Abstract
Eubacterium sp. strain VPI 12708 is an anaerobic intestinal bacterium which possesses inducible bile acid 7-dehydroxylation activity. Several new polypeptides are produced in this strain following induction with cholic acid. Genes coding for two copies of a bile acid-inducible 27,000-dalton polypeptide (baiA1 and baiA2) have been previously cloned and sequenced. We now report on a gene coding for a third copy of this 27,000-dalton polypeptide (baiA3). The baiA3 gene has been cloned in lambda DASH on an 11.2-kilobase DNA fragment from a partial Sau3A digest of the Eubacterium DNA. DNA sequence analysis of the baiA3 gene revealed 100% homology with the baiA1 gene within the coding region of the 27,000-dalton polypeptides. The baiA2 gene shares 81% sequence identity with the other two genes at the nucleotide level. The flanking nucleotide sequences associated with the baiA1 and baiA3 genes are identical for 930 bases in the 5' direction from the initiation codon and for at least 325 bases in the 3' direction from the stop codon, including the putative promoter regions for the genes. An additional open reading frame (occupying from 621 to 648 bases, depending on the correct start codon) was found in the identical 5' regions associated with the baiA1 and baiA3 clones. The 5' sequence 930 bases upstream from the baiA1 and baiA3 genes was totally divergent. The baiA2 gene, which is part of a large bile acid-inducible operon, showed no homology with the other two genes either in the 5' or 3' direction from the polypeptide coding region, except for a 15-base-pair presumed ribosome-binding site in the 5' region. These studies strongly suggest that a gene duplication (baiA1 and baiA3) has occurred and is stably maintained in this bacterium.
Full text
PDF



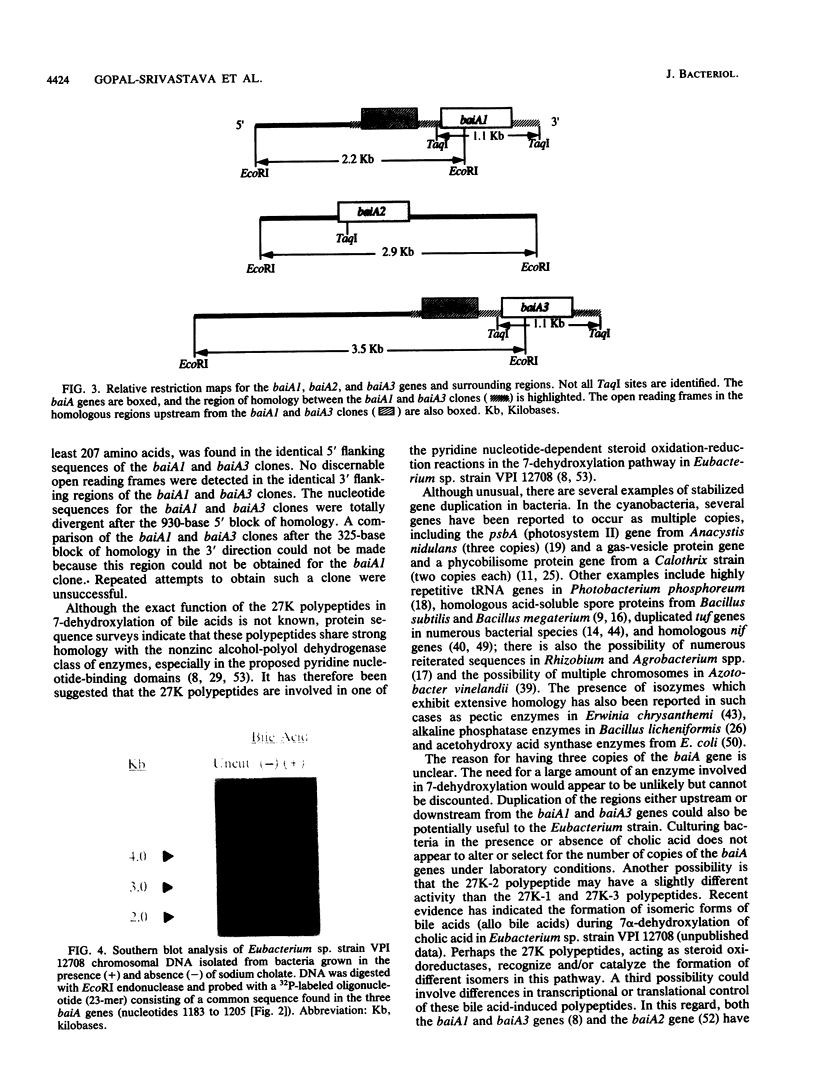


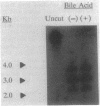
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Roth J. Spontaneous tandem genetic duplications in Salmonella typhimurium arise by unequal recombination between rRNA (rrn) cistrons. Proc Natl Acad Sci U S A. 1981 May;78(5):3113–3117. doi: 10.1073/pnas.78.5.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri S. A., Bolt M. G., Boyer J. L., Palmer R. H. Stimulation of thymidine incorporation in mouse liver and biliary tract epithelium by lithocholate and deoxycholate. Gastroenterology. 1978 Feb;74(2 Pt 1):188–192. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. P., White W. B., Hylemon P. B. Molecular cloning of bile acid 7-dehydroxylase from Eubacterium sp. strain VPI 12708. J Bacteriol. 1987 Apr;169(4):1516–1521. doi: 10.1128/jb.169.4.1516-1521.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. P., White W. B., Lijewski M., Hylemon P. B. Nucleotide sequence and regulation of a gene involved in bile acid 7-dehydroxylation by Eubacterium sp. strain VPI 12708. J Bacteriol. 1988 May;170(5):2070–2077. doi: 10.1128/jb.170.5.2070-2077.