Abstract
A trimeric complex formed by Tub4p, the budding yeast γ-tubulin, and the two spindle pole body components, Spc98p and Spc97p, has recently been characterized in Saccharomyces cerevisiae. We reasoned that crucial functions, such as the control of microtubule nucleation, could be maintained among divergent species. SPC98-related sequences were searched in dbEST using the BLASTN program. Primers derived from the human expressed sequence tag matching SPC98 were used to clone the 5′ and 3′ cDNA ends by rapid amplification of cDNA ends (RACE)-PCR. The human Spc98 cDNA presents an alternative splicing at the 3′ end. The deduced protein possesses 22% identity and 45% similarity with the yeast homologue. We further report that the human Spc98p, like γ-tubulin, is concentrated at the centrosome, although a large fraction is found in cytosolic complexes. Sucrose gradient sedimentation of the cytosolic fraction and immunoprecipitation experiments demonstrate that both γ-tubulin and HsSpc98p are in the same complex. Interestingly, Xenopus sperm centrosomes, which are incompetent for microtubule nucleation before their activation in the egg cytoplasm, were found to contain similar amounts of both Spc98p and γ-tubulin to human somatic centrosomes, which are competent for microtubule nucleation. Finally, affinity-purified antibodies against Spc98p inhibit microtubule nucleation on isolated centrosomes, as well as in microinjected cells, suggesting that this novel protein is indeed required for the nucleation reaction.
Despite differences in size and complexity throughout the evolutionary range of eukaryotes, centrosomes have similar functions. First, they promote microtubule growth from cellular tubulin, the nucleation reaction being either catalyzed or by-passed in their vicinity (Heidemann and McIntosh, 1980; Mitchison and Kirschner, 1984). The precise mechanism ensuring the nucleation reaction for microtubule assembly in vivo remains unknown. Second, they duplicate once during each cell cycle, and this has important implications for microtubule redistribution and spindle morphogenesis at mitosis.
First discovered in Aspergillus nidulans as a suppressor of a temperature-sensitive β-tubulin mutation (Oakley and Oakley, 1989), γ-tubulin is a low abundance protein that shows 35% identity to α- and β-tubulin and has been localized to the spindle pole body of Aspergillus nidulans (Oakley et al., 1990). Homologues of this gene have subsequently been cloned in various eukaryotic species (Stearns et al., 1991; Zheng et al., 1991; Fuchs et al., 1993; Maessen et al., 1993; Sobel and Snyder, 1995; Spang et al., 1996). Disruption of the essential γ-tubulin gene in several organisms prevents the proper microtubule organization (Oakley et al., 1990; Horio et al., 1991; Sunkel et al., 1995; Marschall et al., 1996; Spang et al., 1996). Recent studies have focused on the role of γ-tubulin in microtubule nucleation. In mammalian cells, antibodies directed against γ-tubulin have been shown to block microtubule nucleation, and γ-tubulin overexpression has been reported to induce a reorganization of the microtubule network (Joshi et al., 1992; Shu and Joshi, 1995). Occasionally, γ-tubulin is able to self assemble into γ-tubules (Shu and Joshi, 1995). Although γ-tubulin has been shown to be concentrated at the centrosome (Horio et al., 1991; Stearns et al., 1991; Zheng et al., 1991), a large fraction is not associated with it but belongs to cytoplasmic complexes both in eggs and somatic cells (Raff et al., 1993; Stearns and Kirschner, 1994; Zheng et al., 1995; Moudjou et al., 1996). The so-called γ-tubulin ring complex (γ-TuRC),1 isolated from mitotic Xenopus egg extracts, is able to nucleate microtubules in vitro (Zheng et al., 1995). This complex contains several proteins distinct from γ-tubulin, including α- and β-tubulin, and presents a ring structure with a left-handed helical shape. Ring-like γ-tubulin–containing structures with a diameter similar to microtubules have been observed at the centrosome by tomography at the ultrastructural level (Moritz et al., 1995a ,b). Two opposite models for the structure and activity of such a complex have been proposed (Zheng et al., 1995; Erickson and Stoffler, 1996).
Similar complexes have been found in other species such as the Drosophila embryo (Zheng, Y., D. Agard, R. Milligan, T. Mitchison, and B. Alberts. 1996. Mol. Biol. Cell. 7:207a) as well as in mammalian brain microtubule preparations (Détraves et al., 1997). In the budding yeast Saccharomyces cerevisiae, γ-tubulin–binding proteins have been identified by genetic and biochemical studies. The essential SPC98 gene was first found as a dosage-dependent suppressor of a thermosensible mutation of the TUB4 gene, which codes for γ-tubulin in budding yeast (Geissler et al., 1996). Spc98p has been further found to interact physically with Tub4p. To find new Tub4p partners, Knop et al. (1997) searched for extragenic suppressors of spc98 mutants and identified SPC97, a gene coding for a new spindle pole body (SPB) component. Furthermore, Knop et al. (1997) presented convincing evidences for a trimeric complex formed by Spc98p, Spc97p, and Tub4p. Interestingly, Tub4p, Spc98p, and Spc97p have been localized to the outer and inner plate of the SPB, where microtubules appear to grow. It is noteworthy that these two yeast γ-tubulin–binding proteins have molecular weights compatible with two proteins observed in the γ-TuRC. A similar trimeric complex has also been observed by Zheng et al. (1996. Mol. Biol. Cell. 7:207a) in Drosophila embryos in addition to the large γ-TuRC.
Since important cellular functions are maintained throughout the evolutionary range of eukaryotes, it is reasonable to assume that functional protein complexes are also conserved. This conjecture led to the characterization of the human homologue of the budding yeast CDC31, which is involved in SPB duplication, at molecular and biochemical levels (Middendorp et al., 1997). We have also identified, using a biochemical approach based on immunocytological cross-reaction, a human protein related to the yeast Spc110p (Tassin et al., 1997).
In this work, we were interested in identifying γ-tubulin–binding proteins in mammalian cells. We thus carried out a search in the Expressed Sequence Tag (EST) database for conservation in animal cells of the two yeast γ-tubulin–binding proteins characterized by Knop et al. (1997). We found human EST for both Spc98p and Spc97p. We report here on the isolation and functional characterization of the human homologue of the yeast SPC98 gene.
Materials and Methods
Database Search and Cloning of HsSpc98
SPC98-related sequences were searched in dbEST using the default parameters of the BLASTN program (Genomet, Tokyo, Japan). Primers derived from human ESTs matching SPC98 were used to clone the 5′ and 3′ cDNA ends by rapid amplification of cDNA ends (RACE)-PCR (5′ and 3′ RACE-PCR kit; GIBCO BRL, Gaithersburg, MD) according to the manufacturer's instructions. Briefly, poly-A mRNA obtained from HeLa cells was reverse transcribed using a gene-specific primer or an oligodT primer to obtain the 5′ and 3′ ends of the genes, respectively. Then, single-strand cDNAs corresponding to 5′ ends were elongated with a poly-C tail and amplified by PCR using a gene-specific primer and a poly-G primer. cDNAs corresponding to 3′ ends were directly amplified by PCR using a gene-specific primer and a poly-T primer. Finally, PCR products were submitted to a second round of PCR using a nested gene-specific primer. cDNAs were sequenced by Genomexpress.
Primers used were: 1- 5′ GAGGCGGTACCTGCTT; 2- 5′ ACTTGTCCGTCCAGCTACGA; rev1- 5′ CGAGTAAACACAGTTGCAAT; rev2- 5′ TCGAGGCTGAAGACATCCCAT; rev3- 5′ GCTACAAAAAATTCGTGGTAAGT; rev4- 5′ GCAAGGGTCTTCAGTCGTAT; rev5- 5′ ACCTTTTGAGGTAGTGGCTT; rev6- 5′ CAATGCTGCTGATGCCACT; rev7- 5′ TTAAGAAGCCTCAGCAA; rev8- 5′TAGCGATGAAGACTTTTAGAGA; rev9- 5′ TTTTTCTCATGAATGCTGCTGT; and rev10- 5′TGAAACAGGATGATGATGCCA.
Production and Purification of Recombinant HsSpc98
HsSpc98 partial cDNA (fragment 1006–2025) was cloned in pET3d bacterial expression vector after having introduced by PCR a six-histidine tag at the carboxy terminus of the cDNA to purify the recombinant protein on nickel-chelated agarose beads according to the manufacturer's instructions (Ni2+-NTA; Qiagen, Chatsworth, CA). The vector was then introduced into BL21 DE3 bacteria strain. Protein expression was induced by 1 mM IPTG for 3 h. Protein expression was analyzed by lysing the bacteria pellet in boiling Laemmli buffer followed by sonication.
Polyclonal Antibody Production
The Ni2+-NTA–purified 38-kD protein was injected into two rabbits (200 μg in complete Freund's adjuvant, followed 2 wk later with 100 μg of protein injected in incomplete Freund's adjuvant). The rabbits were injected once a month for an additional 3 mo. Serum was collected and tested in Western blotting of the recombinant protein, cellular extracts, and centrosomes. The serum or affinity-purified IgGs were used for immunofluorescence on HeLa cells.
A polyclonal rabbit anti–γ-tubulin antibody has been produced against a bacterially expressed histidine tag full-length human γ-tubulin. This antibody has been shown to map eight epitopes (collaboration with D. Job, Centre d'Etudes Nucléaires de Grenoble, Grenoble, France).
Northern Blot Analysis
The multiple Tissue Northern blot with the tissues indicated on Fig. 3 was obtained from CLONTECH Laboratories (Palo Alto, CA). Three probes have been used: Probe 1 spans in the common region (1084–1921), probe 2 spans in bp 80–645 of clone 02, and probe 3 spans in bp 2450–2577 of the initial clone. Probes were labeled using redivue [α32P]dCTP (3,000 Ci/ mmol) by random priming using the Rediprime kit from Amersham Corp. (Indianapolis, IN). The membrane has been hybridized with probe 1 using the protocol provided with the nylon membrane and exposed overnight. The membrane was stripped and reprobed with the two other probes located in the divergent part of the sequence.
Figure 3.
