Abstract
The proapoptotic protein BAX contains a single predicted transmembrane domain at its COOH terminus. In unstimulated cells, BAX is located in the cytosol and in peripheral association with intracellular membranes including mitochondria, but inserts into mitochondrial membranes after a death signal. This failure to insert into mitochondrial membrane in the absence of a death signal correlates with repression of the transmembrane signal-anchor function of BAX by the NH2-terminal domain. Targeting can be instated by deleting the domain or by replacing the BAX transmembrane segment with that of BCL-2. In stimulated cells, the contribution of the NH2 terminus of BAX correlates with further exposure of this domain after membrane insertion of the protein. The peptidyl caspase inhibitor zVAD-fmk partly blocks the stimulated mitochondrial membrane insertion of BAX in vivo, which is consistent with the ability of apoptotic cell extracts to support mitochondrial targeting of BAX in vitro, dependent on activation of caspase(s). Taken together, our results suggest that regulated targeting of BAX to mitochondria in response to a death signal is mediated by discrete domains within the BAX polypeptide. The contribution of one or more caspases may reflect an initiation and/or amplification of this regulated targeting.
Keywords: apoptosis, BAX, cytochrome c, caspase, mitochondria
The response of metazoan cells to apoptotic death signals depends on the status of various regulatory checkpoints in the cell. Prominent among these is the BCL-2 family of proteins whose members include dominant suppressors (Ced-9, BCL-2, BCL-XL, BCL-w, A1, MCL-1) and proapopototic inducers (BAX, BAK, BCL-Xs) of cell death, as well as proapoptotic inhibitors of BCL-2/BCL-XL function (BAD, BID; Yang and Korsmeyer, 1996). The relationships among these family members are complex, and in the case of the BCL-2 suppressor and BAX inducer, are further complicated by their apparent ability to function autonomously in regulating cell death (Cheng et al., 1996; Knudson and Korsmeyer, 1997), while at the same time influencing one another's activities via heterodimeric interactions (Oltvai et al., 1993). BCL-2 suppressors function upstream of caspase death effectors such as caspase-3 to inhibit cell death (Boulakia et al., 1996; Chinnaiyan et al., 1996; Armstrong et al., 1996), which is likely accomplished in several ways. These include recruitment and regulation of Ced-4 like molecules (Shaham and Horvitz, 1996; Wu et al., 1997; Chinnaiyan et al., 1997; Spector et al., 1997; James et al., 1997) and Ced-4 like adaptors (Chinnaiyan et al., 1997; Ng and Shore, 1998) that are required for activation of initiator caspases and recruitment of kinases (Wang et al., 1996) and phosphatases (Shibasaki et al., 1997) that may regulate the activity of BCL-2-associated complexes. Moreover, regulation of BCL-2 complexes may influence formation of ion-conducting pores (Minn et al., 1997; Schendel et al., 1997; Schlesinger et al., 1997) or the channel activities of membranes in which BCL-2 resides. While BAX may affect all of these BCL-2–mediated events via heterodimeric modulation, BAX is also capable of autonomous pore formation in lipid bilayers (Schlesinger et al., 1997; Antonnson et al., 1997). The ability of elevated levels of BAX or BAK to initiate cell death in the absence of any additional signal in vivo (Xiang et al., 1996; McCarthy et al., 1997; Rossé et al., 1998) correlates with severe intracellular membrane dysfunction that includes redistribution of mitochondrial cytochrome c to the cytosol and induced mitochondria permeability transition.
Most BCL-2 and BAX family proteins contain at their extreme COOH terminus a single predicted transmembrane segment (TM)1. In the case of BCL-2, the TM functions as a signal anchor that targets and inserts the protein in a Ncyto-Cin orientation into the two main membrane locations for this protein: the mitochondrial outer membrane and the ER/nuclear envelope (Hockenbery et al., 1990; Krajewski et al., 1993; Nguyen et al., 1993; Nguyen et al., 1994). Strikingly, however, the ability of BAX to translocate to membrane sites, including mitochondria, is regulated in certain contexts and depends upon the cell receiving a death signal (Hsu et al., 1997; Wolter et al., 1997). In the absence of such a signal, BAX is found free in the cytosol or peripherally associated with endocellular membrane surfaces. Although the mechanism for regulated targeting of BAX or BAK remains to be elucidated, the fact that transient overexpression (Xiang et al., 1996; Rossé et al., 1998) or forced dimerization (Gross et al., 1998) of the protein can lead to cell death in the absence of other signals implies that the regulatory mechanism can be overridden in certain contexts.
