Abstract
The αvβ3 integrin plays a fundamental role during the angiogenesis process by inhibiting endothelial cell apoptosis. However, the mechanism of inhibition is unknown. In this report, we show that integrin-mediated cell survival involves regulation of nuclear factor-kappa B (NF-κB) activity. Different extracellular matrix molecules were able to protect rat aorta- derived endothelial cells from apoptosis induced by serum withdrawal. Osteopontin and β3 integrin ligation rapidly increased NF-κB activity as measured by gel shift and reporter activity. The p65 and p50 subunits were present in the shifted complex. In contrast, collagen type I (a β1-integrin ligand) did not induce NF-κB activity. The αvβ3 integrin was most important for osteopontin-mediated NF-κB induction and survival, since adding a neutralizing anti-β3 integrin antibody blocked NF-κB activity and induced endothelial cell death when cells were plated on osteopontin. NF-κB was required for osteopontin- and vitronectin-induced survival since inhibition of NF-κB activity with nonphosphorylatable IκB completely blocked the protective effect of osteopontin and vitronectin. In contrast, NF-κB was not required for fibronectin, laminin, and collagen type I–induced survival. Activation of NF-κB by osteopontin depended on the small GTP-binding protein Ras and the tyrosine kinase Src, since NF-κB reporter activity was inhibited by Ras and Src dominant-negative mutants. In contrast, inhibition of MEK and PI3-kinase did not affect osteopontin-induced NF-κB activation. These studies identify NF-κB as an important signaling molecule in αvβ3 integrin-mediated endothelial cell survival.
In recent years it has become evident that integrin- mediated adhesion to extracellular matrix (ECM)1 proteins is required for growth and survival of many cell types. Adhesion to ECM is required for progression of cells through the cell cycle by regulating cyclinD1, cyclinE-Cdk2, and Rb protein activities (Fang et al., 1996). Disruption of adhesion arrests cells in the G1 phase and causes apoptosis (Boudreau et al., 1996; Frisch and Francis, 1994; Howlett and Bissell, 1993; Ingber et al., 1995; Meredith et al., 1993; Re et al., 1994). The requirement of cell–ECM adhesive interactions for cell cycle progression and cell survival is likely to be important in tissue development and involution as a mechanism to regulate cell positioning and cell number (Lin and Bissell, 1993). In addition, anchorage dependence of survival may serve to limit tumor progression by preventing invasion or metastasis of tumor cells (Varner and Cheresh, 1996). Integrin-regulated survival properties have also been shown to be relevant in wound repair since integrin antagonists induced apoptosis of migrating endothelial cells, thereby blocking angiogenesis (Brooks et al., 1994a ; Brooks et al., 1994b ; Friedlander et al., 1996).
The mechanism by which integrin-mediated ECM adhesion is able to prevent apoptosis is unclear and under intense investigation. Focal adhesion kinase (FAK) is phosphorylated in response to cell adhesion, and constitutively activated membrane-targeted FAK was able to rescue cells from suspension-induced cell death (Frisch et al., 1996b ). The JNK pathway is active in detached epithelial cell (Frisch et al., 1996a ), and the Ras, PI3-kinase, Akt pathways are activated and functionally implicated in cell attachment–induced survival (Khwaja et al., 1997). Two groups suggest that integrin engagement positively regulates expression of the antiapoptotic gene bcl-2 in COS and endothelial cells (Stromblad et al., 1996; Zhang et al., 1995). In addition, Stromblad et al. showed that αvβ3 engagement and clustering in endothelial cells, but not β1 or αvβ5 ligation, conferred an antiapoptotic phentoype to endothelial cells. Importantly, the same group showed that inhibition of angiogenesis by anti-αvβ3 antibody correlates with angiogenic endothelial cell apoptosis (Brooks et al., 1994b ).
The transcription factor nuclear factor-kappa B (NF-κB) is a pleiotropic regulator of many genes involved in immune and inflammatory responses. The NF-κB family of proteins consists of homo- or heterodimeric subunits of the Rel family, including p50 and p65. In unstimulated cells, most of the NF-κB is localized in the cytoplasm in complex with an inhibitory protein, IκB(Baldwin, 1996). Upon stimulation, the inhibitory IκB becomes phosphorylated, ubiquinated, and subsequently degraded by the proteosome machinery(Palombella et al., 1994). This allows NF-κB to translocate to the nucleus, bind DNA, and transactivate transcription of specific genes. Recently, several lines of evidence have suggested that NF-κB is an important cell survival factor(Beg and Baltimore, 1996; Liu et al., 1996; Van Antwerp et al., 1996; Wang et al., 1996). However, integrin-mediated survival has never been functionally linked to NF-κB activation.
In the present study, we explored the possibility that integrin-mediated endothelial cell survival is controlled by NF-κB activation. Our experiments indicate that different ECM molecules, including osteopontin, promote endothelial cell survival upon serum deprivation. Moreover, adhesion of endothelial cells to the αvβ3 ligand osteopontin increases nuclear NF-κB activity. Furthermore, we find that β3 integrin mediates osteopontin's protective effect and NF-κB activation. Most importantly, we show that the osteopontin and vitronectin-mediated cell protective effect is abolished by inhibiting NF-κB nuclear translocation. Thus, NF-κB activation is required for endothelial cell protective effects of αvβ3 ligands after serum deprivation– induced apoptosis.
Materials and Methods
Materials
Recombinant wild-type and RGE-mutant mouse osteopontin were expressed in Escherichia coli as glutathione S-transferase fusion proteins as previously described (Liaw et al., 1995). Purified bovine plasma fibronectin was obtained from GIBCO BRL (Gaithersburg, MD); purified rat plasma vitronectin was obtained from Sigma Chemical Co. (St. Louis, MO); purified mouse laminin and rat tail collagen type I were obtained from Collaborative Biomedical Products (Bedford, MA), and polylysine was obtained from Sigma Chemical Co. Mouse monoclonal antibody F11 directed against the rat β3 integrin, and hamster monoclonal antibody Ha2/5 directed against the rat β1 integrin were obtained from PharMingen (San Diego, CA). Rabbit polyclonal antibodies against NF-κB p65, p50 subunits were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibody against poly(ADP-ribose) polymerase was obtained from Upstate Biotechnology Inc. (Lake Placid, NY). Constructs containing the fos gene core promoter by itself (pfLUC) or fused to two NF-κB sites derived from the Igκ promoter (pBIIX-LUC) driving the luciferase gene were a kind gift from D. Baltimore (Massachusetts Institute of Technology, Boston, MA). Dominant negative constructs for Ras (RasN17) and Src (kinase-dead) were a kind gift of Dr. Berk (University of Washington, Seattle, WA). The LY-294002 compound was purchased from Biomol Research Laboratories, Inc. (Plymouth Meeting, PA), and the PD98059 was purchased from Calbiochem-Novabiochem Corp. (La Jolla, CA).
Cell Cultures
Rat aortic endothelial cells (RAEC) were isolated as previously described (Nicosia et al., 1994). Cells were routinely maintained in MCBD 131 medium (GIBCO BRL), supplemented with 10mM l-glutamine, 10% FCS (HyClone Laboratories Inc., Logan, UT), and 100 U/ml each penicillin and streptomycin (GIBCO BRL). Cells were passaged by detaching with 0.05% trypsin and maintained at 37°C with 5% CO2. For all experiments, cells were used between passage 15 and 25. Human microvascular endothelial cells were obtained from Clonetics (San Diego, CA).
Survival Assay
Cells were detached, and equal numbers were replated on permanox slides (Nalge Nunc International, Pittsburgh, PA) either left uncoated or coated with different substrates. Substrates were diluted in PBS and coated onto permanox slides overnight at 4°C. Substrates were used at concentrations giving maximal adhesion and survival. Osteopontin was used at 100 nM; fibronectin, vitronectin, and laminin at 50 nM; collagen type I at 5 μg/ml; and F11 at 500 μg/ml. For the inhibition experiment, antibodies were diluted in serum-free medium and added to the cells ∼2 h after plating. F11 was used at 50 μg/ml; nonimmune mouse IgG at 50 μg/ml; Ha2/5 at 10 μg/ml; and nonimmune hamster IgM at 10 μg/ml. 48 h after plating, nuclei were stained with the membrane-permeant dye Hoechst 33342 (Molecular Bioprobes, Eugene, OR). Live cells were incubated for 30 min at 37°C with a 4 μg/ml solution of the dye. Cells were then fixed with 4% paraformaldehyde and viewed with a fluorescence microscope. Experiments were repeated at least three times. For AnnexinV staining, 106 cells were plated on osteopontin- and polylysine-coated 60-mm tissue culture dishes. 24 and 48 h after plating, cells were detached and incubated with AnnexinV-FITC (PharMingen) according to the manufacturer's instructions and analyzed by flow cytometry.
