ER, Sanderson MJ. Intercellular calcium signaling induced by extracellular adenosine 5′-triphosphate and mechanical stimulation in airway epithelial cells. J Cell Sci. 1993;106:995–1004. doi: 10.1242/jcs.106.4.995. [DOI] [PubMed] [Google Scholar]
- Hassinger TD, Guthrie PB, Atkinson PB, Bennett MVL, Kater SB. An extracellular signaling component in propagation of astrocytic calcium waves. Proc Natl Acad Sci USA. 1996;93:13268–13273. doi: 10.1073/pnas.93.23.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Minnich BN, Moore LK, Burt JM. Oleic acid differentially affects gap junction-mediated communication in heart and vascular smooth muscle cells. Am J Physiol. 1993;265:C1517–1526. doi: 10.1152/ajpcell.1993.265.6.C1517. [DOI] [PubMed] [Google Scholar]
- Jalife J, Sicouri S, Delmar M, Michaels DC. Electrical uncoupling and impulse propagation in isolated sheep Purkinje fibers. Am J Physiol. 1989;257:H179–189. doi: 10.1152/ajpheart.1989.257.1.H179. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Friend DS, Gilula NB. Synthesis and assembly of human β1 gap junctions in BHK cells by DNA transfection with the human β1cDNA. J Cell Sci. 1995;108:3725–3734. doi: 10.1242/jcs.108.12.3725. [DOI] [PubMed] [Google Scholar]
- Mege RM, Goudou D, Giaume C, Nicolet M, Rieger F. Is intercellular communication via gap junctions required for myoblast fusion? . Cell Adhes Commun. 1994;2:329–343. doi: 10.3109/15419069409014208. [DOI] [PubMed] [Google Scholar]
- Miller AG, Zampighi GA, Hall JE. Single-membrane and cell- to-cell permeability properties of dissociated embryonic chick lens cells. J Membr Biol. 1992;128:91–102. doi: 10.1007/BF00231882. [DOI] [PubMed] [Google Scholar]
- Musil L, Goodenough D. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Cunningham BA, Edelman GM, Goodenough DA. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Neyton J, Trautmann A. Single-channel currents of an intercellular junction. Nature. 1985;317:331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- Palmer RK, Yule DI, Shewach DS, Williams JA, Fisher SK. Paracrine mediation of calcium signaling in human SK-N-MCIXC neuroepithelioma cells. Am J Physiol. 1996;271:C4–53. doi: 10.1152/ajpcell.1996.271.1.C43. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signaling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Suter S, Trosko JE, El-Fouly MH, Lockwood LR, Koestner A. Dieldrin inhibition of gap junctional intercellular communication in rat glial cells as measured by the fluorescence photobleaching and scrape loading/ dye transfer assays. Fundam Appl Toxicol. 1987;9:785–794. doi: 10.1016/0272-0590(87)90185-0. [DOI] [PubMed] [Google Scholar]
- Veenstra RD, DeHaan RL. Measurement of single channel currents from cardiac gap junctions. Science. 1986;233:972–974. doi: 10.1126/science.2426781. [DOI] [PubMed] [Google Scholar]
- Venance L, Piomelli D, Glowinski J, Giaume C. Inhibition by anandamide of gap junctions and intercellular calcium signaling in striatal astrocytes. Nature. 1995;376:590–594. doi: 10.1038/376590a0. [DOI] [PubMed] [Google Scholar]
- Warner AE, Guthrie SC, Gilula NB. Antibodies to gap-junctional protein selectively disrupt junctional communication in the early amphibian embryo. Nature. 1984;311:127–131. doi: 10.1038/311127a0. [DOI] [PubMed] [Google Scholar]