Here we demonstrate that BAX targeting to mitochondria can be regulated by zVAD-sensitive caspase(s). Significantly, prevention of BAX targeting in the absence of a death signal has been mapped to two regions of the molecule: the NH2-terminal ART domain and the COOH-terminal TM. Our results indicate a regulated mechanism by which a death signal can cause translocation of BAX to mitochondria where it mediates and amplifies membrane dysfunction (reviewed in Reed, 1997; Hengartner, 1998).
Materials and Methods
Immunocytochemistry
FL5.12 cells were resuspended in fresh media containing 400 nM Mitotracker Green FM (Molecular Probes, Inc., Eugene, OR). After a 15-min incubation, cells were washed and fixed in 3% paraformaldehyde and blocked with 2% normal goat serum (Vector Labs, Inc., Burlingame, CA). Anti-mBAX (P-19) Ab (Santa Cruz Biotechnology, Santa Cruz, CA) and Cy3 goat anti–rabbit Ab (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) were used to detect BAX immunoreactivity. Nuclei were counterstained with Hoechst H33258 (1 μg/ml). Slides were viewed and photographed with a Zeiss Axioskop fluorescence microscope and an MC 100 camera attachment. Confocal laser scanning was performed using a Sarastro 2000 confocal laser scanning microscope (Molecular Dynamics, Inc., Sunnyvale, CA) and Image Space software (Molecular Dynamics).
Plasmids
Using standard recombinant DNA manipulations, cDNA encoding murine BAXΔ1-19 was constructed in pBluescript SK under control of the T7 promoter; the resulting translation product had an initiating methionine located 20 amino acids downstream of the original methionine of the full-length BAX construct. cDNA encoding DHFR-BAX™ was created by PCR in Bluescript SK, and encoded murine dihydrofolate reductase fused to the COOH-terminal 23 amino acids (aa 169–192) of BAX. Similarly, BAX-BCL-2™ was created by fusing amino acids 1–168 of BAX to amino acid 218–239 of human BCL-2.
Mitochondria from Rat Heart
The heart of one male Sprague Dawley rat (∼250 g) was placed in ∼100 ml HIM buffer (0.2% wt/vol BSA, 200 mM mannitol, 70 mM sucrose, 10 mM Hepes-KOH, 1 mM EGTA, pH 7.5) on ice, squeezed several times to force out blood, and transferred to 7.5 ml HIM in a 15-ml corex tube. All subsequent steps were conducted at 4°C. The heart was homogenized with a Polytron homogenizer (Brinkmann Instruments Canada Ltd., Mississauga, ON, Canada) operating for 1 s at setting of 6.5. Nuclei and unbroken cells were pelleted at 1,800 rpm for 10 min in a Sorvall SS34 rotor. The supernatant was centrifuged for 10 min at 7,000 rpm. The resulting pellet was resuspended in HIM (minus BSA), centrifuged at 1,800 rpm, and the mitochondria were collected at 7,000 rpm. The mitochondrial pellet was resuspended in cMRM (250 mM sucrose, 10 mM Hepes-KOH, 1 mM ATP, 5 mM Na succinate, 0.08 mM ADP, 2 mM K2HPO4, pH 7.5) at a concentration of 1 mg of mitochondrial protein per ml, and adjusted to 1 mM dithiothreitol just before use.
Mitochondria from Cultured Cells
Cells were collected, washed twice with PBS, suspended in 2 ml HIM, and subjected to Polytron homogenization for four bursts of 10 s each at a setting of 6.5. Mitochondria were then isolated according to the rat heart protocol.
Mitoplasts
Protein import-competent mitoplasts (mitochondria with a severely disrupted outer membrane) in which the inner membrane and transbilayer electrochemical potential remain intact were prepared from rat liver exactly as described in McBride et al. (1995).