Western Blot
Proteins were extracted from cell monolayers in Laemmli's buffer containing protease inhibitors (0.2 μg/ml aprotinin, 0.2 μg/ml leupeptin, and 0.1 mM PMSF). After centrifugation and boiling, protein concentration was measured using the MicroBCA assay (Pierce, Rockford, IL). 50 μg of total protein were loaded onto 9% SDS-polyacrylamide gel for poly (ADP-ribose) polymerase (PARP) detection. Gels were transferred to a polyvinyldene difluoride membrane (Dupont-NEN, Boston, MA). Peroxidase-conjugated goat anti–rabbit antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was used as secondary antibody. Proteins were visualized by adding chemiluminescence reagent according to the manufacturer's instructions (Dupont-NEN).
Electrophoretic Mobility Shift Assay (EMSA)
Gel shift assays were performed as described by Qwarnstrom et al. (1994). A double-stranded oligonucleotide containing the DNA-binding site for the NF-κB proteins (5′AGTTGAGGGGACTTTCCCAGGC 3′) was obtained from Promega Corp. (Madison, WI), and was end-labeled using [γ32P]ATP according to the manufacturer's protocol (Promega Corp.) and purified using a Sephadex G-25 column (Pharmacia Biotech, Inc., Piscataway, NJ). The binding assay for the proteins was carried out as previously described (Guo et al., 1995). In brief, nuclear extracts were obtained using a modified Dignam protocol (Lee et al., 1988). Cells were scraped, washed with PBS, and resuspended in 20 μl of buffer A (10 mM Hepes, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, and 0.5 mM EDTA, pH 8.0) and allowed to swell on ice for 20 min. The nuclei were separated from the cell lysate by centrifugation at 12,000 g for 5 min in a microfuge. This nuclear pellet was resuspended in 20 μl of buffer C (20 mM Hepes, pH 7.9, 420 mM NaCl, 15 mM MgCl2, 0.2 mM EDTA, pH 8.0, 25% glycerol, 0.5 mM PMSF, and 0.5 mM DTT), and incubated for 10 min in ice. The lysed nuclei were centrifuged for 5 min at 4°C at 12,000 g in a microfuge. The nuclear extracts were assayed for protein content using the Biorad assay method. Aliquots of the extracts were used for the gel-shift assay, or were quick-frozen in dry ice and stored at −70°C. 5–10 μg of nuclear extracts were mixed with 60,000–80,000 cpm of labeled probe along with 300 μg/ml BSA and 133 μg/ml poly dIdC.dIdC (to prevent nonspecific binding). The binding reaction was carried out at room temperature for 15 min, and the samples were run on a 4% nondenaturing acrylamide gel. Reactions containing competitive and uncompetitive oligonucleotides used 10 μg of nuclear extract, and were preincubated for 15 min before adding the labeled probe. For supershift EMSA, the samples were incubated with 6 μl of the proper antibodies for 45 min just before running. The gels were dried, and bands were detected by autoradiography.
Transfections and Reporter Assays
For transfections, 2 × 106 cells in 100-mm dishes were transfected with 10 μg of DNA, using SuperFect™ (QIAGEN Inc., Chatsworth, CA). For cotransfection experiments, equal molar amounts of each plasmid were used for a total of 10 μg of DNA per 100-mm dish. Cells were allowed to recuperate overnight, and were then trypsinized and plated either on osteopontin or polylysine-coated surfaces in serum free medium. Cells were harvested 8 h after plating, and luciferase activity was measured using a luciferase assay system (Promega Corp.) according to the manufacturer's instructions. As a control, transfected cells were treated with IL1-β or vehicle for 1 h. Luciferase activity was normalized relative to human growth hormone secretion as described (Allegro Inc., Madison, WI). Experiments were repeated at least three times.
Immunocytochemistry
Cells were plated either on osteopontin or polylysine-coated permanox slides (Nalge Nunc International) in serum-free medium. 2 h after plating, cells were rinsed with PBS and fixed with cold methanol for 5 min. Endogenous peroxidases were blocked with 0.1% hydrogen peroxide in methanol for 10 min.After rinsing with PBS, nonspecific binding was minimized by blocking with 1.5% normal rabbit serum in PBS. Anti-NF-κB p65 polyclonal antibody at the concentration of 1 μg/ml was used as primary antibody. A biotinylated anti–rabbit antibody was then applied, followed by an avidin-peroxidase conjugate (ABC Elite; Vector Labs, Inc., Burlingame, CA). 3,3′diaminobenzidine was used as detection substrate. Cells were counterstained with Fast Red.
Construction of Nonphosphorylatable IκB Construct and Development of Stable Cell Lines
Murine IκBαcDNA was obtained from Paul Noble (Johns Hopkins, Baltimore, MD). Specific oligonucleotide primers (5′TTGGGATCCATGGACTACAAAGACGATGACGATAAAATGAAGGACGACGAG- TACGACC3′ and 5′CCAGGATCCACTTATAATGTCAGACGCTGGCCT3′) were used to construct an IκBα deletion mutant lacking amino acids 1–37, and to insert a FLAG sequence in frame with amino acid 37. The PCR product was initially cloned in the pCR2.1 vector (Invitrogen Corp., Carlsbad, CA) and then subcloned into the MREpNeo vector (MREpNeoΔN; Searle et al., 1985). Stable lines were obtained by transfecting endothelial cells with MREpNeoΔN using SuperFect™ (Qiagen Inc.). Cells were selected for 10 d with medium containing 500 μg/ml of G418 and 10% Zn-depleted serum(Searle et al., 1985). Five independent clones were isolated. All experiments were repeated with two independent clones (REACΔN2 and REACΔN5). Cell lines were routinely maintained in medium containing 10% Zn-depleted serum.
Results
Osteopontin Rescues Endothelial Cells from Serum Deprivation–induced Apoptosis
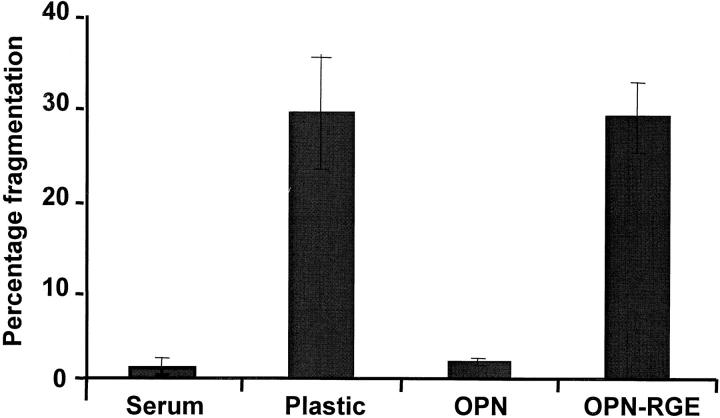
Adhesion to ECM via integrin receptors has previously been shown to protect endothelial cells from suspension-induced apoptosis (Meredith et al., 1993; Re et al., 1994). We now show that ECM may also play a role in cell survival under conditions of serum deprivation. Rat aortic endothelial cells were plated in serum-containing medium and allowed to spread for ∼2 h. Immediately after spreading, the media was changed to serum-free media, and nuclei were scored for fragmentation 48 h after plating. As shown in Fig. 1, cells plated on osteopontin-coated surfaces showed a minimal (<5%) rate of cell death after growth factor withdrawal (Fig. 1). In contrast, cells plated on uncoated surfaces showed 30% cell death. 30% cell death was also observed when cells were plated on a form of osteopontin carrying an inactivating mutation at the RGD sequence, suggesting an integrin-mediated mechanism. Similar results were obtained using human microvascular endothelial cells (data not shown).
Figure 1.
Osteopontin protects endothelial cells from serum deprivation–induced cell death. Endothelial cells were plated in serum on uncoated plastic, or on plastic coated with recombinant wild-type osteopontin (OPN), or osteopontin containing a RGD to RGE mutation (OPN-RGE). Soon after spreading (∼2 h after plating), the serum was withdrawn. 48 h after plating, cells were stained with the nuclear dye Hoechst 33342, and nuclear fragmentation was assessed. In control cultures, serum was not withdrawn from the cells. Data represent the average of triplicates ± SD of a representative experiment.
We then asked if other ECM molecules would promote endothelial cell survival in the same system. We tested vitronectin, fibronectin, laminin, and collagen type I, and all were able to protect endothelial cells from growth factor withdrawal–induced death (data not shown). Since laminin and collagen type I primarily interact with β1-containing integrins, these results suggest that different integrin dimers are able to protect from serum deprivation–induced apoptosis.
