Abstract
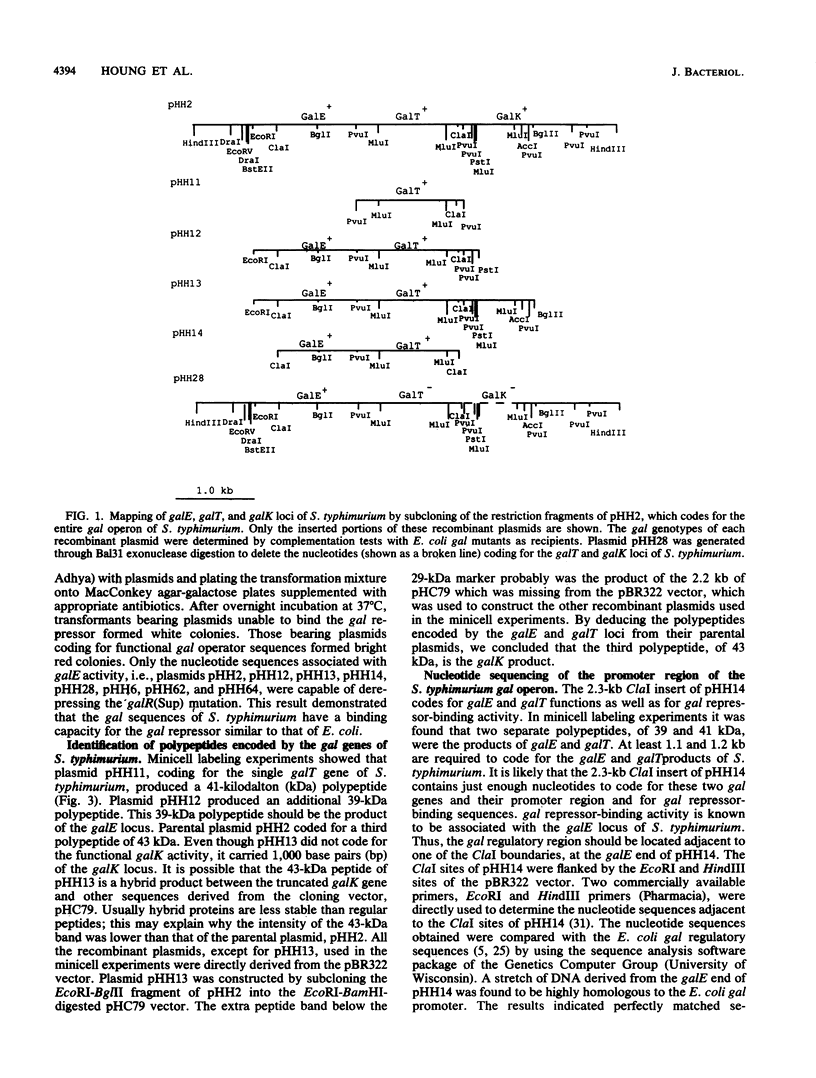
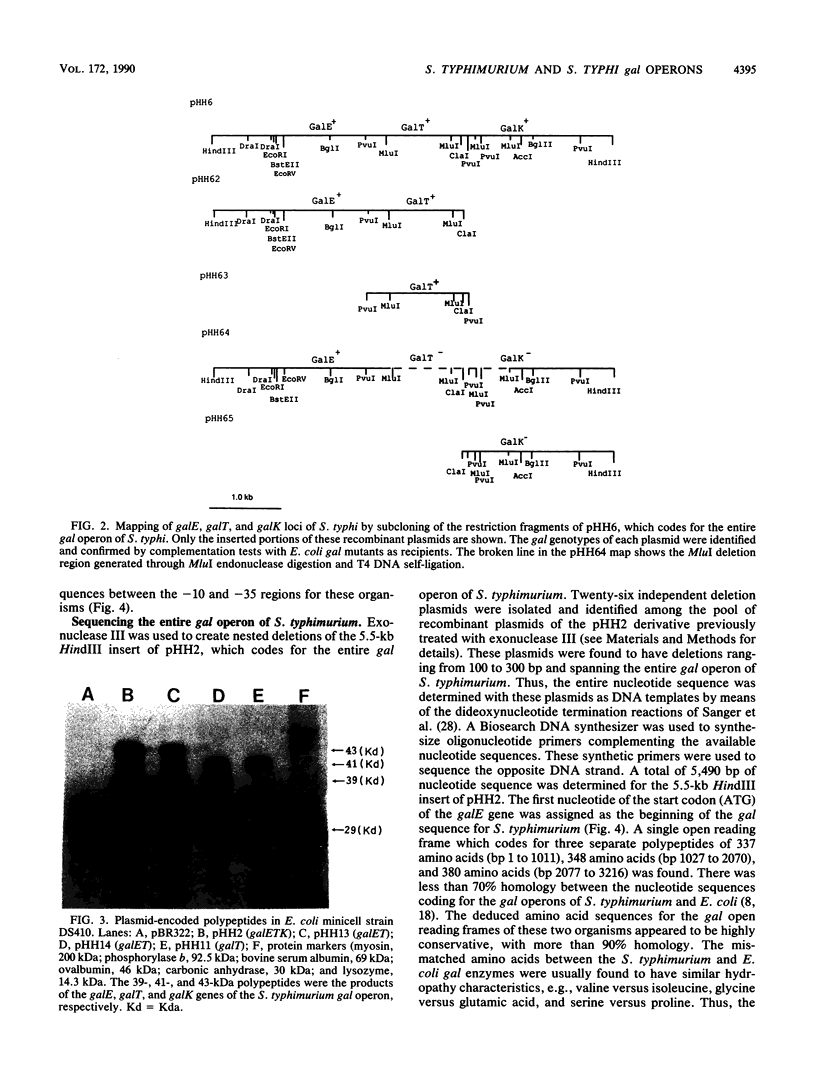
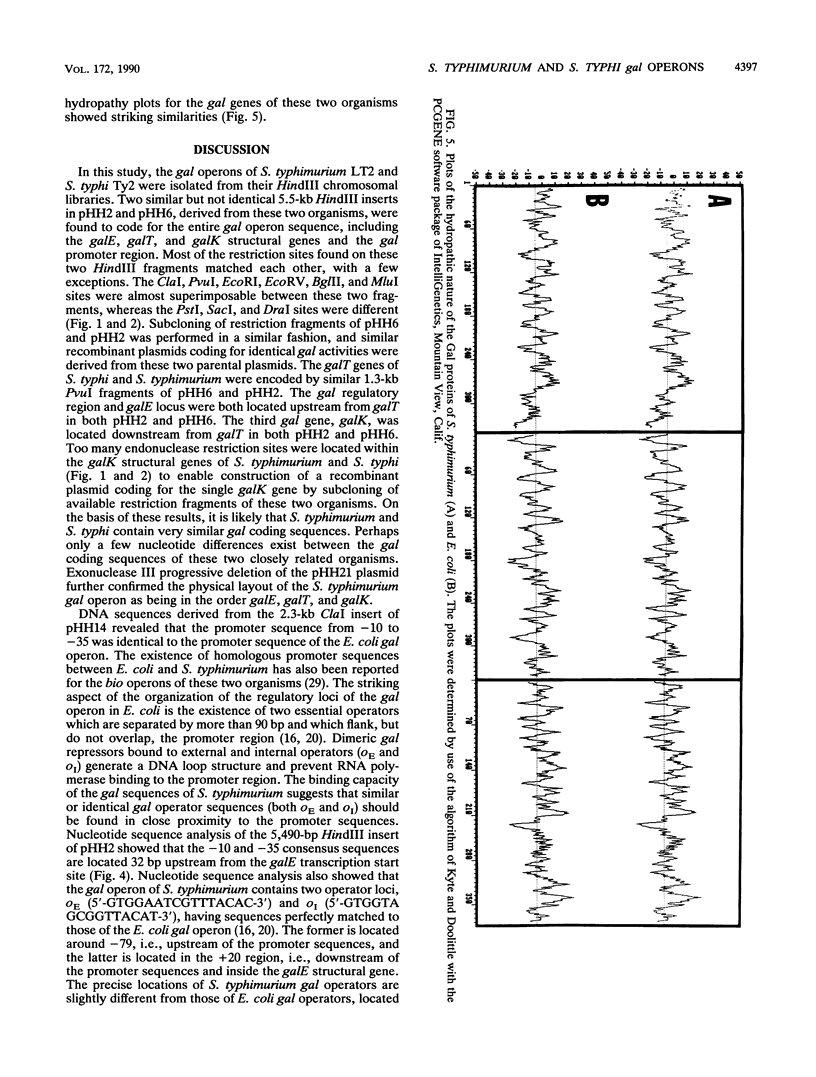
The chromosomally encoded galactose utilization (gal) operons of Salmonella typhimurium and S. typhi were each cloned on similar 5.5-kilobase HindIII fragments into pBR322 and were identified by complementation of Gal- Escherichia coli strains. Restriction endonuclease analyses indicated that these Salmonellae operons share considerable homology, but some heterogeneities in restriction sites were observed. Subcloning and exonuclease mapping experiments showed that both operons have the same genetic organization as that established for the E. coli gal operon (i.e., 5' end, promoter, epimerase, transferase, kinase, and 3' end). Two gal operator regions (oE and oI) of S. typhimurium, identified by repressor titration in an E. coli superrepressor [galR(Sup)] mutant, were sequenced and found to flank the promoter region. This promoter region is identical to the -10 and -35 regions of the E. coli gal operon. Minicell studies demonstrated that the three gal structural genes of S. typhimurium encode separate polypeptides of 39 kilodaltons (kDa) (epimerase, 337 amino acids [aa's]), 41 kDa (transferase, 348 aa's), and 43 kDa (kinase, 380 aa's). Despite functional and organizational similarities, DNA sequence analysis revealed that the S. typhimurium gal genes show less than 70% homology to the E. coli gal operon. Because of codon degeneracy, the deduced amino acid sequences of these polypeptides are highly conserved (greater than 90% homology) as compared with those of the E. coli gal enzymes. These studies have