Mitochondrial Protein Targeting In Vitro
cDNAs were transcribed and translated in the presence of 35S-methionine in a cell-free rabbit reticulocyte lysate system (Promega Corp., Madison, WI) according to the manufacturer's directions. For a standard mitochondrial protein import reaction, 5 μl of translation reaction was incubated with 20 μl of Buffer A (20 mM Hepes-KOH, 10 mM KCl, 2.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, pH 7.5) and 25 μl mitochondria or mitoplasts in cMRM (1 mg protein/ml) at 30°C or 37°C for 30 min to 2 h. The import reaction was then layered on a 500-μl cushion of 1× MRM (250 mM sucrose, 10 mM Hepes-KOH, pH 7.5) and centrifuged for 4 min at top speed in a microfuge at 4°C. For alkali extraction, the mitochondrial pellet was resuspended (0.25 mg protein/ml) in freshly prepared 0.1 M Na2CO3, pH 11.5, and incubated for 30 min on ice. The membranes were then pelleted in an airfuge (Beckman Instruments Canada, Inc., Montreal, PQ, Canada) operating for 10 min at 30 psi. The pellet was analyzed by SDS-PAGE and fluorography. For import assays that included apoptotic cell extract (see below), it replaced buffer A; the buffer used to make the extract, buffer A/ext, did not influence import.
Apoptotic Cell Extract
Extract was prepared at 4°C according to the procedure of Liu et al. (1996) with some minor modifications. In brief, human KB epithelial cells were harvested in PBS containing 1.3 mM Na citrate and 0.6 mM EDTA. The cell pellet was washed in buffer A/ext (50 mM Pipes, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol, 20 μM cytochalasin B, pH 7.4), and was then resuspended in an equal volume of buffer A/ext. The cells were disrupted by five cycles of freeze-thaw interspersed by five strokes with a Wheaton glass homogenizer fitted with a B pestle, and centrifuged at 105 g for 1 h in a Beckman Ti75 rotor. The resulting supernatant contained 10 mg protein/ml. To deplete the supernatant of cytochrome c (Liu et al., 1996), 40 μl of 2G8.B6 anti-cytochrome c antibody (7.7 mg protein/ml) was first incubated with 100 μl of a 1:1 mixture of protein A and protein G Sepharose resuspended in 200 μl of PBS for 4 h at 4°C. For the control reaction, 40 μl of buffer A was substituted for the antibody. The beads were collected, washed with buffer A, and then incubated with 125 μl of KB cell extract for 18 h at 4°C. The beads were recovered and the supernatant was collected.
PARP Cleavage