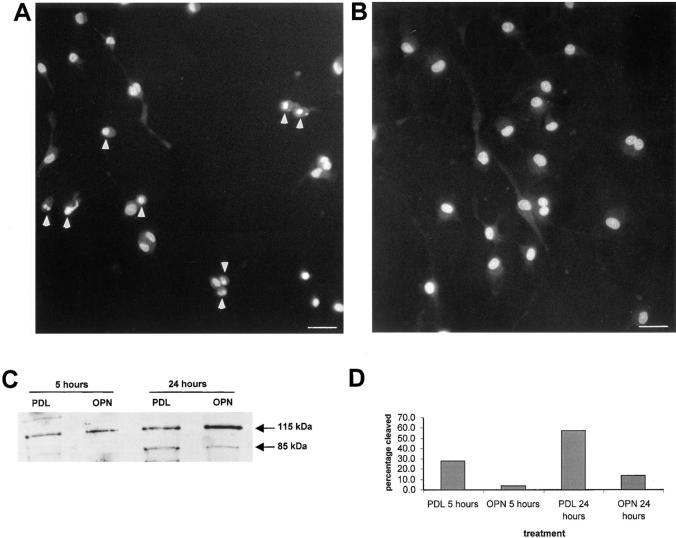
The condensed and fragmented nuclear morphology of the cells scored as dead suggested apoptosis as the mechanism of cell death (Fig. 2, A and B). To obtain a more quantitative assessment of cell death and to verify that apoptosis was indeed the mechanism of cell death, we took several approaches. First, fluorescently labeled AnnexinV (PharMingen, San Diego, CA) was used. AnnexinV binds to the membrane lipid phosphatidylserine, which is translocated to the outer layer of the plasma membrane in cells undergoing apoptosis (Vermes et al., 1995). The results summarized in Table I show a time-dependent increase in AnnexinV binding when cells were plated on polylysine, but not on osteopontin, confirming that the ECM molecule osteopontin promotes endothelial cell survival. Second, we confirmed that caspases were involved in endothelial apoptosis in the absence of serum. The poly (ADP-ribose) polymerase PARP, an enzyme that facilitates repair of DNA strand breaks, is a substrate for caspases. When caspases are active, PARP is cleaved in a typical pattern (Tewari et al., 1995). As shown in Fig. 2, C and D, cells plated on osteopontin demonstrated less PARP cleavage than cells plated on polylysine. These findings strongly suggest that caspase-dependent apoptosis after serum deprivation is the mechanism of death.
Figure 2.
Endothelial cells plated on osteopontin do not show nuclear fragmentation. (A and B) Cells were plated on polylysine- (A) or osteopontin- (B) coated surfaces in serum-free medium. 48 h after plating, cells were stained with the nuclear dye Hoechst 33342, and nuclear morphology was assessed by fluorescence microscope. Arrows indicate the apoptotic nuclei. Bar, 40 μM. (C) Cells were plated on polylysine- (PDL) or osteopontin- (OPN) coated surfaces in serum-free medium. Proteins were extracted 6 and 24 h after plating, electrophoresed, Western blotted, and probed with an anti-PARP antibody. Arrows indicate uncleaved (115 kD) and cleaved (85 kD) forms. (D) The relative intensity of 115 kD and 85 kD was determined with ImageQuant software (Molecular Dynamics, Inc., Sunnyvale, CA), and the percentage of cleaved product (85 kD band) was plotted.
Table I.
AnnexinV Staining
| % negative (peak mean) | % positive (peak mean) | |||
|---|---|---|---|---|
| OPN | ||||
| 24 h | 90.6 (62.59) | 9.4 (106.2) | ||
| 48 h | 88.9 (92.36) | 11.1 (186.97) | ||
| PDL | ||||
| 24 h | 69.9 (76.11) | 30.4 (145.01) | ||
| 48 h | 50.2 (105.52) | 49.08 (192.25) |
Cells were plated on osteopontin (OPN) or polylysine (PDL). 24 and 48 h after plating, cells were harvested and stained with AnnexinV-FITC and analyzed by flow cytometry. Negative and positive percentage values at the two timepoints are indicated. In addition, the peak mean (in parentheses) is also indicated.
β3-containing Integrin Mediates Osteopontin-induced Survival
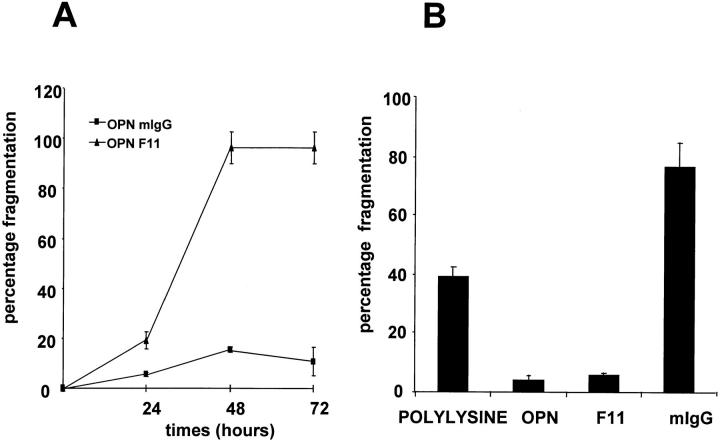
We previously showed that the αvβ3 integrin mediates endothelial cell adhesion and migration to osteopontin (Liaw et al., 1995). To examine if αvβ3 was involved in osteopontin's survival function, endothelial cells were plated in serum-free medium on an osteopontin-coated surface, and were allowed to adhere and spread. Cells were then challenged with either neutralizing integrin subunit antibodies or nonimmune IgG. As shown in Fig. 3 A, treatment with the anti-β3 integrin antibody (F11) caused time-dependent endothelial cell death. The level and the time course of cell death were comparable to those of cells plated on polylysine alone (data not shown). In contrast, cells treated with a nonimmune mIgG or an anti-β1 neutralizing antibody (Ha2/5; data not shown) were fully protected by osteopontin. The F11 antibody used in this study is directed against the β3-integrin subunit. Since no known integrin subunit other than αv can dimerize with β3 in nucleated cells, it is likely that this integrin subunit is heterodimerized to αv. This fact suggests that osteopontin's protective effect was mediated by αvβ3. To confirm this finding, we asked whether β3 ligation and clustering by surface-immobilized F11 could prevent cell death. As shown in Fig. 3 B, F11 was as effective as osteopontin in promoting cell survival, thus strongly suggesting that signaling through αvβ3 receptor was involved.
Figure 3.
αvβ3 integrin mediates osteopontin-induced endothelial cell survival. (A) Cells were plated on osteopontin-coated surfaces in serum-free medium. Soon after spreading (∼2 h after plating), F11 monoclonal antibody (triangles) or mouse IgG (squares) at the concentration of 50 μg/ml were added. Nuclear morphology was assessed at 24, 48, and 72 h. (B) Cells were plated on polylysine, osteopontin (OPN), F11, or mouse IgG (mIgG)-coated surfaces in serum-free medium. 48 h after plating, cells were stained with the nuclear dye Hoechst 33342, and nuclear morphology was assessed. Data represent the average of triplicates ± SD of a representative experiment.
To determine whether αvβ3 was involved in the protective effects of other ECM proteins, we used vitronectin, fibronectin, and laminin as substrates in a second set of similar experiments. As expected, treatment with F11 antibody induced cell death when endothelial cells were plated on vitronectin, but not when cells were plated on fibronectin and laminin (data not shown). Ha2/5 antibody was able to inhibit laminin-induced cell survival, but the combination of the two neutralizing antibodies was necessary in order to inhibit fibronectin-induced survival (data not shown). These findings suggest that the αvβ3 integrin is involved in osteopontin and vitronectin-mediated endothelial cell survival, that a β1-containing integrin-mediated laminin induced cell survival, and finally that fibronectin was able to use both a αvβ3- or β1-containing integrin receptor to signal survival.