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M. J., Howard S., Hoch J., Setlow P. Determination of the chromosomal locations of four Bacillus subtilis genes which code for a family of small, acid-soluble spore proteins. J Bacteriol. 1986 May;166(2):412–416. doi: 10.1128/jb.166.2.412-416.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey F. E., Cronan J. E., Jr Acetohydroxy acid synthase I, a required enzyme for isoleucine and valine biosynthesis in Escherichia coli K-12 during growth on acetate as the sole carbon source. J Bacteriol. 1986 Feb;165(2):453–460. doi: 10.1128/jb.165.2.453-460.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerval T., Houmard J., Guglielmi G., Csiszar K., Tandeau de Marsac N. A developmentally regulated gvpABC operon is involved in the formation of gas vesicles in the cyanobacterium Calothrix 7601. Gene. 1987;54(1):83–92. doi: 10.1016/0378-1119(87)90350-7. [DOI] [PubMed] [Google Scholar]
- Feulner G., Gray J. A., Kirschman J. A., Lehner A. F., Sadosky A. B., Vlazny D. A., Zhang J., Zhao S., Hill C. W. Structure of the rhsA locus from Escherichia coli K-12 and comparison of rhsA with other members of the rhs multigene family. J Bacteriol. 1990 Jan;172(1):446–456. doi: 10.1128/jb.172.1.446-456.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filer D., Dhar R., Furano A. V. The conservation of DNA sequences over very long periods of evolutionary time. Evidence against intergeneric chromosomal transfer as an explanation for the presence of Escherichia coli tuf gene sequences in taxonomically-unrelated prokaryotes. Eur J Biochem. 1981 Nov;120(1):69–77. doi: 10.1111/j.1432-1033.1981.tb05671.x. [DOI] [PubMed] [Google Scholar]
- Finegold S. M., Flora D. J., Attebery H. R., Sutter V. L. Fecal bacteriology of colonic polyp patients and control patients. Cancer Res. 1975 Nov;35(11 Pt 2):3407–3417. [PubMed] [Google Scholar]
- Fliss E. R., Loshon C. A., Setlow P. Genes for Bacillus megaterium small, acid-soluble spore proteins: cloning and nucleotide sequence of three additional genes from this multigene family. J Bacteriol. 1986 Feb;165(2):467–473. doi: 10.1128/jb.165.2.467-473.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores M., González V., Brom S., Martínez E., Piñero D., Romero D., Dávila G., Palacios R. Reiterated DNA sequences in Rhizobium and Agrobacterium spp. J Bacteriol. 1987 Dec;169(12):5782–5788. doi: 10.1128/jb.169.12.5782-5788.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux S., Beaudet J., Cedergren R. Highly repetitive tRNA(Pro)-tRNA(His) gene cluster from Photobacterium phosphoreum. J Bacteriol. 1988 Dec;170(12):5601–5606. doi: 10.1128/jb.170.12.5601-5606.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B. E., Midtvedt T., Norman A. Isolated fecal microorganisms capable of 7-alpha-dehydroxylating bile acids. J Exp Med. 1966 Feb 1;123(2):413–432. doi: 10.1084/jem.123.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Grafstrom R. H., Harnish B. W., Hillman B. S. Tandem duplications resulting from recombination between ribosomal RNA genes in Escherichia coli. J Mol Biol. 1977 Nov 5;116(3):407–428. doi: 10.1016/0022-2836(77)90077-8. [DOI] [PubMed] [Google Scholar]