KB apoptotic cell extract (50–100 μg protein) was incubated with 1 mM dATP in a final volume of 20 μl of buffer A at 30°C for 1 h, and 1 μl of 35S-methionine–labeled PARP derived by transcription-translation in rabbit reticulocyte lysate was added. After 15 min at 30°C, 5 μl of 5× SDS sample buffer was added, and 12.5-μl aliquots of each reaction were analyzed by SDS-PAGE and fluorography.
Results
BAX Integrates into Mitochondrial Membrane after a Death Stimulus In Vivo
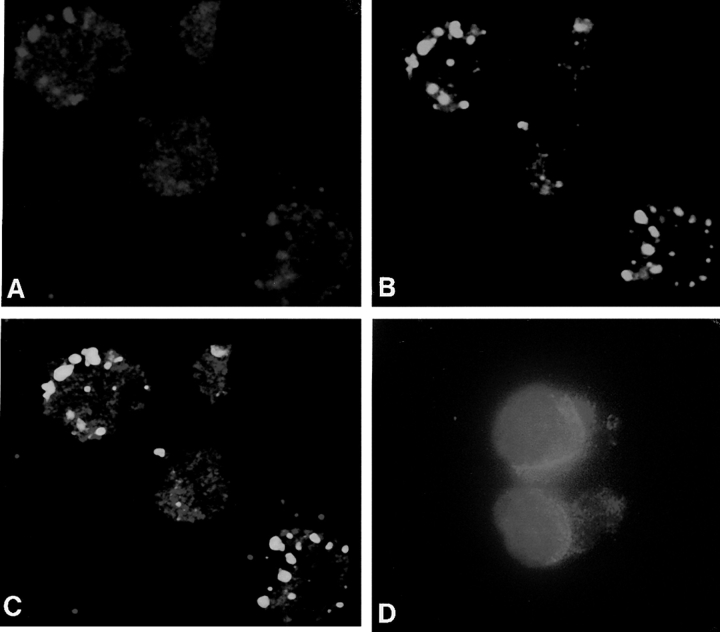
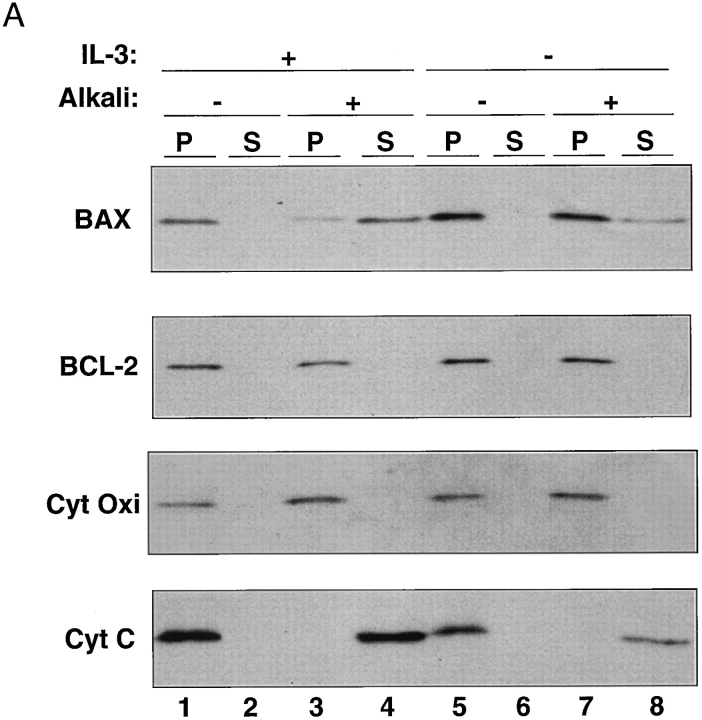
Immunocytochemical analysis of FL5.12 hematopoietic cells revealed BAX in association with mitochondria, as well as distributed throughout the cytoplasm and perinuclear region (Fig. 1). When analyzed by immunoblotting, the mitochondrial fraction isolated from these cells revealed the presence of BAX as well as BCL-2, cytochrome c oxidase subunit iv, and cytochrome c (Fig. 2 A, lanes 1 and 2). In contrast to BCL-2, which was resistant to extraction at alkaline pH as a result of its integration into the membrane lipid bilayer (Nguyen et al., 1993; Nguyen et al., 1994), the majority of BAX was liberated under the same conditions (Fig. 2 A, lanes 3 and 4; Fig. 2 C, lanes 1 and 5), indicative of a peripheral association with the organelle (Fujiki et al., 1982). After induction of cell death upon withdrawal of IL-3 (Hockenbery et al., 1990), however, this situation was reversed and most of the BAX now acquired resistance to alkaline extraction (Fig. 2 A, lanes 5–8; Fig. 2 C, lanes 3 and 7). As expected, cytochrome c was released in response to alkaline extraction, whereas BCL-2 and cytochrome c oxidase subunit iv were retained, and these distributions were unaffected by the death stimulus (Fig. 2 A). We conclude, therefore, that BAX demonstrates a specific response to the death signal in vivo, moving from a membrane-peripheral to a membrane-integrated position.
Figure 1.
Subcellular distribution of BAX. BAX immunoreactivity localizes to mitochondria, perinuclear membrane, and cytosol in FL5.12 cells. Immunofluorescent confocal microscopy of FL5.12 cells using rabbit anti-BAX (P-19) antibody and goat Cy3 anti-rabbit Ig antibody shows both punctate and diffuse cytoplasmic immunoreactivity (A). MitoTracker Green FM shows exclusive punctate labeling (B). Dual exposure of BAX and MitoTracker labeling reveals colocalization of BAX with mitochondria (yellow) as well as nonmitochondrial BAX immunoreactivity (red; C). Conventional fluorescence detection of anti-BAX (P-19) immunoreactivity (Cy3) and Hoechst H33258 nuclear stain shows red cytoplasmic BAX immunoreactivity, blue nuclear Hoechst staining and pink perinuclear signal where the two reactivities overlap (D).