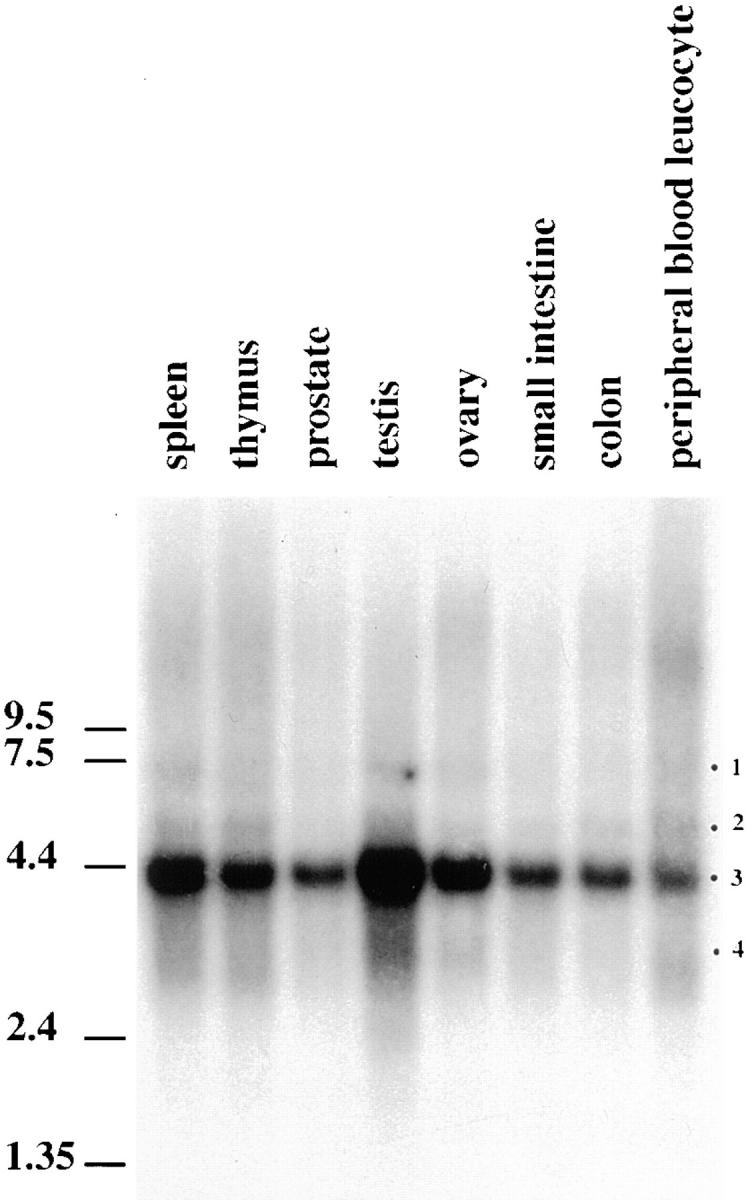
Northern blot analysis on different human tissues (indicated on the top of the figure) using a probe located in the common region (1084–1921). A major messenger at 4.4 kb is observed (band 3). Three minor bands (bands 1, 2, and 4) are also observed. The membrane was further stripped and reprobed with two other probes in the divergent part of the sequence. Probe 2, located in bp 80–645 of clone 02, stained bands 1–3. Probe 3, located in bp 2450–2577 of the initial clone, stained exclusively band 4.
Cell Culture
The human lymphoblastic KE 37 cell line was grown in suspension in RPMI 1640 medium supplemented with 7% FCS at 37°C and cultured in the presence of 5% CO2. HeLa cells were cultured in DME medium containing 10% FCS.
Preparation of the Cytosolic Cell Extracts
Human KE 37 or HeLa cells were harvested and washed twice with PBS buffer at 4°C. The pelleted cells were resuspended at a cell concentration of 1 × 108 cells/ml in cold KHM buffer (50 mM Hepes, pH 7.4, 78 mM KCl, 1 mM MgCl2, 1 mM DTT) containing a mixture of protease inhibitors (aprotinin, leupeptin, and pepstatin, each at 1 mg/ml, and 1 mM PMSF). Cells were then disrupted using a Bill Balch homogenizer with a clearance of 33 μm at 4°C. All the subsequent steps were performed at 4°C. The homogenate was immediately centrifuged at 150,000 g in an ultracentrifuge (Beckman Instruments, Fullerton, CA) with a swinging bucket rotor (model SW55; Beckman Instruments) for 30 min. After this centrifugation, the supernatant representing the cytosolic fraction was recovered. This fraction was quick frozen as aliquots using liquid nitrogen and stored at −80°C. Mitotic HeLa cell extract was obtained as described above except that cells were first blocked in mitosis with a 24-h nocodazole block (5 × 10−7 M).
Sucrose Gradient Centrifugation
Cytosolic cell extract (2–3 ml) was applied to a 15–40% sucrose linear gradient (10 ml) prepared in KHM buffer in ultraclear rotor tubes (model SW41; Beckman Instruments). Centrifugation was performed using a swinging bucket rotor (model SW41; Beckman Instruments) at 100,000 g for 16 h. After centrifugation, ∼26 fractions (400 μl each) were collected, starting from the bottom of the gradient. A sample from each fraction was taken out and diluted with eightfold concentrated SDS-PAGE sample buffer (without glycerol) and heated at 100°C for 5 min. The remainder of the fractions were stored at −80°C after freezing in liquid nitrogen.
In some experiments, the γ-tubulin–enriched fractions from the first SW41 sucrose gradient were pooled, concentrated by dialysis/concentration step against KHM buffer, and then layered on the top of a 4-ml 15– 40% sucrose gradient made in ultraclear rotor tubes (model SW55; Beckman Instruments). The gradient was centrifuged at 100,000 g for 16 h, and 300-μl fractions were collected from the bottom of the gradient and treated as described above for the SW41 sucrose gradient.
Immunoprecipitation Experiments
The fractions from the bottom of the SW41 sucrose gradient (which were enriched in γ-tubulin) were simultaneously concentrated/dialyzed overnight against 1D buffer (50 mM Tris-HCl, pH 7.4, 4.5 mM EDTA, 0.25 M NaCl, 0.5% NP-40). After a rapid centrifugation to eliminate trace aggregates, the primary antibody preadsorbed to protein G–Sepharose beads (Pharmacia Biotech, Piscataway, NJ) was added to the sample, and the mixture was incubated for 2 h at 4°C. Protein G–Sepharose beads were then sedimented. The supernatants were precipitated with cold methanol, and the proteins were resuspended in SDS-PAGE sample buffer (100°C, 5 min). The sedimented protein G–Sepharose beads were washed (five times) with 500 μl of 1D buffer and with 1 ml of double distilled water three times. The immunoprecipitates were solubilized from the Sepharose beads by incubation with the SDS-PAGE sample buffer (100°C, 5 min) and centrifuged before loading the supernatant.
Cellular Fractionation
Centrosomes were isolated from KE 37 cells as previously described (Komesli et al., 1989; Moudjou and Bornens, 1994).
Soluble and insoluble protein fractions were prepared as follows: KE37 cells were washed in PBS and lysed in a PHEM buffer containing 1% Triton X-100 and protease inhibitors (1 mg/ml leupeptin, 1 mg/ml pepstatin, and 10 mg/ml aprotinin). Insoluble proteins were pelleted at 300 g, solubilized in SDS-PAGE sample buffer (Laemmli, 1970), and then boiled for 5 min. Soluble proteins were precipitated with 9 vol of methanol at 4°C for 1 h and pelleted. The pellet was resuspended in the same amount of sample buffer that was used for the insoluble proteins.
5 × 105 Xenopus sperm heads were diluted in XB-CSF buffer (100 mM KCl, 0.1 mM CaCl2, 2 mM MgCl2, 10 mM potassium Hepes, pH 7.7, 50 mM sucrose, 5 mM EGTA, pH 7.7) and centrifuged for 15 min at 10,000 g, and the pellet was resuspended in 20 μl of SDS-sample buffer, sonicated, boiled for 5 min, and processed for protein analysis.
Centrosome Subfractionation
Centrosomes containing fractions were diluted in 10 mM K-Pipes, pH 7.2, and centrifuged at 10,000 g for 15 min. Pelleted centrosomes were incubated for 1 h in extraction buffer (20 mM Tris, pH 7.4, 2 mM EDTA) containing either 1 M NaCl (NaCl); 0.5% NP-40 (1D); 0.5% NP-40 and 0.5% deoxycholate (2D); 0.5% NP-40, 0.5% deoxycholate, and 0.1% SDS (3D); or 8 M Urea (Urea). After treatment, centrosomal proteins were fractionated into pellet and supernatant by centrifugation at 10,000 g for 15 min and solubilized in SDS-sample buffer.
Protein Analysis
SDS-PAGE was performed according to Laemmli using either an 8 or a 6–15% acrylamide gradient gel. Immunoblotting experiments were performed according to Towbin et al. (1979). Nitrocellulose filters were saturated in TBS (10 mM Tris, pH 7.4, 150 mM NaCl) containing 5% nonfat dry milk for 1 h at 37°C. Primary antibodies were incubated for 1 h at 37°C. Phosphatase alkaline–conjugated secondary antibodies were purchased from Promega Corp. (Madison, WI), and the peroxidase antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Immunofluorescence Microscopy
HeLa cells were grown on coverslips, washed in PBS, and fixed in methanol at −20°C for 6 min. Alternatively, after a PBS wash, cells were extracted in a PHEM buffer (45 mM Pipes, 45 mM Hepes, 10 mM EGTA, 5 mM MgCl2 adjusted to pH 6.9, and 1 mM PMSF) containing 1% Triton X-100 and then fixed as described above. Cells were rinsed in PBS containing 0.1% Tween 20. Primary antibodies diluted in PBS containing 3% BSA were added for 1 h at room temperature. Three washes were performed in PBS-Tween, and the fluorescein- or rhodamine-labeled secondary antibodies were applied (Jackson ImmunoResearch Laboratories). Cells were finally dehydrated in ethanol and mounted in Citifluor (City University, London, England). Observations were done on a confocal microscope. All documents presented are two-dimensional projections of images collected at all relevant z-axes.