Endothelial Cell Adherence to Osteopontin Induces NF-κB Activation
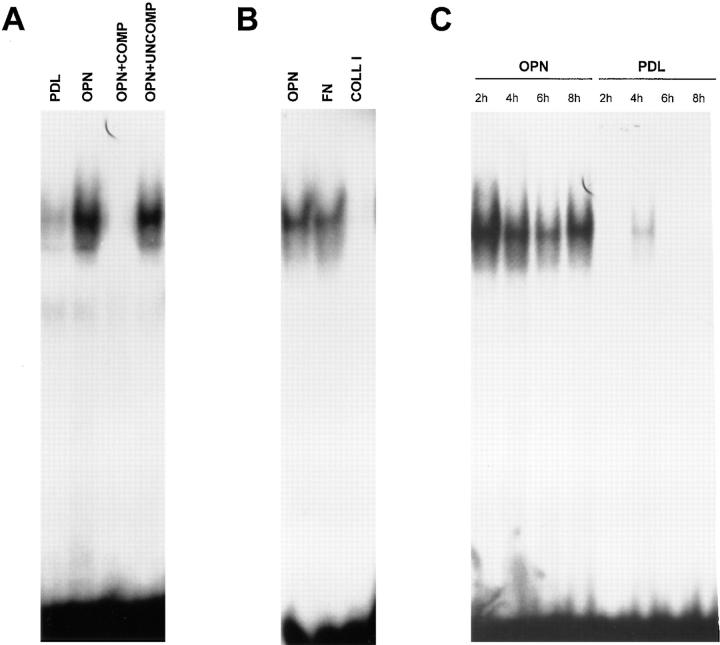
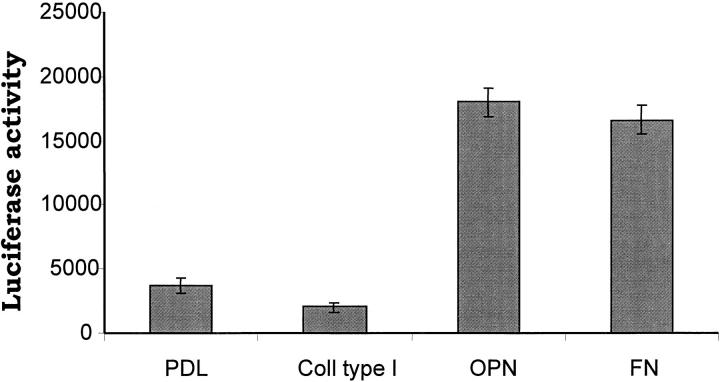
The p65 subunit of the NF-κB transcription factor has been shown recently to be necessary for cell survival (Beg and Baltimore, 1996; Liu et al., 1996; Van Antwerp et al., 1996; Wang et al., 1996). We hypothesized that adhesion of endothelial cells to an ECM substrate was capable of activating the NF-κB pathway, thereby activating a protective pathway in the cell. To test this hypothesis, we used three different approaches. First, endothelial cells were plated on osteopontin or polylysine-coated surfaces. Nuclear extracts were prepared at various time points, and NF-κB activity was examined by EMSA. As shown in Fig. 4 A, nuclear extract prepared from endothelial cells plated on osteopontin contained significant levels of NF-κB consensus oligomer-binding activity. In contrast, control cells showed very little activity (Fig. 4 A). The DNA–protein complex formed in cells adherent to osteopontin was specific, since excess unlabeled NF-κB consensus oligomer inhibited, whereas an unrelated sequence had no effect (Fig. 4 A). In contrast, the pure β1–integrin ligand, collagen type I, did not induce NF-κB binding activity, as shown in Fig. 4 B. As expected, the mixed ligand fibronectin, which can interact with both β3 and β1 integrins, was able to induce NF-κB binding activity (Fig. 4 B). Finally, the osteopontin-induced NF-κB–binding activity was sustained up to 8 h (Fig. 4 C).
Figure 4.
Endothelial cells plated on osteopontin have elevated NF-κB binding activity. (A) Cells were plated on polylysine (PDL) or osteopontin (OPN)-coated surfaces in serum-free medium. 4 h after plating, nuclear protein extracts were harvested, and EMSA was performed using a double-stranded 32P-labeled consensus NF-κB oligomer. Adding a 100-fold excess of unlabeled consensus NF-κB oligomer (COMP) completely inhibited binding, and a 100-fold excess of unlabeled unrelated sequence (UNCOMP) had no effect. (B) Cells were plated on osteopontin (OPN), fibronectin (FN), and collagen type I (COLL I). 4 h after plating, nuclear protein extracts were harvested, and EMSA was performed using a double-stranded 32P-labeled consensus NF-κB oligomer. (C) Cells were plated on polylysine (PDL) or osteopontin (OPN)-coated surfaces in serum-free medium. Nuclear protein extracts were harvested at 2, 4, 6, and 8 h after plating, and EMSA was performed using a double-stranded 32P-labeled consensus NF-κB oligomer.
The transcription factor Rel family of genes is composed of several NF-κB protein subunits (p65 or RelA, p50/p105 or NF-κB1, p52/p100 or NF-κB2, RelB, and the protooncogene c-rel; Baldwin, 1996). To establish the composition of the band that was shifted, we performed super-shift EMSA using direct antibodies against the p65, p50, p52, and c-rel subunits. As shown in Fig. 5, the shifted complex formed when the cells were plated on an osteopontin-coated surface was composed of the p65 and p50 NF-κB subunits, since the combination of these antibodies completely ablated the primary band. Antibodies directed against the p52 and c-rel subunits did not affect the primary band (data not shown). Second, we used a reporter construct containing two NF-κB consensus binding sites in the promoter in order to verify that NF-κB was transcriptionally active. A fourfold induction of the reporter construct was observed when cells were plated on osteopontin and fibronectin compared with control polylysine (Fig. 6). In contrast, no reporter induction was seen when cells were plated on collagen type I (Fig. 6). Finally, we observed nuclear translocation of the p65 subunit when endothelial cells were plated on osteopontin. As shown in Fig. 7 A, most of the p65 NF-κB subunit was localized in the nucleus when endothelial cells were plated on osteopontin. On the contrary, the staining remained localized to the cytoplasm when cells were plated on polylysine (Fig. 7 B). No staining was observed when nonimmune antibody was used (Fig. 7 C). These data suggest that osteopontin is able to activate NF-κB activity in endothelial cells.
Figure 5.
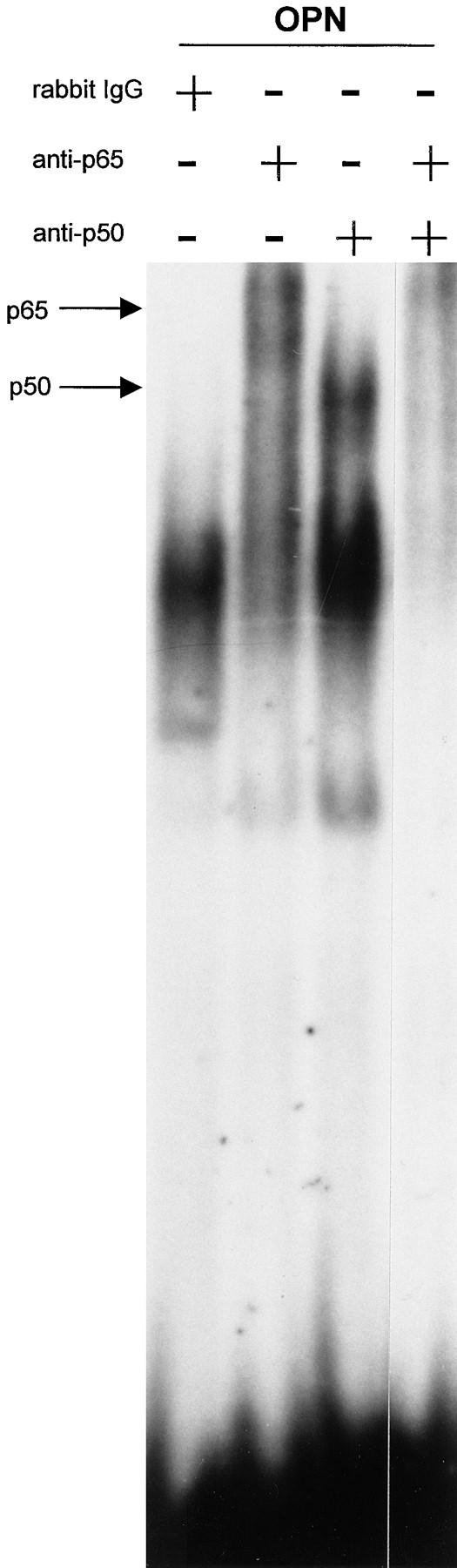
The p65 and p50 subunits form the NF-κB complex induced by osteopontin. Nuclear extract from endothelial cells plated on osteopontin were incubated with a double-stranded 32P-labeled consensus NF-κB oligomer, followed by incubation with polyclonal antibody against the p65 and p50 NF-κB subunits singly and combined, and with control rabbit IgG. Arrows indicate the shifted bands.
Figure 6.
Osteopontin induces NF-κB–dependent luciferase gene expression. Endothelial cells were transfected with a NF-κB–responsive luciferase reporter construct. Cells were plated on polylysine (PDL), collagen type I (Coll type I), osteopontin (OPN), and fibronectin (FN)-coated surfaces in serum-free medium. Cell lysates were harvested, and luciferase activity was measured 8 h after plating on immobilized substrates. Data represent the average of triplicates ± SD of a representative experiment.
Figure 7.