Figure 2.
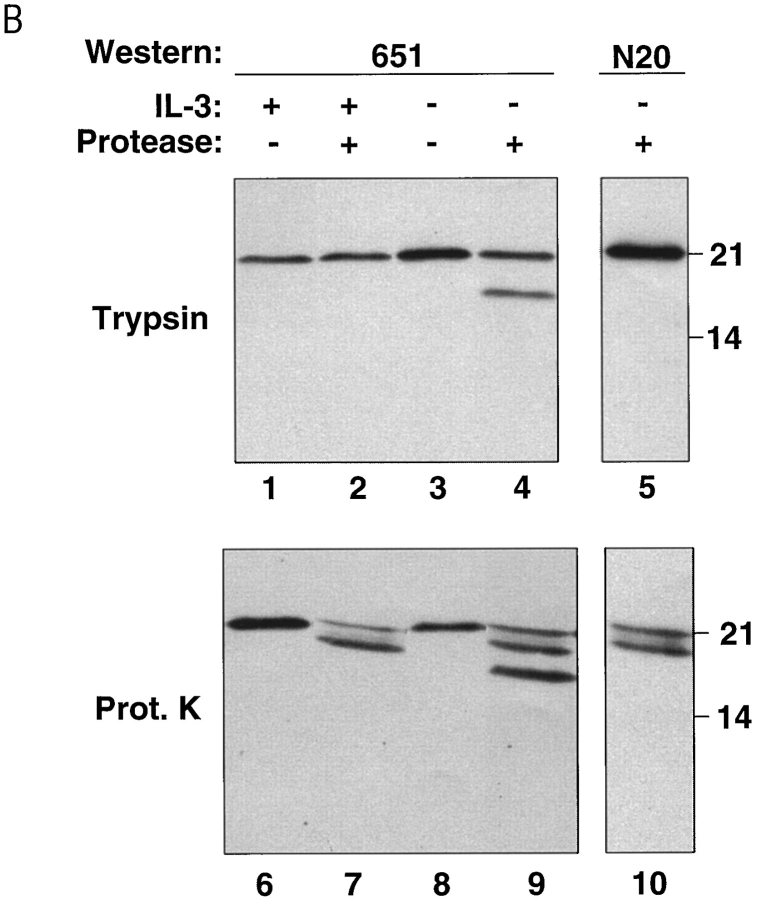
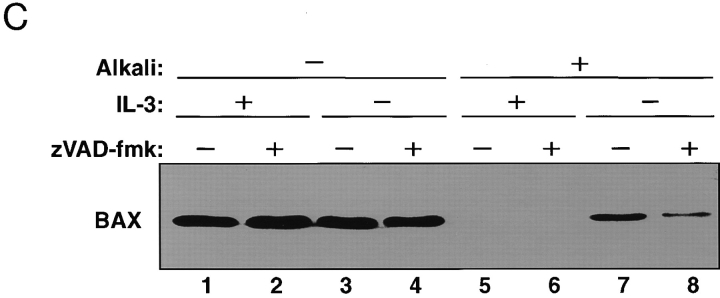
Mitochondrial BAX changes its alkali and protease sensitivity after a death stimulus. (A) BAX becomes alkali-resistant after a death stimulus. Heavy membranes enriched in mitochondria were prepared from FL5.12 cells cultured in IL-3 (lanes 1–4) or deprived of IL-3 for 12 h (lanes 5–8), incubated in isotonic buffer (lanes 1 and 2 and lanes 5 and 6), or in 0.1 M Na2CO3, pH 11.5 (lanes 3 and 4 and lanes 7 and 8), and centrifuged at 200,000 g for 45 min to yield supernatant (S) and pellet (P). The fractions were analyzed by immunoblot for BAX, BCL-2, cytochrome c oxidase subunit iv, and cytochrome c. (B) The NH2-terminus of BAX is further exposed after a death stimulus. The mitochondrial fraction prepared from FL5.12 cells in IL-3 (lanes 1 and 2 and lanes 6 and 7) or deprived of IL-3 for 12 h (lanes 3–5, and lanes 8–10) were incubated in isotonic buffer and treated with trypsin (30 μg/ml; lanes 2, 4, and 5) or proteinase K (100 μg/ml; prot. K, lanes 7, 9, and 10), for 20 min at 4°C. Trypsin was inactivated by adding a 30-fold weight excess of soybean trypsin inhibitor, and proteinase K was inactivated with phenylmethylsulfonylfluoride (1 mM). The fractions were analyzed by immunoblot with anti-BAX 651 antibody (amino acids 43–61; lanes 1–4 and lanes 6–9) or with anti-BAX N20 antibody (amino acids 11–30; Santa Cruz Biotechnology; lanes 5 and 10). (C) zVAD-fmk reduces mitochondrial membrane integration of BAX after IL-3 withdrawal. The mitochondrial fraction prepared from FL5.12 cells maintained in IL-3 (lanes 1, 2, 5, and 6) or deprived of IL-3 for 12 h (lanes 3, 4, 7, and 8) in the absence (lanes 1, 3, 5, and 7) or presence (lanes 2, 4, 6, and 8) of 50 μM zVAD-fmk for 12 h, were analyzed either directly (lanes 1–4) or after extraction with 0.1 M Na2CO3, pH 11.5 (lanes 5–8), as described in A.