Permeabilized Xenopus sperm heads were prepared according to the method of Murray (1991). For immunofluorescence, sperm heads were placed on poly-lysine coverslips, fixed for 6 min in methanol at −20°C, and processed for immunofluorescence as described above.
Electron Microscopy
Centrosomes were sedimented onto glass coverslips at 20,000 g, fixed with methanol, and processed for preembedding immunogold staining with affinity-purified polyclonal antibody against HsSpc98p or γ-tubulin. Glutaraldehyde postfixation was used before processing the coverslips for electron microscopy.
Microtubule Nucleation Test
The microtubule nucleating activity of isolated centrosomes was tested according to Mitchison and Kirschner (1984) using bovine brain tubulin purified on phosphocellulose. Nucleation was monitored by immunofluorescence using antitubulin and anticentrosome antibodies. Tubulin (10 μM final concentration) and 1 mM GTP were added to the centrosome suspension, and the temperature was raised to 37°C for 4 min. After glutaraldehyde fixation and sedimentation on glass coverslips, microtubules were visualized using an anti–α-tubulin antibody. To test the effect of anti-Spc98p antibodies on microtubule nucleation, we affinity-purified the rabbit serum against HsSpc98p and preincubated the pAb HsSpc98 immunoglobulins with centrosomes for 30 min at 4°C. Microtubules were then allowed to regrow for 5 min.
Microinjection Experiments
Cells were seeded onto glass coverslips for at least 2 d until 50% confluent. Coverslips were transferred into fresh medium before injection of anti-HsSpc98p IgG (1.2 mg/ml) into the cytoplasm of interphase cells. As a control, anti-Cen3p IgG were microinjected at the same concentration. Injection was carried out using an Eppendorf (Madison, WI) semiautomatic microinjection device coupled to the Zeiss Automated Injection System (Thornwood, NY). Cells were allowed to recover for 2 h before adding 5 × 10−6 M nocodazole. After 2 h, nocodazole is washed out, and microtubules are allowed to regrow for 10 min. Cell fixation (using methanol) and immunofluorescence were routinely performed after microtubules regrowth. Injected immunoglobulins were detected with the corresponding secondary antibody. In parallel, microtubules were decorated using a monoclonal antibody directed against α-tubulin.
Results
Cloning the Human Homologue of ScSPC98
One human SPC98-related sequence (No. T55505) was found in dbEST, showing 38% identity and 58% similarity to the yeast protein. We cloned the corresponding full-length cDNA by reverse transcription PCR. Three successive rounds of reverse transcription using internal primers, as described in Fig. 1, were necessary to obtain the 5′ end. We further explored dbEST and GenBank using the full-length cDNA sequence. Some of the human ESTs found in this way are reported in Fig. 1. Surprisingly, we found one EST (No. AA081954) and one sequence already cloned (accession number L13801 L12708) that from now on will be referred to as clone 02, which showed 100% homology to 52 and 78 bp, respectively, with the human cDNA and which were completely divergent in the 3′ region (see Fig. 1). From information obtained from the database, clone 02 is predicted to have a poly-A tail 1.1 kb from the 3′ end of the sequenced region, suggesting a different mRNA than that previously found. Several possibilities could explain this divergence at the 3′ end. To confirm the possibility that the new sequence was related to the previously characterized cDNA, we performed a PCR reaction using the 5′ primers that were already used to isolate the first 3′ end (Fig. 1 A, 1 and 2) and 3′ primers found in clone 02 (Fig. 1 A, rev7 and rev8). We also set up PCR reactions, using primers 1 and 2 and primers rev9 and rev10, to confirm our first 3′ end. Using the two sets of primers, we obtained bands of the expected sizes. We further cloned each of them in Bluescript vector and sequenced them. The sequence obtained for the new 3′ end is 100% homologous on 694 bp in its 5′ region, suggesting the possibility of an alternative splicing. This possibility was further tested using Northern blot analysis (see below). The protein derived from our first cDNA is slightly smaller than the one obtained from the second version. These proteins have predicted molecular masses of 94 and 103.7 kD, respectively, and pI's of 7.85 and 8.35, respectively.
Figure 1.
(A) Cloning strategy for identifying the HsSPC98 full-length sequence. The location of the primers used, as well as the length of the fragments obtained by RACE-PCR, are reported. Some ESTs homologous to the human sequence have been also indicated. (B) Predicted protein sequence for the two major forms of HsSpc98p. The arrowheads in the sequence indicate where the sequences differ. The minor form of the protein is written below the major one. These sequence data for HsSpc98p major form and minor form have been submitted to the EMBL Nucleotide Sequence Database under accession numbers AJ003061 and AJ003062.
Sequence comparison between the human and the budding yeast Spc98p showed that these proteins are poorly conserved (22% identity, 45% similarity). However, the regions of homology are clustered in the central part of the protein (Fig. 2 A). Interestingly, ESTs from three other species, including zebrafish and plant, are located in the same region. The highly conserved domain of Spc98p is present in those different species (Fig. 2 B), suggesting that a conserved function is probably located in this domain. The three ESTs are closer to the human sequence than to the yeast one (see Table I). It is interesting to point out that HsSpc98p also presented homology (22% identity, 46% similarity) with the yeast Spc97p (Fig. 2 A), although the conserved regions are spread over the entire sequence.
Figure 2.

(A) Alignment sequence of HsSpc98p with the yeast Spc98p and Spc97p (indicated as Sc). The underlined sequence corresponds to the NLS in yeast ScSpc98p. (B) Alignment of the conserved sequence of HsSpc98p with ScSpc98p and with mouse (Mm), zebrafish (zf), and rice ESTs. These comparisons suggest that the protein is highly conserved in this small region. Alignments were performed using the Pileup program (Infobiogen, Villejuif, France). Comparisons to ScSpc98p, ScSpc97p and the ESTs were performed using the Boxshade program (Institut Suisse de Recherche Experimentales sur le Cancer, Lausanne, France). Identical amino acids are boxed in black. Conservative changes are boxed in gray.
Table I.
Sequence Comparison of Human and S. cerevisiae Spc98p and EST Derived from Mouse, Zebrafish, and Rice
| ScSpc98 | MmSpc98 | zbSpc98 | riceSpc98 | |||||
|---|---|---|---|---|---|---|---|---|
| HsSpc98 | 22/45 | 98.3/99.4 | 91.6/96.1 | 49/72 | ||||
| ScSpc98 | 100 | 30.7/54.8 | 31/52 | 28/52 | ||||
| MmSpc98 | 100 | 92.9/96.8 | 49.1/72.5 | |||||
| zbSpc98 | 100 | 53/73 | ||||||
| riceSpc98 | 100 |
Percentages of identity and similarity are indicated.
Northern Blot Analysis
To substantiate the possibility of an alternative splicing of the 3′ end region, Northern blot analysis was performed. Three parts of the human sequence were used as probes to screen the Northern blot. The first probe was located in the common region (1084–1921), the second probe corresponded only to the divergent part of clone 02 (bp 80– 645), and the last one was in the second divergent region (bp 2450–2577 of the initial clone). Fig. 3 shows the Northern blot obtained with the first probe. All tissues tested expressed an abundant messenger RNA of 4.4 kb in addition to three minor ones (Fig. 3, bands 1, 2, and 4). The second probe labeled band 1–3, while band 4 was only stained with the third probe (data not shown). These results suggested that the most abundant messenger RNA contained in those tissues has the 3′ end found in clone 02. The two minor forms represented by bands 1 and 2 were probably minor spliced messenger RNAs. Our initial 3′ end represented a minor and shorter spliced messenger RNA that was favored by the RACE-PCR cloning approach.
Cellular Distribution of the Human Spc98p
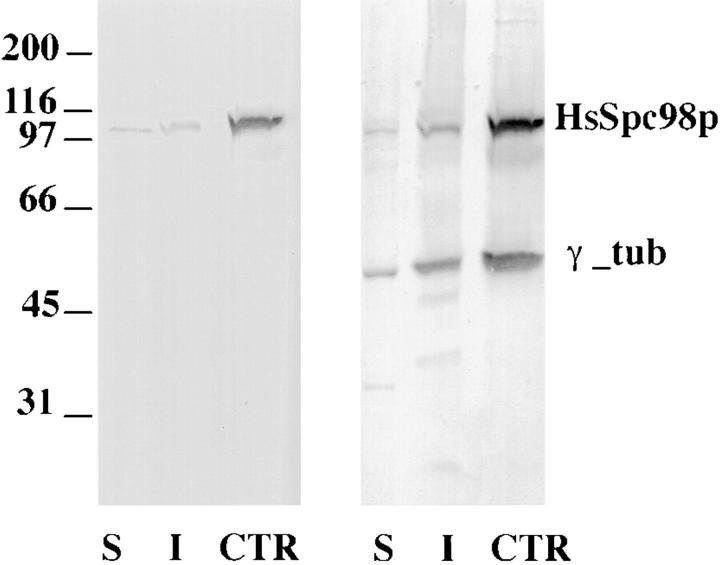
To characterize the human homologue of Spc98p at a cellular and biochemical level, we raised polyclonal antibodies against the most conserved domain of the protein (amino acids 362–673). This peptide was produced in bacteria in fusion with a histidine tag at the COOH terminus and purified on a nickel column before rabbit immunization. Sera strongly reactive with the recombinant peptide were used to analyze the cellular distribution of the protein in human cells after further affinity purification. When Western immunoblotting was carried out on Triton X-100–soluble and -insoluble fractions (10 μg protein each) and centrosomal protein (0.6 μg protein), affinity-purified immunoglobulins reacted in all fractions with a unique band with an apparent molecular mass of 103 kD. This was in good agreement with the predicted size of the most abundant form of the protein. We further observed that HsSpc98p was highly enriched in the centrosomal fraction but that a significant signal was also detected in the Triton X-100–soluble and -insoluble fractions. Such a distribution is similar to that of γ-tubulin (Fig. 4) and was expected for a γ-tubulin–binding protein. Isolated centrosomes can be easily counted by immunofluorescence. As they are single-copy organelles, we could normalize with respect to cell number, the semiquantitative analysis obtained by a National Institutes of Health image program of the signals observed in the soluble, insoluble, and centrosomal fractions. Using our purest centrosome preparation, we could roughly establish that ∼6% of the HsSpc98p was associated with the centrosome, whereas 30 and 70% of the protein were present in the insoluble and soluble fraction respectively.