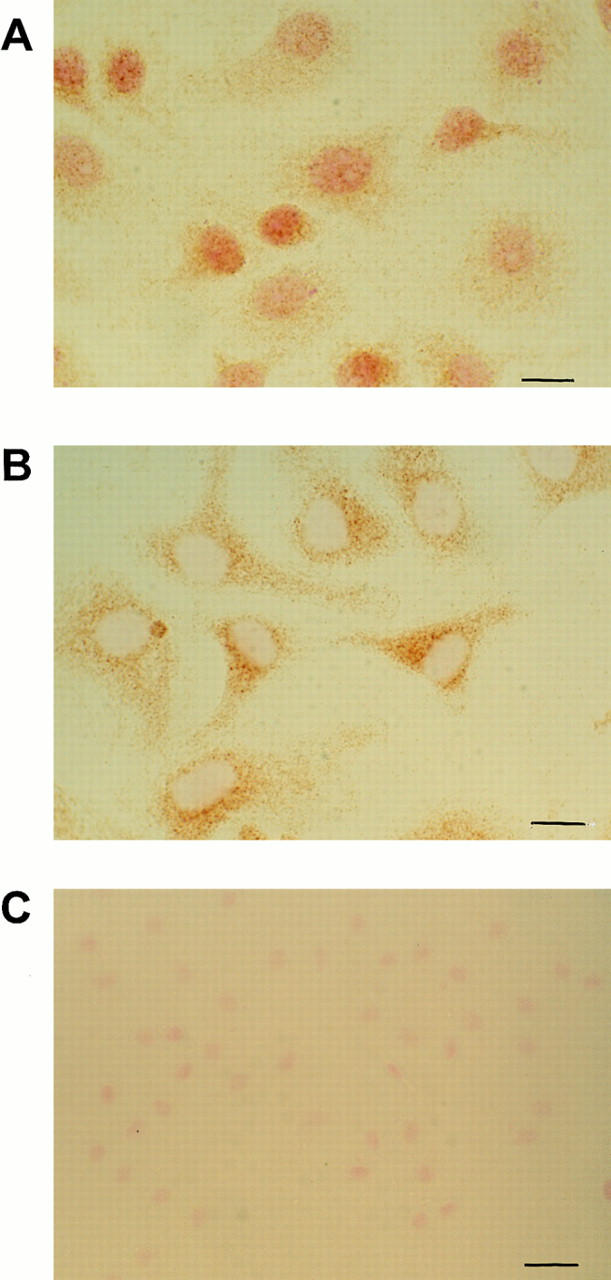
Osteopontin induces nuclear translocation of the p65 subunit. Cells were plated on osteopontin (A)- or polylysine (B)- coated surfaces in serum-free medium. 2 h after plating, cells were fixed and stained with a polyclonal anti-p65 subunit antibody. Bar, 10 μM. (C) Staining of cells plated on osteopontin with control nonimmune serum. Bar, 20 μM.
Osteopontin Induction of NF-κB is αvβ3-dependent
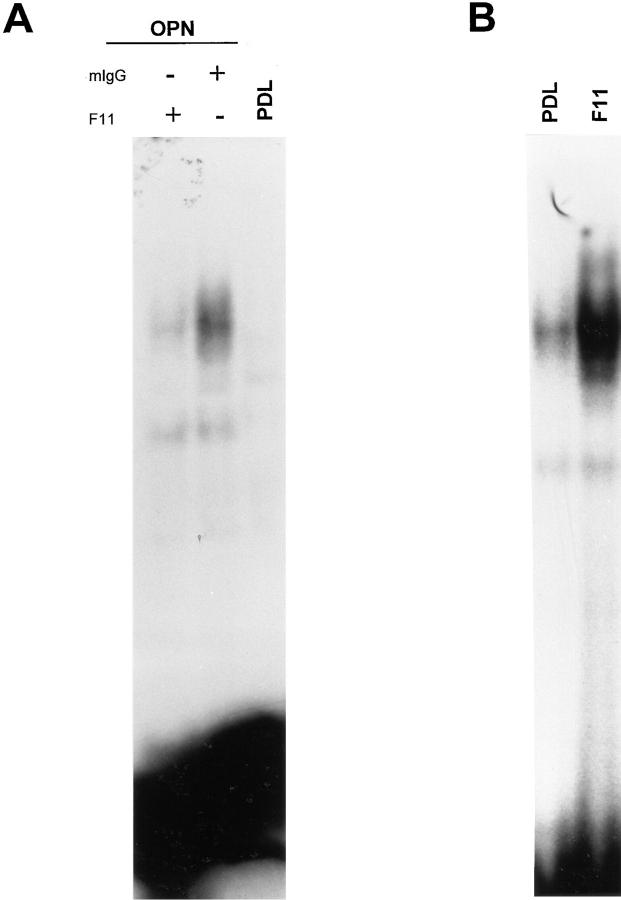
We then asked if osteopontin-induced NF-κB activity was αvβ3-dependent. Endothelial cells plated on osteopontin were treated with F11 or a control antibody. As shown in Fig. 8 A, when cells were treated for 8 h with F11 antibody, NF-κB activity was markedly reduced compared with control treatment. This result suggests that the αvβ3 integrin mediated osteopontin-induced NF-κB activity. We next explored whether endothelial cell adhesion to immobilized F11 was able to similarly regulate NF-κB. Fig. 8 B shows that endothelial cells plated on surface-immobilized F11 showed a considerably higher binding activity than control cells. These results show that αvβ3 ligation alone is sufficient to induce NF-κB activity.
Figure 8.
Osteopontin-induced NF-κB binding activity is inhibited by the β3 integrin antagonist F11. (A) Cells were plated on osteopontin-coated (OPN) surfaces in serum-free medium. Soon after spreading (∼2 h after plating), F11 monoclonal antibody or mouse-IgG (mIgG) were added at the concentration of 50 μg/ml. 10 h after plating, nuclear protein extracts were harvested, and EMSA was performed using a double-stranded 32P-labeled consensus NF-κB oligomer. (B) Cells were plated on polylysine (PDL) or immobilized F11. 4 h after plating, nuclear protein extracts were harvested, and EMSA was performed using a double-stranded 32P-labeled consensus NF-κB oligomer.
Inhibition of NF-κB Activity Abolishes Osteopontin-mediated Survival
To determine if nuclear translocation of the NF-κB heterodimer was necessary for αvβ3-mediated survival function, we overexpressed a nondegradable form of IκBα in endothelial cells. Overexpression of a mutant form of IκBα, in which the two serines (S 32 and 36) critical for phosphorylation and subsequent degradation were mutated to alanines, has been recently successfully used to inhibit NF-κB nuclear translocation (Brockman et al., 1995). We generated a murine IκBα deletion construct where the first 37 amino acids were deleted, thereby eliminating both Ser 32 and Ser 36. This cDNA was cloned in MRE-pNeo expression vector (Searle et al., 1985) downstream of a synthetic, Zn-inducible promoter. We then transfected endothelial cells and generated stable cell lines. NF-κB inducibility was lost in two clones (RAECΔN2 and RAECΔN5) upon treatment with 100 μM ZnSO4 for 16 h, followed by treatment with IL1-β as measured by reporter assay (Fig. 9 A). 100 μM of ZnSO4 was chosen since this concentration gave maximal expression of the IκBα mutant in serum-containing medium as determined by Western blot analysis (data not shown).
Figure 9.
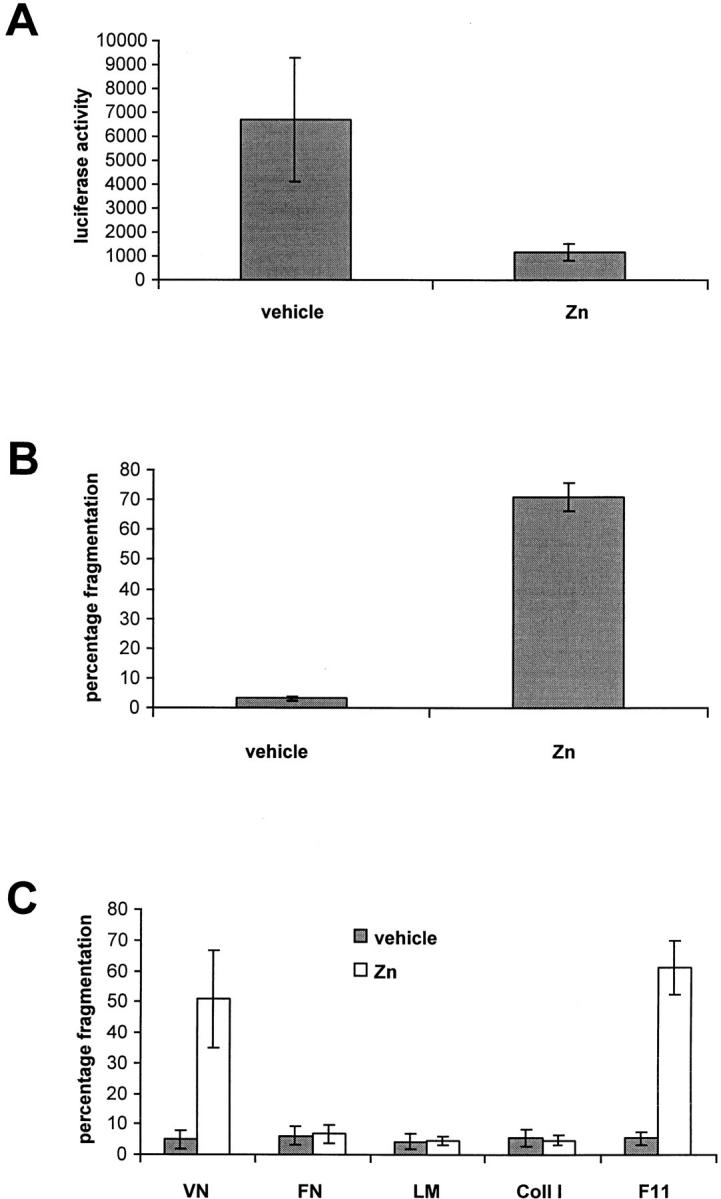
Inhibition of NF-κB nuclear translocation abolishes osteopontin-induced endothelial cell survival. (A) RAECΔN2 clone was transfected with a NF-κB–responsive luciferase reporter construct. Cells were then treated for 16 h with either vehicle or 100 μM of ZnSO4 (Zn) followed by a 1-h treatment with IL1-β. Cell lysates were harvested, and luciferase activity was measured. (B) RAECΔN2 clone was plated on osteopontin-coated surfaces in serum-free medium in the absence or presence of 20 μM ZnSO4 (Zn). 16 h later, cells were stained with the nuclear dye Hoechst 33342, and nuclear fragmentation was assessed. (C) RAECΔN2 clone was plated on vitronectin (VN), fibronectin (FN), laminin (LM), collagen type I (Coll I), and F11 monoclonal antibody–coated surfaces in serum-free medium in the absence (vehicle) or presence of 20 μM ZnSO4 (Zn). 16 h later, cells were stained with the nuclear dye Hoechst 33342, and nuclear fragmentation was assessed. Data represent the average of triplicates ± SD of a representative experiment. Every experiment was repeated with a separate clone (RAECΔN5), and identical results were obtained.