Of note, integration of BAX into mitochondrial membrane after IL-3 withdrawal was also accompanied by an apparent change at the extreme NH2 terminus of the protein. In isolated mitochondria, this region of the BAX molecule was now accessible to limited proteolysis by exogenous trypsin or proteinase K, as revealed by the loss of immunoreactivity of the cleavage product to an antibody (N20) directed against amino acids 11–30 of BAX (Fig. 2 B). The remaining protease-resistant BAX fragment, on the other hand, retained immunoreactivity to an antibody (651) directed against amino acids 43–61.
Finally, BAX integration into mitochondrial membrane in response to IL-3 withdrawal from FL5.12 cells for 12 h was partly blocked by the wide spectrum caspase inhibitor, zVAD-fmk. Whereas the inhibitor did not significantly influence recovery of total BAX associated with mitochondria (Fig. 2 C, lanes 3 and 4), it reduced the amount of alkaline-resistant membrane-integrated BAX that was recovered with the organelle (Fig. 2 C, lanes 7 and 8). This suggests that caspases, which are activated in response to IL-3 withdrawal, stimulate mitochondrial membrane integration of BAX in vivo.
An Apoptotic Cell Extract Stimulates BAX Targeting to Mitochondria In Vitro
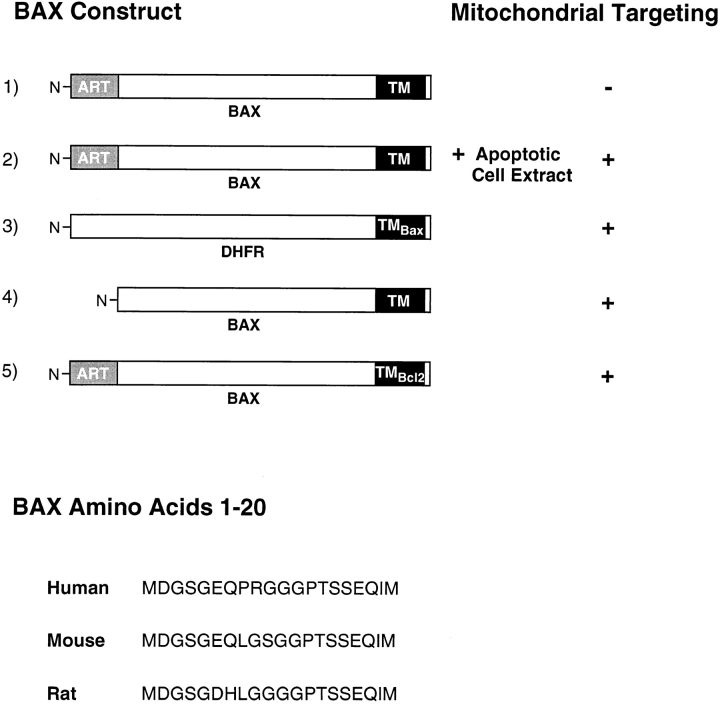
To examine BAX targeting into mitochondria in vitro, we used the 35S-labeled transcription-translation product of BAX cDNA in rabbit reticulocyte lysate combined with intact mitochondria isolated from rat heart. This is a well-documented system that faithfully reflects in vivo import of diverse mitochondrial proteins, including insertion of integral proteins into the mitochondrial outer membrane (Li and Shore, 1992; McBride et al., 1992). The various BAX constructs that were used for these assays, and their targeting competence in vitro, are summarized in Fig. 3.