Figure 4.
Western blot analysis of low-speed, Triton X-100–soluble (S) and -insoluble (I) protein fractions from unsynchronized KE37 cells and of a highly enriched centrosome preparation (CTR). Proteins were probed with affinity-purified HsSpc98p IgG (left). A band at 103 kD is observed in all fractions, while highly enriched in the centrosome fraction. The same blot was subsequently probed with anti–γ-tubulin (right). Note the similar partition of both proteins in all fractions. 10 μg of Triton X-100–soluble and -insoluble proteins representing 2 × 105 and 6 × 105 cells, respectively, and ∼3 × 107 centrosomes were loaded.
Immunolocalization of HsSpc98p
To further characterize the cellular distribution of HsSpc98p, we performed an immunolocalization experiment of HsSpc98p using a double immunofluorescence approach on methanol-fixed HeLa cells with or without Triton X-100 permeabilization. Affinity-purified antibody directed against HsSpc98p showed a centrosomal staining in all cells over a weak and homogenous background (Fig. 5). However, the labeling appeared more restricted to the centrioles than the one observed for pericentriolar material (PCM) with mAb CTR453 (Bailly et al., 1989; see Fig. 5 C). At the G2/M transition, an increase in centrosomal HsSpc98p labeling was observed concomitant with the increase of the microtubule nucleation activity (Fig. 5, B and D). In addition to the centrosome staining, a specific decoration of the polar microtubules in metaphase (Fig. 5 E) and of the midbody during anaphase was observed (Fig. 5 A, inset). All those features were reminiscent of that described for γ-tubulin (Lajoie-Mazenc et al., 1994; Moudjou et al., 1996).
Figure 5.

Double immunostaining of HeLa cells with anti-HsSpc98p affinity-purified antibody and a monoclonal antibody CTR453, which recognizes the PCM observed by confocal microscopy. All documents presented are two-dimensional projections of images collected at all relevant z-axes. (A and B) Anti-HsSpc98p antibody recognizes the centrosome in all cells as demonstrated by the colocalization of both staining. The inset in A shows the accumulation of HsSpc98-positive material at the midbody during anaphase. (C–E) Representative blow ups of cells at different stages during the cell cycle. In G2 cells (C), HsSpc98 staining is restricted to the centrioles, while CTR453 presented in addition of the centrioles an accumulation of PCM. During prophase (D), HsSpc98p accumulates to the centrosome concomitant with the increase of microtubule nucleation activity. During metaphase (E), HsSpc98p antibodies recognize the centrosome as well as the polar microtubules, as described elsewhere for γ-tubulin. Bars, 10 μm.
When we attempted to compare the localization of γ-tubulin and HsSpc98p at the ultrastructural level, we realized that the epitopes recognized by anti-Spc98p and anti–γ-tubulin antibodies were either destroyed or occluded after aldehyde fixation, thus precluding postembedding immunolocalization as carried out previously (Moudjou et al., 1996). Preembedding staining has several drawbacks including a differential accessibility of antigens depending on their location, the most peripheral being preferentially recognized. We thus attempted a compromise by using preembedding staining after methanol fixation. Using this approach, we observed reasonably well preserved centrosomes (Fig. 6) in which HsSpc98p and γ-tubulin had similar localization, as expected for interacting proteins: in both cases, gold particles were observed within the pericentriolar material (PCM), sometimes close to the centriole wall. It is interesting to note that both HsSpc98p and γ-tubulin seemed to be present at the tip of subdistal appendages surrounding the mother centriole. No gold particles were observed when the first antibody was omitted.
Figure 6.
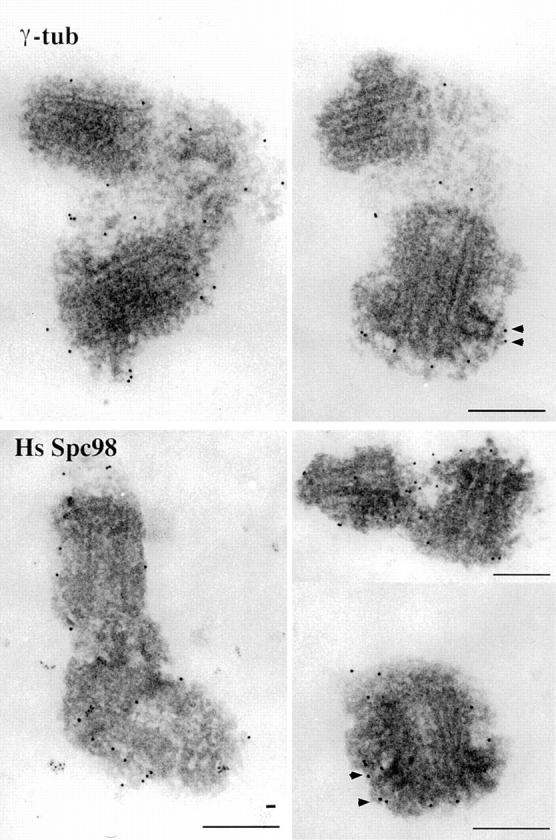
Preembedding immunostaining with affinity-purified anti-Spc98p or anti–γ-tubulin antibodies on isolated centrosomes after a methanol fixation. Note that gold particles are associated with the PCM close to the centrioles regardless of the antibody. Note also the particles at the tip of the subdistal appendages (arrowheads). Bars, 200 μm.
HsSpc98p and γ-Tubulin Belong to the Same Cytosolic Complex
The immunofluorescence localization of HsSpc98p and the cell fractionation suggested a possible interaction of HsSpc98p with γ-tubulin in both the cytosolic and the centrosomal fractions. Biochemical experiments were undertaken to test this hypothesis. We first focused our attention on the cytosolic form of HsSpc98p and γ-tubulin. It has been previously reported that γ-tubulin from somatic cells is present in a cytosol fraction obtained by mechanical breaking of cells in the absence of detergent and subsequent high-speed centrifugation (Moudjou et al., 1996). We checked for the presence of HsSpc98p in those cytosolic fractions after sedimenting them for 16 h at 100,000 g on a 15–40% sucrose gradient. HsSpc98p cosedimented with γ-tubulin (data not shown). To improve the resolution of those gradients, the γ-tubulin–rich fractions were loaded on a second sucrose gradient. Interestingly, γ-tubulin and HsSpc98p still fully cosedimented (Fig. 7 A).
Figure 7.
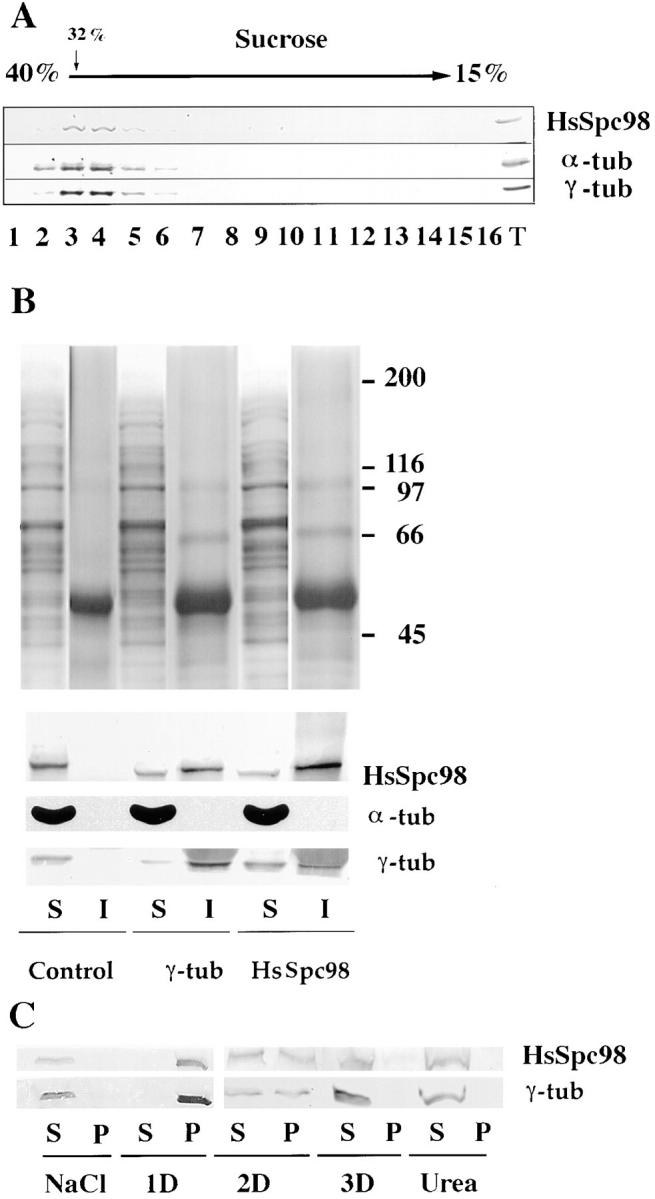
The cytosolic as well as the centrosomal form of HsSpc98p are associated with γ-tubulin. (A) G1/S cytosol from HeLa cells are loaded on a 15–40% sucrose gradient (10 ml in SW41 tubes) and centrifuged at 100,000 g for 16 h. The γ-tubulin– rich fractions were reloaded on the top of another small 15–40% sucrose gradient (4 ml in SW55 tubes). HsSpc98p cosediments with γ-tubulin as well as with α-tubulin. (B) Immunoprecipitation experiments of the γ-tubulin–rich fractions obtained from sucrose gradient as presented in A with preimmune (Control), anti– γ-tubulin (γ-tub), and anti-HsSpc98p (HsSpc98) immunoglobulins coupled to protein G beads. The proteins precipitated with the beads are indicated as “I,” while the proteins not associated with the beads are referred as “S.” The top figure shows the silver staining of the immunoprecipitate. Note that no proteins are detected with the control beads and that anti–γ-tubulin or anti-HsSPc98p antibodies precipitate the same proteins. The lower part shows the immunostaining of the different fractions with γ-tubulin, α-tubulin, and HsSpc98 antibodies. Anti–γ-tubulin as well as anti-HsSpc98 immunoglobulins precipitate both γ-tubulin and HsSpc98p. However, no α-tubulin is detected in the immunoprecipitates, suggesting that α-tubulin is not part of the complex. (C) Biochemical extraction of the centrosomal HsSpc98p. Soluble (S) and insoluble (P) centrosomal protein fractions obtained in different extraction conditions (NaCl, 1 M; 1D, 0.5% NP-40; 2D, 0.5% NP-40 and 0.5% deoxycholate; 3D, 0.5% NP-40, 0.5% deoxycholate, and 0.1% SDS; Urea, 8 M) were immunodetected with anti–γ-tubulin or anti-HsSpc98p antibodies. Note that the same extraction conditions are necessary to solubilize both γ-tubulin and HsSpc98p. Observe in 2D buffer that 50% of the protein is soluble while 50% is insoluble.