defined basic genetic parameters of the gal genes of two medically important Salmonella species, and our findings support the hypothesized divergent evolution of E. coli and Salmonella spp. from a common ancestral parent bacterium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHCROFT M. T., RITCHIE J. M., NICHOLSON C. C. CONTROLLED FIELD TRIAL IN BRITISH GUIANA SCHOOL CHILDREN OF HEAT-KILLED-PHENOLIZED AND ACETONE-KILLED LYOPHILIZED TYPHOID VACCINES. Am J Hyg. 1964 Mar;79:196–206. doi: 10.1093/oxfordjournals.aje.a120376. [DOI] [PubMed] [Google Scholar]
- Adhya S. L., Shapiro J. A. The galactose operon of E. coli K-12. I. Structural and pleiotropic mutations of the operon. Genetics. 1969 Jun;62(2):231–247. doi: 10.1093/genetics/62.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbás P., Soberón X., Merino E., Zurita M., Lomeli H., Valle F., Flores N., Bolivar F. Plasmid vector pBR322 and its special-purpose derivatives--a review. Gene. 1986;50(1-3):3–40. doi: 10.1016/0378-1119(86)90307-0. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby S., Aiba H., de Crombrugghe B. Mutations in the Escherichia coli operon that define two promoters and the binding site of the cyclic AMP receptor protein. J Mol Biol. 1982 Jan 15;154(2):211–227. doi: 10.1016/0022-2836(82)90061-4. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck C., Riccio A., Schumperli D., McKenney K., Jeffers J., Hughes C., Rosenberg M., Heusterspreute M., Brunel F., Davison J. Structure of the galactokinase gene of Escherichia coli, the last (?) gene of the gal operon. Nucleic Acids Res. 1985 Mar 25;13(6):1841–1853. doi: 10.1093/nar/13.