- Hirano S., Nakama R., Tamaki M., Masuda N., Oda H. Isolation and characterization of thirteen intestinal microorganisms capable of 7 alpha-dehydroxylating bile acids. Appl Environ Microbiol. 1981 Mar;41(3):737–745. doi: 10.1128/aem.41.3.737-745.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J., Capuano V., Coursin T., Tandeau de Marsac N. Genes encoding core components of the phycobilisome in the cyanobacterium Calothrix sp. strain PCC 7601: occurrence of a multigene family. J Bacteriol. 1988 Dec;170(12):5512–5521. doi: 10.1128/jb.170.12.5512-5521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulett F. M., Wang P. Z., Sussman M., Lee J. W. Two alkaline phosphatase genes positioned in tandem in Bacillus licheniformis MC14 require different RNA polymerase holoenzymes for transcription. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1035–1039. doi: 10.1073/pnas.82.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H., von Bahr-Lindström H., Jany K. D., Ulmer W., Fröschle M. Extended superfamily of short alcohol-polyol-sugar dehydrogenases: structural similarities between glucose and ribitol dehydrogenases. FEBS Lett. 1984 Jan 9;165(2):190–196. doi: 10.1016/0014-5793(84)80167-2. [DOI] [PubMed] [Google Scholar]
- Kulkarni M. S., Heidepriem P. M., Yielding K. L. Production by lithocholic acid of DNA strand breaks in L1210 cells. Cancer Res. 1980 Aug;40(8 Pt 1):2666–2669. [PubMed] [Google Scholar]
- Lin R. J., Capage M., Hill C. W. A repetitive DNA sequence, rhs, responsible for duplications within the Escherichia coli K-12 chromosome. J Mol Biol. 1984 Jul 25;177(1):1–18. doi: 10.1016/0022-2836(84)90054-8. [DOI] [PubMed] [Google Scholar]
- Low-Beer T. S., Nutter S. Colonic bacterial activity, biliary cholesterol saturation, and pathogenesis of gallstones. Lancet. 1978 Nov 18;2(8099):1063–1065. doi: 10.1016/s0140-6736(78)91800-7. [DOI] [PubMed] [Google Scholar]
- Mori M., Tanimoto A., Yoda K., Harada S., Koyama N., Hashiguchi K., Obinata M., Yamasaki M., Tamura G. Essential structure in the cloned transforming DNA that induces gene amplification of the Bacillus subtilis amyE-tmrB region. J Bacteriol. 1986 Jun;166(3):787–794. doi: 10.1128/jb.166.3.787-794.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal P., Jafri S., Reddy M. A., Das H. K. Multiple chromosomes of Azotobacter vinelandii. J Bacteriol. 1989 Jun;171(6):3133–3138. doi: 10.1128/jb.171.6.3133-3138.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa T., Magadia N. E., Weisburger J. H., Wynder E. L. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N'-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974 Oct;53(4):1093–1097. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- Paone D. A., Hylemon P. B. HPLC purification and preparation of antibodies to cholic acid-inducible polypeptides from Eubacterium sp. V.P.I. 12708. J Lipid Res. 1984 Dec 1;25(12):1343–1349. [PubMed] [Google Scholar]