In an attempt to directly demonstrate the association of γ-tubulin and HsSpc98p in a complex, we carried out immunoprecipitation experiments on the pooled sucrose gradient fractions. Silver staining of the immunoprecipitates using anti–γ-tubulin or anti-HsSpc98p showed a similar protein profile in which one could recognize some of the γ-TurC proteins (Zheng, Y., D. Agard, R. Milligan, T. Mitchison, and B. Alberts. 1996. Mol. Biol. Cell. 207a). In control experiments with preimmune immunoglobulins, neither HsSpc98p nor γ-tubulin was immunoprecipitated as determined by Western blotting. However, anti-HsSpc98 as well as anti–γ-tubulin antibodies coprecipitated HsSpc98p and γ-tubulin, demonstrating the participation of both proteins to the same complex (Fig. 7 B). α–β-tubulin, which was present in the same gradient fractions, was not at a detectable level in the immunoprecipitate obtained with either anti-HsSpc98p or anti–γ-tubulin immunoglobulins. By contrast, immunoprecipitation carried out with anti–α-tubulin antibodies precipitated a different protein profile as judged by silver staining (data not shown). In that case, however, both γ-tubulin and HsSpc98p, although in a low amount, were present in addition to α-tubulin (data not shown).
The Centrosomal Forms of HsSpc98p and γ-Tubulin
We were interested to ascertain whether both proteins participated in the same complex in the centrosome as well. However, it is difficult to carry out coimmunoprecipitation experiments from centrosome preparations that require extreme dissociating conditions to be partially solubilized (Moudjou et al., 1996). These conditions are incompatible with the stability of most protein–protein interactions. Therefore, we investigated the interaction of both proteins in the centrosome by analyzing their behavior during centrosomal fractionation under dissociating conditions. Isolated centrosomes were incubated in different solubilizing buffers including salts, detergents, and chaotropic agents such as urea. Whatever the dissociating agent used, both proteins behaved in a similar manner, suggesting that they had similar solubility properties (Fig. 7 C). Interestingly, when a partial solubilization was produced by treatment with detergents, the proportion that was solubilized appeared to be the same for both proteins. These results suggest that about half of both proteins are not very tightly associated to the centrosome, whereas the rest are more tightly bound.
HsSpc98p as well as γ-Tubulin Are Present in the Inactive Centrosome from Xenopus Sperm Cells
To understand the function of Spc98p, we first ask whether this protein could act as an anchoring protein of γ-tubulin on the centrosome. To address this question, we looked at whether Spc98p was associated with centrosomes incompetent for microtubule nucleation, i.e., to those present in Xenopus sperm cells, which have been reported to be devoid of γ-tubulin (Felix et al., 1994; Stearns and Kirschner, 1994). Our anti-HsSpc98p antibody was expected to react with the Xenopus Spc98p homologue, as it is directed against the most conserved part of the protein (Fig. 2 B), which is also conserved in the Xenopus protein as recently reported by Gunawardane and Zheng (1997. Mol. Biol. Cell. 8:1553a). In agreement with this prediction, Xenopus egg extracts were shown to contain a protein reacting with our anti-HsSpc98p and migrating at the expected molecular weight. Therefore, a double immunofluorescence was performed on Xenopus sperm heads using affinity-purified anti-HsSpc98p antibody and the monoclonal antibody GT335 directed against the polyglutamylated forms of tubulin (Wolff et al., 1992). GT335 distinctly stained the two centrioles and a piece of axoneme attached to the distal centriole (Fig. 8, F and I). Anti-Spc98p antibody showed a distinct labeling surrounding each centriole with elongated X-shaped filaments corresponding to striated rootlets observed at the electron microscopy level (Fig. 8 H). This result suggested that Spc98p could act as an anchoring protein for γ-tubulin. We attempted to confirm the absence of γ-tubulin in Xenopus sperm centrosomes. However, we observed that anti–γ-tubulin antibodies stained the Xenopus sperm centrosome in the absence of egg extract, in a manner strikingly similar to that observed with anti-Spc98p antibodies (Fig. 8, A and E). To fully demonstrate the presence of Spc98p and γ-tubulin in Xenopus sperm heads, 5 × 105 sperm heads and 5 × 105 centrosomes from KE37 cells were analyzed by SDS-PAGE and immunoblotting. Interestingly, similar amounts of Spc98p and of γ-tubulin were revealed in the centrosomes from sperm and KE37 cells (Fig. 8 K). A similar observation was made for Cen3p (Middendorp et al., 1997), which we used as an internal control (see Fig. 8 K). We thus conclude that Spc98p codistributes with γ-tubulin in sperm centrosomes also.
Figure 8.
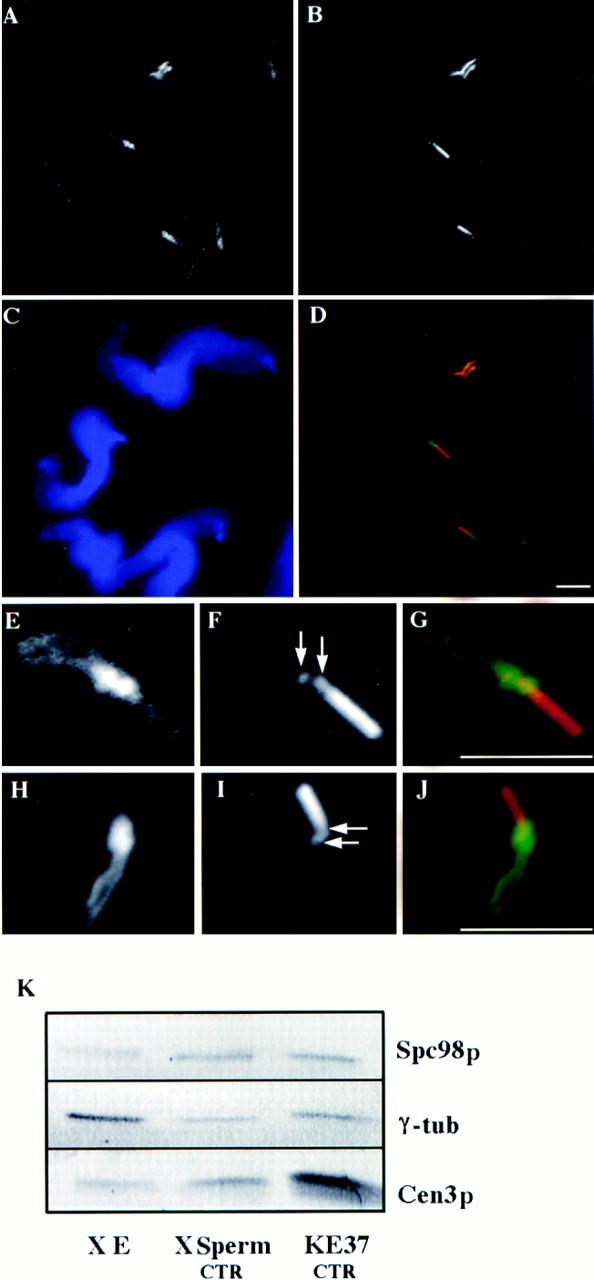
(A–J) Decoration of Xenopus sperm centrosome before activation in Xenopus egg extract. Double labeling with either anti–γ-tubulin antibodies (A and E) or with anti-HsSpc98p (H) and with GT335, a monoclonal antibody recognizing polyglutamylated tubulin (B, F, and I; arrows point to the centrioles). D, G, and J are the superposition of the respective labelings. (C) DAPI staining of the sperm nuclei. (A–D) A field of Xenopus sperm heads. (E–J) High magnification of a single sperm head. Note that both γ-tubulin and Spc98p accumulate around the two centrioles and along the striated rootlets. (K) Western blot analysis of Xenopus egg extract (X E), Xenopus sperm centrosomes (X Sperm CTR), and human somatic centrosomes (KE37 CTR). Proteins were probed with affinity-purified HsSpc98p IgG, anti–γ-tubulin antibody, or anti-HsCen3p antibody. Note the presence of similar amounts of both Spc98p and γ-tubulin in Xenopus sperm centrosomes and human somatic centrosomes. Bars, 5 μm.