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont H. L., Hornick R. B., Snyder M. J., Libonati J. P., Woodward T. E. Immunity in typhoid fever: evaluation of live streptomycin-dependent vaccine. Antimicrob Agents Chemother (Bethesda) 1970;10:236–239. [PubMed] [Google Scholar]
- Germanier R., Fürer E. Immunity in experimental salmonellosis. II. Basis for the avirulence and protective capacity of gal E mutants of Salmonella typhimurium. Infect Immun. 1971 Dec;4(6):663–673. doi: 10.1128/iai.4.6.663-673.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Hone D. M., Attridge S. R., Forrest B., Morona R., Daniels D., LaBrooy J. T., Bartholomeusz R. C., Shearman D. J., Hackett J. A galE via (Vi antigen-negative) mutant of Salmonella typhi Ty2 retains virulence in humans. Infect Immun. 1988 May;56(5):1326–1333. doi: 10.1128/iai.56.5.1326-1333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone D., Morona R., Attridge S., Hackett J. Construction of defined galE mutants of Salmonella for use as vaccines. J Infect Dis. 1987 Jul;156(1):167–174. doi: 10.1093/infdis/156.1.167. [DOI] [PubMed] [Google Scholar]
- Irani M. H., Orosz L., Adhya S. A control element within a structural gene: the gal operon of Escherichia coli. Cell. 1983 Mar;32(3):783–788. doi: 10.1016/0092-8674(83)90064-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemaire H. G., Müller-Hill B. Nucleotide sequences of the gal E gene and the gal T gene of E. coli. Nucleic Acids Res. 1986 Oct 10;14(19):7705–7711. doi: 10.1093/nar/14.19.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A., Adhya S. Demonstration of two operator elements in gal: in vitro repressor binding studies. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6100–6104. doi: 10.1073/pnas.81.19.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Michaelis G., Starlinger P. Sequential appearance of the galactose enzymes in E. coli. Mol Gen Genet. 1967;100(2):210–215. doi: 10.1007/BF00333607. [DOI] [PubMed] [Google Scholar]
- Musso R., Di Lauro R., Rosenberg M., de Crombrugghe B. Nucleotide sequence of the operator-promoter region of the galactose operon of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jan;74(1):106–110. doi: 10.1073/pnas.74.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland J. W., Green B. A., Foulds J., Holmes R. K. Cloning of extracellular DNase and construction of a DNase-negative strain of Vibrio cholerae. Infect Immun. 1985 Mar;47(3):691–696. doi: 10.1128/iai.47.3.691-696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiuan D., Campbell A. Transcriptional regulation and gene arrangement of Escherichia coli, Citrobacter freundii and Salmonella typhimurium biotin operons. Gene. 1988 Jul 30;67(2):203–211. doi: 10.1016/0378-1119(88)90397-6. [DOI] [PubMed] [Google Scholar]
- Wahdan M. H., Sérié C., Cerisier Y., Sallam S., Germanier R. A controlled field trial of live Salmonella typhi strain Ty 21a oral vaccine against typhoid: three-year results. J Infect Dis. 1982 Mar;145(3):292–295. doi: 10.1093/infdis/145.3.292. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Suggs S. V., Miyoshi K., Bhatt R., Itakura K. A set of synthetic oligodeoxyribonucleotide primers for DNA sequencing in the plasmid vector pBR322. Gene. 1981 Dec;16(1-3):21–26. doi: 10.1016/0378-1119(81)90057-3. [DOI] [PubMed] [Google Scholar]