We then asked whether overexpression of the mutant IκBα and subsequent inhibition of NF-κB translocation could abolish osteopontin-induced survival upon growth factor withdrawal. To test this hypothesis, RAECΔN2 cells were plated in serum-free medium on osteopontin-coated surface, and were treated either with vehicle or 20 μM ZnSO4. In serum-free medium, 20 μM ZnSO4 was sufficient to induce maximal expression of the IκBα mutant (data not shown). As shown in Fig. 9 B, upon treatment the osteopontin protective effect was completely abolished, strongly suggesting that NF-κB activation by osteopontin is necessary for endothelial cell survival. Cells that were transfected with vector alone did not show any appreciable cell death when plated on osteopontin and treated with vehicle or 20 μM ZnSO4 (data not shown), thus confirming the specificity of the effect seen with the mutant IκBα construct.
In a similar set of experiments, we tested whether inhibition of NF-κB translocation could abolish the protective effect observed with other integrin ligands. As shown in Fig. 9 C, only the protective effects of vitronectin and immobilized β3 antibody were abolished. In contrast, RAECΔN2 cells plated on fibronectin, laminin, and collagen type I continued to be protected (Fig. 9 C). Thus, these results indicate that NF-κB activation is necessary for αvβ3-mediated endothelial cell survival, but a different and at the moment unknown survival pathway is engaged by β1-containing integrins.
Ras and Src, but not MEK and PI3-Kinase, Mediate NF-κB Induction by Osteopontin
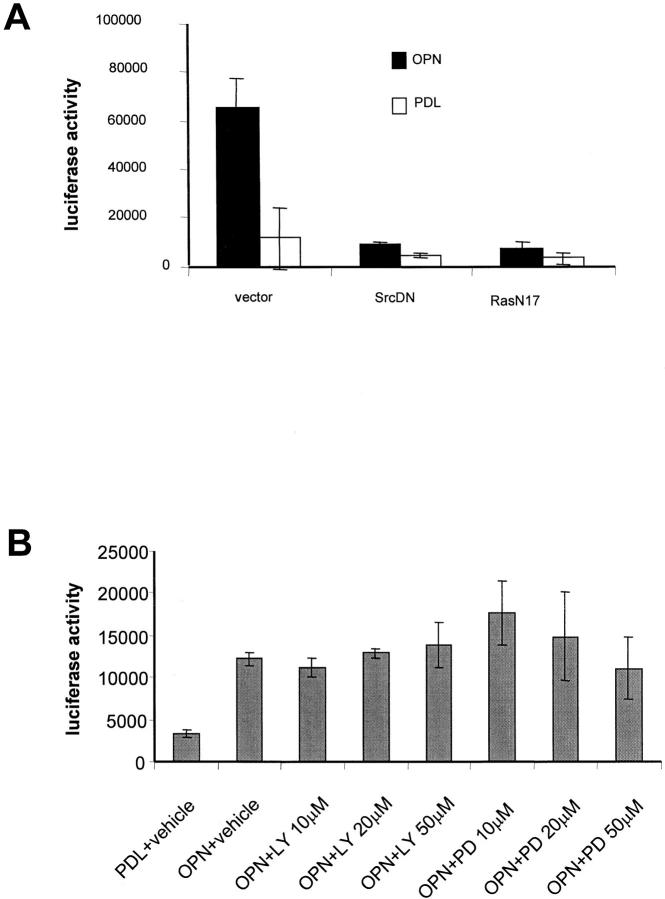
In an effort to identify membrane proximal signal by which αvβ3 integrin ligation leads to NF-κB activation, dominant negative constructs for the GTP-binding protein Ras (Ras-N17), for the kinase Src (kinase dead), and a vector control were cotransfected with the NF-κB reporter construct. As shown in Fig. 10 A, osteopontin-induced NF-κB activity was completely inhibited by expression of Ras-N17 and Src dominant negative constructs. In contrast, the specific MEK inhibitor PD58095 and the specific PI3-kinase inhibitor LY-294002 did not effect osteopontin-mediated NF-κB activity (Fig. 10 B). These inhibitors were shown to be effective by Western blot (data not shown). These results suggest that Ras and Src are proximal mediators of αvβ3-induced NF-κB activation.
Figure 10.
Ras and Src mediate osteopontin-induced NF-kB activation. (A) Cells were cotransfected with the NF-κB–responsive luciferase reporter construct and either the RasN17 construct, the Src kinase dead (SrcDN) construct, or vector alone. Cells were then plated on polylysine (PDL)- or osteopontin (OPN)-coated surfaces in serum-free medium. Cell lysates were harvested, and luciferase activity was measured 8 h after plating on immobilized substrates. (B) Cells were transfected with the NF-κB–responsive luciferase reporter construct. Cells were then plated on polylysine (PDL)- or osteopontin (OPN)-coated surfaces in serum-free medium. Cells plated on osteopontin were treated with the indicated concentrations of LY-294002 and PD98059 compounds. Cell lysates were harvested, and luciferase activity was measured 8 h after plating. Data represent the average of triplicates ± SD of a representative experiment.
Discussion
We have investigated the ability of ECM proteins to protect endothelial cells against apoptosis induced by serum withdrawal. We found that different ECM molecules played a role in cell survival under conditions of serum withdrawal, suggesting that different classes of integrins (β1 and β3) could mediate this function. In addition, we were able to show that a neutralizing β3 integrin antibody induced rounding and apoptosis when added in soluble form to cells plated on osteopontin, a known αvβ3 ligand. In contrast, a neutralizing β1 antibody had no effect. These data support the hypothesis that osteopontin promotes endothelial cell survival uniquely via the αvβ3 integrin. To explore the downstream mechanism of protection, we examined the activity of NF-κB, a recently described cell survival factor(Beg and Baltimore, 1996; Liu et al., 1996; Van Antwerp et al., 1996; Wang et al., 1996). We found that integrin receptor ligation resulted in rapid NF-κB activation in endothelial cells. Furthermore, we give functional evidence that NF-κB nuclear translocation was required for osteopontin-induced endothelial cell survival, but not for β1 integrin-mediated survival. Finally, we provide evidence that the small GTP-binding protein Ras and the tyrosine kinase Src are required for osteopontin-induced NF-κB activity. These results define a novel integrin-mediated survival pathway leading from αvβ3 integrin through Ras and Src to NF-κB in endothelial cells.
In vivo, inhibition of the αvβ3 integrin in angiogenic vessels causes endothelial cell apoptosis(Brooks et al., 1994b ), suggesting that a specific interaction between endothelial cells and an αvβ3 ligand(s) is required for survival. Here we show that αvβ3 ligands also protect endothelial cells from apoptosis induced by serum withdrawal in vitro.Osteopontin, vitronectin, and fibronectin were all able to promote endothelial cell survival after serum withdrawal.Moreover, the β1 ligands fibronectin, laminin, and collagen type I were also able to protect against apoptosis induced by serum deprivation, consistent with previous studies in which apoptosis was induced by suspension(Meredith et al., 1993; Re et al., 1994). Thus, different classes ofintegrins and ligands are able to facilitate endothelial survival under diverse conditions.
The mechanism by which integrin ligation leads to cell survival is under intense investigation.It has been proposed that integrin regulation of bcl2-family members(Stromblad et al., 1996; Zhang et al., 1995), the PI3-kinase/ Akt pathway(Khwaja et al., 1997), and the MEKK-1/JNK pathway(Cardone et al., 1997) may be important for cell protective effects. Moreover, Day et al. (1997) suggest that the activity of the tumor suppressor Rb is required for induction of apoptosis in cells maintained in suspension (anoikis), and some evidence suggests that αvβ3 integrin ligation in endothelial cells suppresses p53 binding activity(Stromblad et al., 1996). These findings suggest that multiple and potentially overlapping integrin-mediated survival pathways exist.