Anti-HsSpc98p Immunoglobulins Inhibit Microtubule Nucleation by Somatic Centrosomes
We further tested the involvement of HsSpc98p in microtubule nucleation using an in vitro nucleation assay. Therefore, isolated human centrosomes were preincubated with the affinity-purified HsSpc98p immunoglobulins (1.2 mg/ml) on ice, while control centrosomes were resuspended with PBS or control immunoglobulins for the same time period. Then, phosphocellulose-tubulin was added together with a GTP-containing buffer, and microtubules were allowed to polymerize for 4 min at 37°C. Centrosomes and microtubules were fixed and processed for double immunofluorescence using antitubulin and anti-PCM antibodies. Most centrosomes nucleated a microtubule aster in the control experiment (Fig. 9), whereas only one or two short microtubules were nucleated from centrosomes preincubated with anti-HsSpc98p IgG (Fig. 9). To confirm this result in vivo, we microinjected purified anti-HsSpc98p immunoglobulins into HeLa cells. After 2 h of cell recovery, microtubules were depolymerized for 2 h by nocodazole and allowed to regrow for 10 min. Cells were fixed and processed for immunofluorescence using anti–α-tubulin. The microinjected cells were visualized using an anti–rabbit immunoglobulin antibody. In cells heavily loaded with IgGs (Fig. 9, bottom, arrows), a few or no microtubules were observed, while noninjected cells had a centrosome-growing aster of microtubules. The situation was not so clear when cells were injected with less antibody: Some cells presented no microtubule, while others possessed a normal microtubule network. These results obtained in vitro as well as in vivo argue for the involvement of HsSpc98 in the microtubule nucleation reaction. The control for the microinjection has been performed using another rabbit antibody that recognizes a centrosomal protein (anti-Cen3p). This protein is probably not involved in microtubule nucleation indeed, but injecting this antibody represents a good technical control for our experiment. In all microinjected cells, the microtubule regrowth was similar to nonmicroinjected cells.
Figure 9.
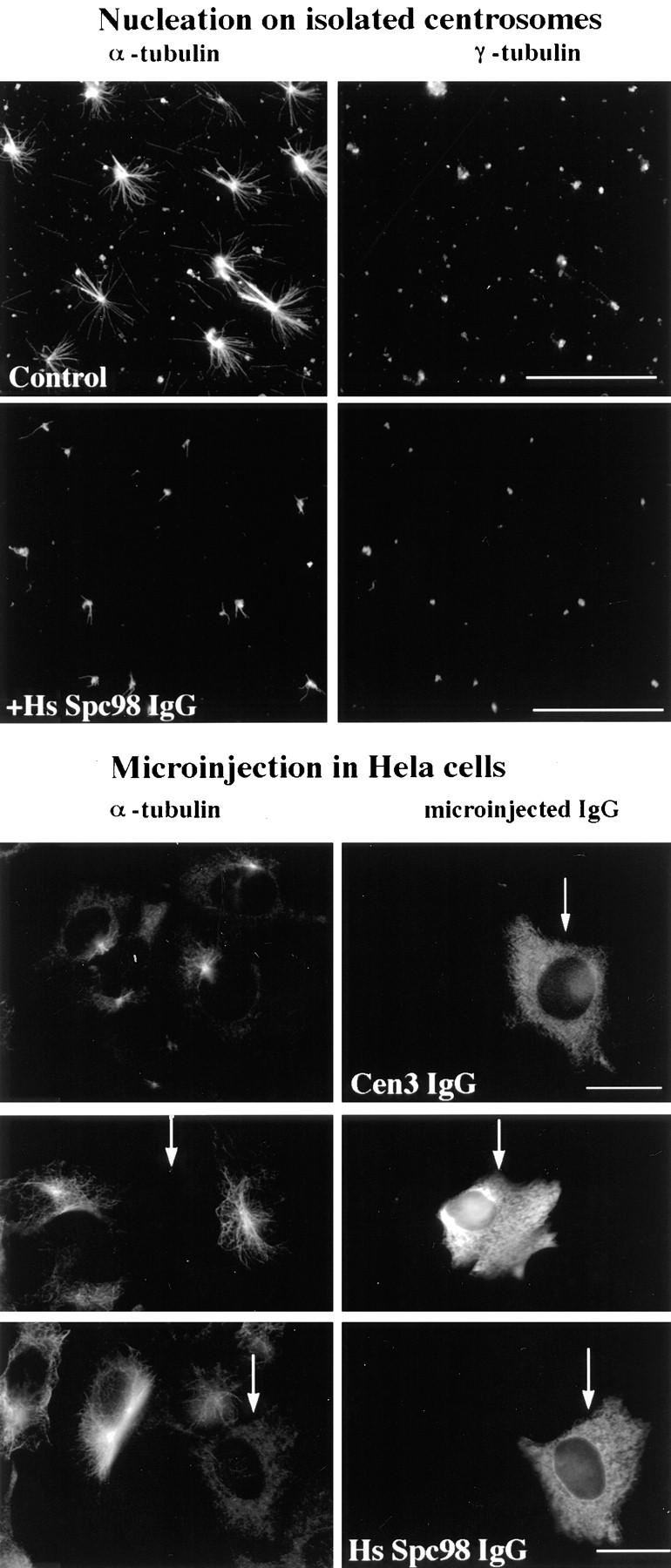
Affinity-purified anti-HsSpc98p antibodies inhibit microtubule nucleation in vitro (top) and in vivo (bottom). (Top) Microtubule nucleating activity of isolated centrosomes was monitored by double immunofluorescence with anti–α-tubulin and with anti–γ-tubulin. Centrosomes were preincubated with preimmune immunoglobulins (Control) or with anti-HsSpc98p immunoglobulins (+HsSpc98 IgG) on ice for 30 min and subsequently incubated in PC-tubulin. Microtubules were allowed to grow for 4 min. Note the specific inhibition of aster formation after 4 min of growth with HsSpc98 immunoglobulins, while the control centrosomes were growing typical microtubule asters. (Bottom) Anti-HsSpc98 immunoglobulins were microinjected into HeLa cells (HsSpc98 IgG). 2 h after microinjection, microtubules were depolymerized for 2 h with 5 × 10−6 M nocodazole. After washing out the nocodazole, microtubules were allowed to regrow for 10 min. Cells were fixed with methanol and processed for immunofluorescence with monoclonal anti–α-tubulin antibodies followed by the mouse and rabbit secondary antibodies. In this way, only the microinjected cells are detected with the fluorescein anti–rabbit secondary antibody. Note that the nonmicroinjected cells presented the usual centrosome-growing microtubule aster, while heavily microinjected cells did not show any microtubule (arrows). Note that in cells micronjected with an unrelated antibody (anti-Cen3p IgG), microtubule regrowth is not affected. Bars, 10 μm.
Discussion
Our aim was to obtain a molecular characterization of γ-tubulin–interacting proteins in mammalian cells. The discovery of the Saccharomyces cerevisiae γ-tubulin gene, TUB4, by the sequencing genome program coupled with the powerful genetic approach in this system has led to the identification of a functional trimeric complex containing two SPB components, namely Spc98p and Spc97p, in addition to Tub4p. These three proteins have been localized to the inner and outer plates of the SPB, which appear to be the site of microtubule nucleation. We report the molecular characterization of the human homologue of the yeast Spc98p. This has been achieved by searching an EST presenting homology with the yeast Spc98p and by isolating the human full-length sequence by reverse transcription PCR. By comparing the obtained sequence with dbEST and GenBank, we identified a 3′ divergent sequence, suggesting the existence of alternative spliced mRNA.
Comparison of the human sequence with the yeast sequence shows that Spc98p is poorly conserved (22% identity), although a conserved central domain is found in the two proteins. The conservation of this domain is likely to be functionally significant because ESTs from divergent species located in the central domain show high homology with the human sequence. It does not come as a surprise that γ-tubulin–binding proteins are more divergent in yeast, since TUB4 itself is the most divergent γ-tubulin gene among species. It will be interesting to isolate the full-length SPC98 cDNA from other species to appreciate the level of protein conservation during evolution. The presence of a central conserved domain in HsSpc98p suggests that the γ-tubulin–binding domain is probably located in this region. It is interesting to note that HsSpc98p, like yeast Spc98p, has also some homology with the yeast ScSpc97p. This result suggests that the two yeast genes SPC97 and SPC98 might be derived from a common ancestor.
It is noteworthy that proteins involved in microtubule nucleation appear less conserved between human and budding yeast than are proteins involved in centrosome duplication (see Middendorp et al., 1997). This would suggest that centrosome duplication is under more evolutive constraint than microtubule nucleation.
HsSpc98p Is a γ-Tubulin–binding Protein
The strategy we used to isolate the human homologue of the yeast Spc98p assumed that HsSpc98p would be a γ-tubulin–binding protein as its yeast homologue. As expected, HsSpc98p was shown to have exactly the same cellular localization and the same fate as γ-tubulin during cell fractionation. Both proteins are concentrated at the centrosome throughout the cell cycle. Like γ-tubulin, HsSpc98p is localized to the polar microtubules in addition to the centrosome during metaphase. It is interesting to note that the NLS consensus sequence (Dingwall and Laskey, 1991) observed in yeast Spc98p (amino acids 580–595; Knop et al., 1997) is modified in the human protein. This sequence may not be functional since we did not observe significant nuclear staining, although, depending on the fixation procedure, some nuclear decoration could be detected. At the ultrastructural level, using preembedding immunostaining after methanol fixation, both proteins appear colocalized in the PCM, close to the centrioles. This type of fixation is not the best for ultrastructural preservation but permits a good accessibility to the antigens: We could observe, for example, that the tip of the subdistal appendages were decorated with both anti–γ-tubulin and anti-HsSpc98p antibodies. This result was not obtained previously using aldehyde fixation (see Moudjou et al., 1996). These appendages could be sites where microtubules grow (Chrétien et al., 1997).
Like γ-tubulin, a large pool of HsSpc98p is not associated with the centrosome. The partition of both proteins in the different fractions are strikingly similar, suggesting that both proteins participate in a complex. This has been demonstrated by sucrose gradient sedimentation and by coimmunoprecipitation. Both anti–γ-tubulin and anti-HsSpc98p antibodies were found to coprecipitate a similar complex as judged by silver staining. It is noteworthy that the pattern was reminiscent of the γ-TuRC described by Zheng et al. (1995) in Xenopus eggs, or Détraves et al. (1997) in sheep brain. Interestingly, α–β-tubulin did not participate in this complex, although it was present in significant amount in the density gradient fractions used to perform immunoprecipitation experiments (see Fig. 7 A). When immunoprecipitation was performed with anti– α-tubulin, the immunoprecipitate showed a very distinct protein profile, as observed by silver staining, and contained a slight amount of both γ-tubulin and HsSpc98p only (data not shown; see also Moudjou et al., 1996). These results suggest that different α-, β-, γ-tubulin– and HsSpc98p-containing complexes can exist in the cell with similar sedimentation properties (Melki et al., 1993; Archer et al., 1995; Tian et al., 1996, 1997; Geissler et al., 1998). Several possibilities could explain the fact that α–β-tubulin was not found in the immunoprecipitate obtained by anti– γ-tubulin or anti-HsSpc98p antibodies: complexes without α–β-tubulin are the most abundant within the cell, and/or γ-tubulin and HsSpc98p are not accessible in the α–β-tubulin–containing complex. Alternatively, a partial dissociation of the complex could take place.