- Quinto C., De La Vega H., Flores M., Leemans J., Cevallos M. A., Pardo M. A., Azpiroz R., De Lourdes Girard M., Calva E., Palacios R. Nitrogenase reductase: A functional multigene family in Rhizobium phaseoli. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1170–1174. doi: 10.1073/pnas.82.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoedel C., Collmer A. Evidence of homology between the pectate lyase-encoding pelB and pelC genes in Erwinia chrysanthemi. J Bacteriol. 1986 Jul;167(1):117–123. doi: 10.1128/jb.167.1.117-123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela S., Yogev D., Razin S., Bercovier H. Duplication of the tuf gene: a new insight into the phylogeny of eubacteria. J Bacteriol. 1989 Jan;171(1):581–584. doi: 10.1128/jb.171.1.581-584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stellwag E. J., Hylemon P. B. 7alpha-Dehydroxylation of cholic acid and chenodeoxycholic acid by Clostridium leptum. J Lipid Res. 1979 Mar;20(3):325–333. [PubMed] [Google Scholar]
- Vagner V., Ehrlich S. D. Efficiency of homologous DNA recombination varies along the Bacillus subtilis chromosome. J Bacteriol. 1988 Sep;170(9):3978–3982. doi: 10.1128/jb.170.9.3978-3982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Z., Chen J. S., Johnson J. L. The presence of five nifH-like sequences in Clostridium pasteurianum: sequence divergence and transcription properties. Nucleic Acids Res. 1988 Jan 25;16(2):439–454. doi: 10.1093/nar/16.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Hauser C. A., Hatfield G. W. The nucleotide sequence of the ilvBN operon of Escherichia coli: sequence homologies of the acetohydroxy acid synthase isozymes. Nucleic Acids Res. 1985 Jun 11;13(11):3995–4010. doi: 10.1093/nar/13.11.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Lipsky R. L., Fricke R. J., Hylemon P. B. Bile acid induction specificity of 7 alpha-dehydroxylase activity in an intestinal Eubacterium species. Steroids. 1980 Jan;35(1):103–109. doi: 10.1016/0039-128x(80)90115-4. [DOI] [PubMed] [Google Scholar]
- White W. B., Coleman J. P., Hylemon P. B. Molecular cloning of a gene encoding a 45,000-dalton polypeptide associated with bile acid 7-dehydroxylation in Eubacterium sp. strain VPI 12708. J Bacteriol. 1988 Feb;170(2):611–616. doi: 10.1128/jb.170.2.611-616.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White W. B., Franklund C. V., Coleman J. P., Hylemon P. B. Evidence for a multigene family involved in bile acid 7-dehydroxylation in Eubacterium sp. strain VPI 12708. J Bacteriol. 1988 Oct;170(10):4555–4561. doi: 10.1128/jb.170.10.4555-4561.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. R., Morgan A. E. Chromosomal-DNA amplification in Bacillus subtilis. J Bacteriol. 1985 Aug;163(2):445–453. doi: 10.1128/jb.163.2.445-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young M., Ehrlich S. D. Stability of reiterated sequences in the Bacillus subtilis chromosome. J Bacteriol. 1989 May;171(5):2653–2656. doi: 10.1128/jb.171.5.2653-2656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]