Recently, several groups have suggested a key role of NF-κB p65 in apoptosis inhibition(Beg and Baltimore, 1996; Liu et al., 1996; Van Antwerp et al., 1996; Wang et al., 1996). In the p65 knockout mouse, embryonic lethality is seen, and is most likely due to extensive apoptosis and necrosis of the liver (Beg et al., 1995). In addition, cells with functionally inactive NF-κB undergo apoptosis when challenged with TNFα(Beg and Baltimore, 1996; Liu et al., 1996; Van Antwerp et al., 1996; Wang et al., 1996). However, the potential role of NF-κB in integrin-mediated survival has not previously been reported. In the present studies, we observed a rapid and sustained activation of NF-κB when endothelial cells were plated on osteopontin, as measured by EMSA, nuclear translocation of the p65 subunit, and luciferase reporter assay. We also determined that αvβ3 was required for osteopontin-induced NF-κB activity, since adding F11 antibody to endothelial cells spread on osteopontin inhibited this activity, and cells plated on immobilized F11 showed elevated NF-κB activity. This result correlated with the observation that soluble β3 antagonist inhibited osteopontin-induced endothelial cell survival. Indeed, NF-κB activity was required for osteopontin-induced survival, since inhibition of NF-κB nuclear translocation by the nonphosphorylatable IκBα completely blocked the protective effect of osteopontin.While several previous studies have correlated NF-κB activation with integrin ligation(McGilvray et al., 1997; Qwarnstrom et al., 1994; Rosales and Juliano, 1996), ours is the first to demonstrate a functional role for integrin-induced NF-κB in cell survival.
Several signaling pathways leading from integrin ligation to cell function have been recently identified (Schlaepfer and Hunter, 1996). Engagement of integrins by the ECM causes organization of a complex structure, termed focal adhesion, on the cytoplasmic side of the plasma membrane (Schwartz et al., 1995). The tyrosine kinase FAK appears to play a central role in integrin signal transduction. FAK activation has been linked to cytoskeletal organization, regulation of small GTP-binding proteins like Rho and Ras, and interaction with intracellular proteins like c-Src and c-Fyn, Grb2/Sos, and PI-3 kinase. These data suggest that FAK and its downstream targets are important relays in integrin-mediated signaling (Schaller and Parsons, 1994).
Our studies indicate that both Ras and Src are required for αvβ3-mediated NF-κB activation in endothelial cells. Overexpression of Ras and Src dominant negative mutants specifically inhibited osteopontin-induced NF-κB activation.In contrast, inhibitors of PI-3 kinase and MEK, which were previously shown to block suspension-induced death(Khwaja et al., 1997; Wary et al., 1996), failed to block osteopontin-induced NF-κB activation. These data suggest that the survival pathway initiated by αvβ3 is distinct from those previously identified(Khwaja et al., 1997; Wary et al., 1996).In addition, in the present studies, β1 ligation was unable to induce NF-κB activation, and the protective effect of β1 ligands was not blocked when NF-κB was inhibited by nonphosphorylatable IκBα. These findings indicate that at least two distinct ECM-mediated protective pathways exist in endothelial cells: one mediated by β3 integrins, and another mediated by β1 integrins.
Our studies are consistent with previous studies showing that overexpression of Ras induced activation of NF-κB in NIH3T3 fibroblasts and Jurkat T-lymphoma cells(Perona et al., 1997). However, overexpression of activated Ras and Src appeared to inhibit β1 ligation-induced NF-κB activation in human monocytic cells(Rosales and Juliano, 1996), and MAPK appeared to mediate α4β1-induced NF-κB activation in the same cell type(McGilvray et al., 1997). Thus, the effect of integrins on NF-κB activation and the signaling pathways involved may be cell type–specific.
Our studies support the hypothesis that intracellular signaling initiated by ligating αvβ3 leads to NF-κB activation. NF-κB normally exists in an inactive form in the cytoplasm complexed to its inhibitor, IκB, a family of related ankyrin-containing proteins.Phosphorylation of IκB promotes its ubiquitination and subsequent degradation by the proteosome machinery, thereby unmasking the NF-κB nuclear targeting sequence.It is likely that αvβ3 integrin ligation indirectly regulates IκB phosphorylation. Several kinases have been shown potentially to regulate IkB phosphorylation: protein kinase C, MEKK1, NIK (a member of the MAPK family; Baldwin, 1996; Lee et al., 1997; Malinin et al., 1997), and the recently identified IKK-1 and IKK-2(Mercurio et al., 1997). Which, if any, of these kinases is involved in NF-κB activation in our system is currently under investigation.
How might NF-κB regulate cell survival? Previous studies showing that cells lacking NF-κB become sensitive to TNFα treatment suggest that NF-κB may regulate antiapototic genes. TNFα regulates many genes, and some of them are known to suppress apoptosis like the endothelial-specific bcl-2 homolog A1(Karsan et al., 1996). Indeed, Karsan et al. (1996) showed that A1 inhibits not only TNFα-induced endothelial cell death, but also ceramide-induced endothelial cell apoptosis. At the moment, we do not know if A1 or other antiapoptotic bcl-2 family members are regulated by NF-κB, but it is a possibility since integrin ligation correlates with their upregulation(Stromblad et al., 1996; Zhang et al., 1995). Another candidate is manganous superoxide dismutase. This enzyme has been shown to be antiapoptotic and TNFα-induced (Liu et al., 1996). Interestingly, MnSOD Drosophila homolog has been shown to have NF-κB–like sites in the 5′-untranslated region(Duttaroy et al., 1997). Baculovirus inhibitors of apoptosis mammalian homologs (cIAPs) are also candidates(Uren et al., 1996). cIAPs are caspase inhibitors probably by directly binding and inactivating these enzymes(Deveraux et al., 1997). Recently, cIAP1 has been shown to be NF-κB inducible(Chu et al., 1997). Finally, a new class of molecules (casper/I-FLICE/cFlip) containing caspase and death effector domain has been describedrecently (Hu et al., 1997; Irmler et al., 1997; Shu et al., 1997). It appears that different splice variants of these molecules can either inhibit or stimulate apoptosis. Nothing is known about the regulation casper/I-FLICE/cFlip; therefore, it will be a challenge to explore a possible NF-κB involvement.
From this study, a pathway linking αvβ3 integrin ligation, NF-κB, and endothelial cell survival has emerged. This pathway may be important in the angiogenesis process since the αvβ3 integrin is expressed in proliferating angiogenic endothelial cell, and is required for their survival(Brooks et al., 1994a ; Brooks et al., 1994b ). Moreover, αvβ3 antagonists prevent vessel maturation in developing quail(Drake et al., 1995).While it is not yet clear if NF-κB is generally important in angiogenesis in vivo, NF-κB activation has been described in regenerating rat aortic endothelial cells in vivo(Lindner and Collins, 1996), and nuclear NF-κB was detected in activated endothelial cells overlying atherosclerotic plaques(Brand et al., 1996). Furthermore, in vitro studies support a role for NF-κB in angiogenic processes. Both capillary tube formation and NF-κB activity were induced by 12(R)-HETrE in cultured rabbit coronary microvascular endothelial cells. Moreover, inhibition of NF-κB activation in these cells resulted in inhibition of 12(R)-HETrE-induced tube formation(Stoltz et al., 1996).Similarly, human microvascular endothelial cells were shown to undergo both NF-κB activation and tubular morphogenesis in response to hydrogen peroxide treatment, and NF-κB antisense oligonucleotides completely blocked hydrogen peroxide-induced tube formation (Shono et al., 1996).
Osteopontin and its interactions with endothelial cells via the αvβ3 integrin are of particular interest since we recently identified osteopontin as an endothelial cell product in a subset of vasa vasorum in the human atherosclerotic plaqueand granulation tissue(O'Brien et al., 1994). αvβ3 was also expressed in the same type of vessels(Hoshiga et al., 1995). We have also shown that osteopontin synthesis was dramatically increased in regenerating large vessel endothelium in vivo, and the β3 integrin subunit was coordinately upregulated(Liaw et al., 1995). As mentioned above, nuclear translocation of the NF-κB p65 subunit was recently observed in regenerating endothelial cells using the same animal model(Lindner and Collins, 1996).
In conclusion, we have provided evidence that osteopontin mediates endothelial cell survival uniquely via the αvβ3 integrin. Furthermore, we have presented functional evidence that the NF-κB pathway is fundamental for β3-mediated inhibition of apoptosis. Given the critical role of αvβ3 integrin during the angiogenesis process (Brooks et al., 1994a ; Brooks et al., 1994b ; Brooks et al., 1995; Drake et al., 1995; Friedlander et al., 1996), it is tempting to speculate that osteopontin may act as one of the endogenous ligands for αvβ3 integrin during angiogenesis. Finally, we have demonstrated that β1 ligand–mediated endothelial survival is not NF-κB dependent, suggesting that distinct β3 and β1-mediated survival pathways exists.
Acknowledgments
This study was supported by National Institutes of Health grants HL-18645 and DK-47659 (to C.M. Giachelli), HL-52585 (to R.F. Nicosia), by the National Science Foundation grant EEC9529161 (to C.M. Giachelli), and by the National Cancer Institute grant CA-70131 (to N. Fausto). Michelle Chaisson was supported by the National Cancer Institute training grant CA-09437. Dr. Giachelli is an established investigator of the American Heart Association.