The significance of such a cytosolic pool of γ-tubulin– and HsSpc98p-containing complexes in somatic cells is an important question. One possibility is suggested by recent reports that have significantly modified the current view on microtubule network turnover: many microtubules, and in some cells the majority of them (up to 75%), can be free in the cytoplasm, because of either their release from the centrosome, their breaking, or their spontaneous assembly (Keating et al., 1997; Rodionov and Borisy, 1997; Vorobjev et al., 1997; Waterman-Storer and Salmon, 1997b ; Yvon and Wadsworth, 1997; for review see Waterman-Storer and Salmon, 1997a ). In many cases, the minus end of these free microtubules can be stable for long periods of time, or can disassemble, generating treadmilling. This suggests the presence of minus end regulatory factors. γ-tubulin– and Spc98p-containing cytosolic complexes are indeed candidates for such a role, as already suggested by others (Vorobjev et al., 1997; Waterman-Storer and Salmon, 1997a ). This could also explain why we find complexes free of α–β-tubulin, and others containing α–β-tubulin, which could represent the minus end–terminal subunits of free microtubules associated with the complexes.
Does HsSpc98p Participate in the Microtubule Nucleation Reaction?
This work demonstrates that although individual sequences for γ-tubulin and Spc98p are divergent between yeast and human, the complexes formed by the two proteins are maintained during the evolution. Homologues of Spc98p have also been isolated in Xenopus and Drosophila (Wiesc, C., O. Martin, and Y. Zheng. ASCB/ EMBO/H. Dudley Wright Conference on Centrosome and Spindle Pole Bodies. Santa Cruz, CA). Antibodies directed against the yeast Spc98p recognize the nucleus-associated body from Dictyostelium discoideum in immunofluorescence experiments (Euteneuer, U., personal communication). HsSpc98p could play a structural role defining a ring structure to which γ-tubulin is binding (Zheng et al., 1995), or it could play a more direct role in the microtubule nucleation reaction. Our results show that in vitro as well as in vivo, antibodies directed against HsSpc98p inhibit the microtubule nucleation reaction or interfere with the stability of the microtubules, resulting in an absence of microtubule growth. However, these experiments do not permit us to distinguish between these two potential functions of HsSpc98p. It is interesting to point out that in yeast, the analysis of the phenotype of both tub4-1 and spc98-1 mutants (absence of a mitotic spindle under restrictive temperature) suggests that both proteins play an essential role in mitotic spindle formation.
Regulation of the Nucleation Reaction and the Centrosome Inheritance
The presence of a large cytosolic pool of HsSpc98p and of γ-tubulin contrasts with the fact that the nucleation reaction is restricted to the centrosome. As emphasized previously (see for example Oakley, 1995), this suggests that a regulatory event is able to turn on and off the nucleation reaction. A naive view would propose that an important parameter of this regulation is the local concentration of the components participating in the nucleating complex. In other words, a critical concentration of γ-tubulin and of γ-tubulin–interacting proteins should be reached before any microtubule nucleation can take place. This might be necessary but is not sufficient because we have shown in this work that inactive centrosomes from Xenopus sperm cells contain comparable amounts of Spc98p and γ-tubulin to active human somatic centrosomes. The main differences with previous works (Felix et al., 1994; Stearns and Kirschner, 1994) are probably the affinity of the anti–γ-tubulin antibody used, the number of sperm heads analyzed, and more importantly, that an equivalent number of active centrosomes have been used as an internal standard. Recent studies on human or frog sperm cells has also reported the presence of γ-tubulin (Navara et al., 1997; Merdes and Cleveland, 1998). We thus conclude that a specific regulatory event must take place at fertilization to turn on the nucleating activity of the sperm centrosome.
It has been proposed that the essential step of the centrosome activation reaction in Xenopus eggs is the γ-tubulin recruitment from egg cytoplasm (Felix et al., 1994; Stearns and Kirschner, 1994). Our data do not eliminate the possibility that γ-tubulin–containing complexes are recruited at the centrosome, but this cannot be the limiting step. This view is reminiscent of the conclusions of Masuda and Shibata (1996), who showed that heterologous interphase centrosomes from Schizosaccharomyces pombe, which do contain γ-tubulin but are inactive, can be activated by a Xenopus mitotic extract fraction that does not contain γ-tubulin.
Our results also call for a reappraisal of centrosome inheritance during fertilization. Felix et al. (1994) as well as Stearns and Kirschner (1994) have put forward the idea that the centrosome is assembled at fertilization by a sort of complementation in which the centriole pair, inherited from the male gamete, binds nucleating components from the female gamete. Since sperm centrosomes, although inactive for microtubule nucleation, do not differ from active somatic centrosomes with respect to the presence of both Spc98p and of γ-tubulin (this work), and since they apparently possess all the other centrosomal components that have been looked at so far, such as centrin (this work), CTR2611 antigen (Felix et al., 1994), and Pericentrin (Doxsey et al., 1994; Stearns and Kirschner, 1994), we propose to return to the more classical view, according to which the centrosome organelle, and not simply part of it, is paternally inherited, and that fertilization triggers a switch in its activity.
In conclusion, we report the molecular characterization of a γ-tubulin–binding protein in mammalian cells and its involvement in the microtubule nucleation reaction. The precise function of this protein in the microtubule nucleation reaction will have to be further characterized. Many questions are still to be answered, the most important one being the definition of the real nucleation complex, which could be either the trimeric complex, as observed in yeast and Drosophila, or the large γ-TuRC. The function of the other proteins in the complex is another important issue. The molecular characterization of all proteins of the γ-TuRC complex will be necessary to understand the microtubule nucleation mechanism. How this complex is anchored to the centrosome remains unknown. Our results concerning the extractibility of both γ-tubulin and HsSpc98p from the centrosome suggest that at least part of the complex is not tightly bound to the centrosome. In light of the data reported in yeast (Knop and Schiebel, 1997), a possible candidate as an anchoring protein could be the human homologue of the yeast Spc110p (Tassin et al., 1997). The precise function of HsSpc98p in the microtubule nucleation reaction will require further experiments. Mapping the interaction of HsSpc98p with γ-tubulin might be rewarding.
Acknowledgments
We thank Dr. E. Schiebel for providing us the yeast Spc98p and Spc97p sequence before their publication and for enlightening discussions. We thank Dr. M. Paintrand for performing the electron microscopy experiments. We gratefully acknowledge N. Bordes for her help in the immunoprecipitation experiments and for the affinity purification of the antibodies, A. Echard for his help in the use of the alignment program, S. Middendorpp for discussions all along this work, and M. Piel for teaching us cell microinjections. We thank V. Doye, A. Rousselet, and S. Holmes for helpful comments and critical reading of the manuscript. S. Holmes and J. Mogg are thanked for the English version of the manuscript. We thank Daniel Meur for the art work.
This work has been supported by the CNRS (UMR144) and by Institut Curie.