Abbreviations used in the paper
- cIAP
baculovirus inhibitors of apoptosis mammalian homologs
- ECM
extracellular matrix
- EMSA
electrophoretic mobility shift assay
- FAK
focal adhesion kinase
- NF-κB
nuclear factor-kappa B
- PARP
poly(ADP-ribose) polymerase
- PI3
phosphotidylinositol 3
- RAEC
rat aortic endothelial cells
Footnotes
Address all correspondence to Marta Scatena, Department of Pathology, University of Washington, Box 357335, Seattle, WA. Tel.: 206 685 4288; Fax: 206 685 3662; E-mail: mscatena@u.washington.edu
References
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci USA. 1996;93:3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, Ballard DW. Coupling of a signal response domain in I kappa B alpha to multiple pathways for NF-kappa B activation. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994a;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994b;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone MH, Salvesen GS, Widmann C, Johnson G, Frisch SM. The regulation of anoikis: MEKK-1 activation requires cleavage by caspases. Cell. 1997;9:315–323. doi: 10.1016/s0092-8674(00)80339-6. [DOI] [PubMed] [Google Scholar]
- Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci USA. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day ML, Foster RG, Day KC, Humphrey P, Swamson P, Postigo AA, Zhang SH, Dean DC. Cell anchorage regulates apoptosis through the retinoblastoma tumor suppressor/E2F pathway. J Biol Chem. 1997;272:8125–8128. doi: 10.1074/jbc.272.13.8125. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Cheresh DA, Little CD. An antagonist of integrin alpha v beta 3 prevents maturation of blood vessels during embryonic neovascularization. J Cell Sci. 1995;108:2655–2661. doi: 10.1242/jcs.108.7.2655. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Parkes T, Emtage P, Kirby K, Boulianne GL, Wang XD, Hilliker AJ, Phillips JP. The manganese superoxide dismutase gene of Drosophila: structure, expression, and evidence for regulation by MAP kinase. DNA Cell Biol. 1997;16:391–399. doi: 10.1089/dna.1997.16.391. [DOI] [PubMed] [Google Scholar]
- Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, Cheresh DA. Involvement of integrins alpha(v)beta(3) and alpha(v)beta(5) in ocular neovascular diseases. Proc Natl Acad Sci USA. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Kelaita D, Sicks S. A role for Jun-N-Terminal kinase in anoikis: suppression by bcl-2 and crmA. J Cell Biol. 1996a;135:1377–1382. doi: 10.1083/jcb.135.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, ChanHui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996b;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhang YP, Mitchell DA, Denhardt DT, Chambers AF. Identification of a ras-activated enhancer in the mouse osteopontin promoter and its interaction with a putative ETS-related transcription factor whose activity correlates with the metastatic potential of the cell. Mol Cell Biol. 1995;15:476–487. doi: 10.1128/mcb.15.1.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiga M, Alpers CE, Smith LL, Giachelli CM, Schwartz SM. Alpha-v beta-3 integrin expression in normal and atherosclerotic artery. Circ Res. 1995;77:1129–1135. doi: 10.1161/01.res.77.6.1129. [DOI] [PubMed] [Google Scholar]
- Howlett AR, Bissell MJ. The influence of tissue microenvironment (stroma and extracellular matrix) on the development and function of mammary epithelium. Epithelial Cell Biol. 1993;2:79–89. [PubMed] [Google Scholar]
- Hu SM, Vincenz C, Ni J, Gentz R, Dixit VM. I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD-95-induced apoptosis. J Biol Chem. 1997;272:17255–17257. doi: 10.1074/jbc.272.28.17255. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Prusty D, Sun ZQ, Betensky H, Wang N. Cell shape, cytoskeletal mechanics, and cell cycle control in angiogenesis. J Biomech. 1995;28:1471–1484. doi: 10.1016/0021-9290(95)00095-x. [DOI] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, Schneider P, Hofmann B, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- Karsan A, Yee E, Kaushansky K, Harlan JM. Cloning of human Bcl-2 homologue: inflammatory cytokines induce human A1 in cultured endothelial cells. Blood. 1996;87:3089–3096. [PubMed] [Google Scholar]
- Khwaja A, Rodriguez P, Viciana, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO (Eur Mol Biol Organ) J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Hagler J, Chen ZJ, Maniatis T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- Lee KA, Bindereif A, Green MR. A small-scale procedure for preparation of nuclear extracts that support efficient transcription and pre-mRNA splicing. Gene Anal Tech. 1988;5:22–31. doi: 10.1016/0735-0651(88)90023-4. [DOI] [PubMed] [Google Scholar]
- Liaw L, Lindner V, Schwartz SM, Chambers AF, Giachelli CM. Osteopontin and beta 3 integrin are coordinately expressed in regenerating endothelium in vivo and stimulate Arg-Gly-Asp-dependent endothelial migration in vitro. Circ Res. 1995;77:665–672. doi: 10.1161/01.res.77.4.665. [DOI] [PubMed] [Google Scholar]
- Lin CQ, Bissell MJ. Multi-faceted regulation of cell differentiation by extracellular matrix. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- Lindner V, Collins T. Expression of NF-kappa B and I kappa B-alpha by aortic endothelium in an arterial injury model. Am J Pathol. 1996;148:427–438. [PMC free article] [PubMed] [Google Scholar]
- Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappa B induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- McGilvray ID, Lu Z, Bitar R, Dackiw APB, Davreux CJ, Rotstein OD. VLA-4 integrin cross-linking on human monocytic THP-1 cells induces tissue factor expression by a mechanism involving mitogen-activated protein kinase. J Biol Chem. 1997;272:10287–10294. doi: 10.1074/jbc.272.15.10287. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia RF, Villaschi S, Smith M. Isolation and characterization of vasoformative endothelial cells from the rat aorta. In Vitro Cell Dev Biol Anim. 1994;6:394–399. doi: 10.1007/BF02634360. [DOI] [PubMed] [Google Scholar]
- O'Brien ER, Garvin MR, Stewart DK, Hinohara T, Simpson JB, Schwartz SM, Giachelli CM. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler Thromb. 1994;14:1648–1656. doi: 10.1161/01.atv.14.10.1648. [DOI] [PubMed] [Google Scholar]
- Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Perona R, Montaner S, Saniger L, Sánchez-Pérez I, Bravo R, Lacal JC. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- Qwarnstrom EE, Ostberg CO, Turk GL, Richardson CA, Bomsztyk K. Fibronectin attachment activates the NF-kappa B p50/p65 heterodimer in fibroblasts and smooth muscle cells. J Biol Chem. 1994;269:30765–30768. [PubMed] [Google Scholar]
- Re F, Zanetti A, Sironi M, Polentarutti N, Lanfrancone L, Dejana E, Colotta F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J Cell Biol. 1994;127:537–546. doi: 10.1083/jcb.127.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales C, Juliano R. Integrin signaling to NF-kappa B in monocytic leukemia cells is blocked by activated oncogenes. Cancer Res. 1996;56:2302–2305. [PubMed] [Google Scholar]
- Schaller MD, Parsons JT. Focal adhesion kinase and associated proteins. Curr Opin Cell Biol. 1994;6:705–710. doi: 10.1016/0955-0674(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Signal transduction from the extracellular matrix--a role for the focal adhesion protein-tyrosine kinase FAK. Cell Struct Funct. 1996;21:445–450. doi: 10.1247/csf.21.445. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Ann Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Searle PF, Stuart GW, Palmiter RD. Building a metal-responsive promoter with synthetic regulatory elements. Mol Cell Biol. 1985;5:1480–1489. doi: 10.1128/mcb.5.6.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shono T, Ono M, Izumi H, Jimi SI, Matsushima K, Okamoto T, Kohno K, Kuwano M. Involvement of the transcription factor NF-kappaB in tubular morphogenesis of human microvascular endothelial cells by oxidative stress. Mol Cell Biol. 1996;16:4231–4239. doi: 10.1128/mcb.16.8.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HB, Halpin DR, Goeddel DV. Casper is a FADD- and caspase-related inducer of apoptosis. Immunity. 1997;6:751–763. doi: 10.1016/s1074-7613(00)80450-1. [DOI] [PubMed] [Google Scholar]
- Stoltz RA, Abraham NG, Laniado M, Schwartzman The role of NF-kappaB in the angiogenic response of coronary microvessel endothelial cells. Proc Natl Acad Sci USA. 1996;93:2832–2837. doi: 10.1073/pnas.93.7.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin alphaVbeta3 during angiogenesis. J Clin Invest. 1996;98:426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari M, Quan LT, O'Rourke K, Desnoyers S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS, Dixit VM. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Uren AG, Pakusch M, Hawkins CJ, Puls KL, Vaux DL. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc Natl Acad Sci USA. 1996;93:4974–4978. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- Vermes I, Haanen C, Steffens H, Nakken, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]