Abbreviations used in this paper
- EST
expressed sequence tag
- PCM
pericentriolar material
- RACE
rapid amplification of cDNA ends
- SPB
spindle pole body
- γ-TuRC
γ-tubulin ring complex
Footnotes
Address all correspondence to Anne-Marie Tassin, Institut Curie, Section Recherche, UMR 144 du Centre National de la Recherche Scientifique (CNRS), 26 rue d'Ulm, 75248 Paris Cedex 05, France. Tel.: (33) 1 42346403. Fax: (33) 1 42346421. E-mail: atassin@curie.fr
References
- Archer J, Vega LR, Solomon F. Rbl2p, a yeast protein that binds to β-tubulin and participates in microtubule function in vivo. Cell. 1995;82:425–434. doi: 10.1016/0092-8674(95)90431-x. [DOI] [PubMed] [Google Scholar]
- Bailly E, Doree M, Nurse P, Bornens M. p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO (Eur Mol Biol Organ) J. 1989;8:3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrétien D, Buendia B, Fuller DF, Karsenti E. Reconstruction of the centrosome cycle from cryoelectron micrographs. J Struct Biol. 1997;120:117–133. doi: 10.1006/jsbi.1997.3928. [DOI] [PubMed] [Google Scholar]
- Détraves C, Mazarguil H, Lajoie-Mazenc I, Julian M, Raynaud-Messina B, Wright M. Protein complexes containing γ-tubulin are present in mammalian brain microtubule protein preparations. Cell Motil Cytoskel. 1997;36:179–189. doi: 10.1002/(SICI)1097-0169(1997)36:2<179::AID-CM7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dingwall, C., and R.A. Laskey. 1991. Nuclear targeting sequences—a consensus? Trends Biochem. Sci. 478–481. [DOI] [PubMed]
- Doxsey SJ, Stein P, Evans L, Calarco PD, Kirschner M. Pericentrin, a highly conserved centrosome protein involved in microtubule organization [see comments] Cell. 1994;76:639–650. doi: 10.1016/0092-8674(94)90504-5. [DOI] [PubMed] [Google Scholar]
- Erickson HP, Stoffler D. Protofilaments and rings, two conformations of the tubulin family conserved from bacterial FtsZ to α/β and γ tubulin. J Cell Biol. 1996;135:5–8. doi: 10.1083/jcb.135.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix MA, Antony C, Wright M, Maro B. Centrosome assembly in vitro: role of γ-tubulin recruitment in Xenopussperm aster formation. J Cell Biol. 1994;124:19–31. doi: 10.1083/jcb.124.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs U, Moepps B, Maucher HP, Schraudolf H. Isolation, characterization and sequence of a cDNA encoding γ-tubulin from the fern Anemia phyllitidisL Sw. Plant Mol Biol. 1993;23:595–603. doi: 10.1007/BF00019306. [DOI] [PubMed] [Google Scholar]
- Geissler S, Pereira G, Spang A, Knop M, Soues S, Kilmartin J, Schiebel E. The spindle pole body component Spc98p interacts with the γ-tubulin-like Tub4p of Saccharomyces cerevisiaeat the sites of microtubule attachment. EMBO (Eur Mol Biol Organ) J. 1996;15:3899–3911. [PMC free article] [PubMed] [Google Scholar]
- Geissler S, Siegers K, Schiebel E. A novel protein complex promoting formation of functional α- and γ-tubulin. EMBO (Eur Mol Biol Organ) J. 1998;17:952–966. doi: 10.1093/emboj/17.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann SR, McIntosh JR. Visualization of the structural polarity of microtubules. Nature. 1980;286:517–519. doi: 10.1038/286517a0. [DOI] [PubMed] [Google Scholar]
- Horio T, Uzawa S, Jung MK, Oakley BR, Tanaka K, Yanagida M. The fission yeast γ-tubulin is essential for mitosis and is localized at microtubule organizing centers. J Cell Sci. 1991;99:693–700. doi: 10.1242/jcs.99.4.693. [DOI] [PubMed] [Google Scholar]
- Joshi HC, Palacios MJ, McNamara L, Cleveland DW. γ-Tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature. 1992;356:80–83. doi: 10.1038/356080a0. [DOI] [PubMed] [Google Scholar]
- Keating TJ, Peloquin JG, Rodionov VI, Momcilovic D, Borisy GG. Microtubule release from the centrosome. Proc Natl Acad Sci USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M, Schiebel E. Spc98p and Spc97p of the yeast γ-tubulin complex mediate binding to the spindle pole body via their interaction with Spc110p. EMBO (Eur Mol Biol Organ) J. 1997;16:6985–6995. doi: 10.1093/emboj/16.23.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M, Pereira G, Geissler S, Grein K, Schiebel E. The spindle pole body component Spc97p interacts with the γ-tubulin of Saccharomyces cerevisiaeand functions in microtubule organization and spindle pole body duplication. EMBO (Eur Mol Biol Organ) J. 1997;16:1550–1564. doi: 10.1093/emboj/16.7.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komesli S, Tournier F, Paintrand M, Margolis R, Job D, Bornens M. Mass isolation of calf thymus centrosomes: identification of a specific configuration. J Cell Biol. 1989;109:2869–2878. doi: 10.1083/jcb.109.6.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lajoie-Mazenc I, Tollon Y, Detraves C, Julian M, Moisand A, Gueth-Hallonet C, Debec A, Salles-Passador I, Puget A, Mazarguil H, et al. Recruitment of antigenic γ-tubulin during mitosis in animal cells: presence of γ-tubulin in the mitotic spindle. J Cell Sci. 1994;107:2825–2837. doi: 10.1242/jcs.107.10.2825. [DOI] [PubMed] [Google Scholar]
- Maessen S, Wesseling JG, Smits MA, Konings RNH, Schoemakers JGG. The γ-tubulin gene of the malaria parasite Plasmodium falciparum. . Mol Biochem Parasitol. 1993;60:27–36. doi: 10.1016/0166-6851(93)90025-s. [DOI] [PubMed] [Google Scholar]
- Marschall LG, Jeng RL, Mulholland J, Stearns T. Analysis of Tub4p, a yeast γ-tubulin–like protein: implications for microtubule-organizing center function. J Cell Biol. 1996;134:443–454. doi: 10.1083/jcb.134.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Shibata T. Role of γ-tubulin in mitosis-specific microtubule nucleation from the Schizosaccharomyces pombespindle pole body. J Cell Sci. 1996;109:165–177. doi: 10.1242/jcs.109.1.165. [DOI] [PubMed] [Google Scholar]
- Melki R, Vainberg IE, Chow RL, Cowan NJ. Chaperonin-mediated folding of vertebrate actin-related protein and γ-tubulin. J Cell Biol. 1993;122:1301–1310. doi: 10.1083/jcb.122.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A, Cleveland DW. The role of NuMA in the interphase nucleus. J Cell Sci. 1998;111:71–79. doi: 10.1242/jcs.111.1.71. [DOI] [PubMed] [Google Scholar]
- Middendorp S, Paoletti A, Schiebel E, Bornens M. Identification of a new mammalian centrin gene, more closely related to Saccharomyces cerevisiaeCDC31 gene. Proc Natl Acad Sci USA. 1997;94:9141–9146. doi: 10.1073/pnas.94.17.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984;312:232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Fung JC, Sedat JW, Alberts BM, Agard DA. Three-dimensional structural characterization of centrosomes from early Drosophilaembryos. J Cell Biol. 1995a;130:1149–1159. doi: 10.1083/jcb.130.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Sedat JW, Alberts BM, Agard DA. Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature. 1995b;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Moudjou, M., and M. Bornens. 1994. Isolation of centrosomes from cultured animal cells. In Cell Biology: A Laboratory Handbook. J.E. Celis, editor. Academic Press, New York. 595–604.
- Moudjou M, Bordes N, Paintrand M, Bornens M. γ-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J Cell Sci. 1996;109:875–887. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- Navara CS, Hewitson LC, Simerly CR, Sutovsky P, Schatten G. The implications of a paternally derived centrosome during human fertilization: consequences for reproduction and the treatment of male factor infertility. Am J Reprod Immunol. 1997;37:39–49. doi: 10.1111/j.1600-0897.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Oakley BR. Cell biology. A nice ring to the centrosome [news] Nature. 1995;378:555–556. doi: 10.1038/378555a0. [DOI] [PubMed] [Google Scholar]
- Oakley BR, Oakley CE, Yoon Y, Jung MK. γ-Tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. . Cell. 1990;61:1289–1301. doi: 10.1016/0092-8674(90)90693-9. [DOI] [PubMed] [Google Scholar]
- Oakley CE, Oakley BR. Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. . Nature. 1989;338:662–664. doi: 10.1038/338662a0. [DOI] [PubMed] [Google Scholar]
- Raff JW, Kellog DR, Alberts BM. Drosophilaγ-tubulin is part of a complex containing two previously identified centrosomal MAPs. J Cell Biol. 1993;121:823–835. doi: 10.1083/jcb.121.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov VI, Borisy GG. Microtubule treadmilling in vivo. Science. 1997;275:215–218. doi: 10.1126/science.275.5297.215. [DOI] [PubMed] [Google Scholar]
- Shu HB, Joshi HC. γ-Tubulin can both nucleate microtubule assembly and self-assemble into novel tubular structures in mammalian cells. J Cell Biol. 1995;130:1137–1147. doi: 10.1083/jcb.130.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel SG, Snyder M. A highly divergent γ-tubulin gene is essential for cell growth and proper microtubule organization in Saccharomyces cerevisiae. . J Cell Biol. 1995;131:1775–1788. doi: 10.1083/jcb.131.6.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A, Geissler S, Grein K, Schiebel E. γ-Tubulin–like Tub4p of Saccharomyces cerevisiaeis associated with the spindle pole body substructures that organize microtubules and is required for mitotic spindle formation. J Cell Biol. 1996;134:429–441. doi: 10.1083/jcb.134.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T, Kirschner M. In vitro reconstitution of centrosome assembly and function: the central role of γ-tubulin [see comments] Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Stearns T, Evans L, Kirschner M. γ-Tubulin is a highly conserved component of the centrosome. Cell. 1991;65:825–836. doi: 10.1016/0092-8674(91)90390-k. [DOI] [PubMed] [Google Scholar]
- Sunkel CE, Gomes R, Sampaio P, Perdigao J, Gonzalez C. γ-Tubulin is required for the structure and function of the microtubule organizing centre in Drosophila neuroblasts. EMBO (Eur Mol Biol Organ) J. 1995;14:28–36. doi: 10.1002/j.1460-2075.1995.tb06972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin AM, Celati C, Paintrand M, Bornens M. Identification of an Spc110p-related protein in vertebrates. J Cell Sci. 1997;110:2533–2545. doi: 10.1242/jcs.110.20.2533. [DOI] [PubMed] [Google Scholar]
- Tian G, Huang Y, Vandekerckhove J, Ampe C, Cowan NJ. Pathway leading to correctly folded β-tubulin. Cell. 1996;86:287–296. doi: 10.1016/s0092-8674(00)80100-2. [DOI] [PubMed] [Google Scholar]
- Tian G, Lewis SA, Feierbach B, Stearns T, Rommelaere H, Ampe C, Cowan NJ. Tubulin subunits exist in an activated conformational state generated and maintained by protein cofactors. J Cell Biol. 1997;138:821–832. doi: 10.1083/jcb.138.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoresis transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev IA, Svitkina TM, Borisy GG. Cytoplasmic assembly of microtubules in cultured cells. J Cell Sci. 1997;110:2635–2645. doi: 10.1242/jcs.110.21.2635. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Microtubule dynamics: treadmilling comes around again. Curr Biol. 1997a;7:R369–R372. doi: 10.1016/s0960-9822(06)00177-1. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J Cell Biol. 1997b;139:417–434. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff A, de Néchaud B, Chillet D, Mazarguil H, Desbruyès E, Audebert S, Eddé B, Gros F, Denoulet P. Distribution of glutamylated α- and β-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur J Cell Biol. 1992;59:425–432. [PubMed] [Google Scholar]
- Yvon AMC, Wadsworth P. Non-centrosomal microtubule formation and measurement of minus end microtubule dynamics in A498 cells. J Cell Sci. 1997;110:2391–2401. doi: 10.1242/jcs.110.19.2391. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Jung MK, Oakley BR. γ-Tubulin is present in Drosophila melanogaster and Homo sapiensand is associated with the centrosome. Cell. 1991;65:817–823. doi: 10.1016/0092-8674(91)90389-g. [DOI] [PubMed] [Google Scholar]
- Zheng YX, Wong ML, Alberts B, Mitchison T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]