Figure 3.
BAX constructs and their competence for mitochondrial targeting in vitro. ART domain (gray box); TM (black box); +, targeting-competent; −, targeting-incompetent. Amino acids 1–20 of BAX from the indicated species are shown. See text for additional details.
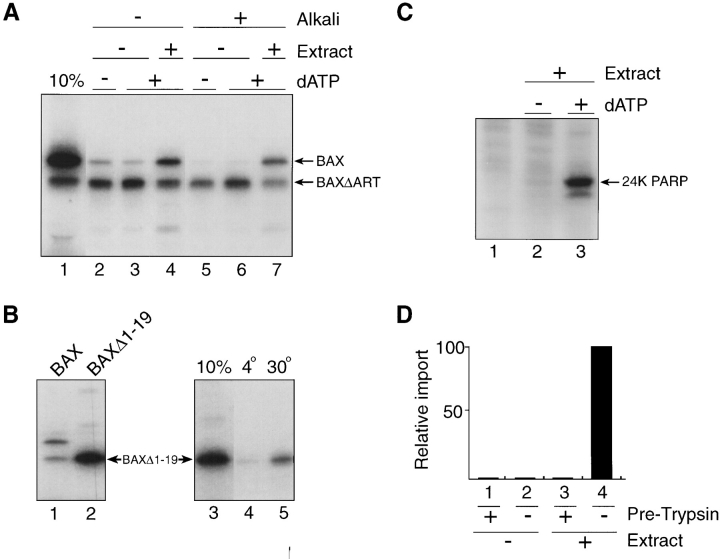
Transcription-translation of BAX cDNA yielded full-length protein, as well as a prominent product ∼2 kD smaller and apparently arising from an internal translation initiation, resulting in an NH2-terminal truncated BAX (designated BAXΔART). However, only the truncated BAXΔART was membrane-integrated, as judged by acquired resistance to extraction at alkaline pH (Fig. 4 A; compare lanes 2 and 5). Insertion of this product was temperature-sensitive; it occurred at 30°C, but not at 4°C (Fig. 5, lanes 2–5), indicative of membrane integration rather than tight but nonspecific binding (McBride et al., 1992). Inspection of the BAX sequence revealed the presence of an internal methionine at codon position 20 (Fig. 3). Enforced translation initiation from met codon 20 was achieved by deleting codon 1, which yielded a product that comigrated in SDS PAGE with BAXΔART and demonstrated temperature-sensitive membrane integration (Fig. 4 B). We conclude, therefore, that amino acids 1–19 of full-length BAX harbors a domain, designated ART, that is required for retention of BAX in a membrane insertion– incompetent state. This domain is rich in glycine and hydroxylated amino acid residues (Fig. 3).
Figure 4.
Apoptotic cell extract supports BAX targeting to mitochondria in vitro. (A) 35S-labeled transcription-translation products of BAX cDNA in rabbit reticulocyte lysate (BAX and BAXΔART) were incubated with purified intact mitochondria from rat heart in a standard protein import reaction supplemented with 1 mM dATP (lanes 3, 4, 6, and 7) or 1 mM dATP and 200 μg apoptotic cytosol protein (Extract; lanes 4 and 7) in a volume of 50 μl. At the end of the reaction, mitochondria were recovered by centrifugation, and the pellets were analyzed by SDS PAGE and fluorography either directly or after extraction with 0.1 M Na2CO3, pH 11.5 (Alkali; lanes 5–7). Lane 1, 10% of input translation product. (B) As in A except that import was conducted with BAXΔ1-19 cDNA transcription product (lane 2) at 4°C or 30°C as indicated, and was analyzed after extraction of the mitochondria with 0.1M Na2CO3, pH 11.5 (lanes 4 and 5). (C) 35S-labeled transcription-translation product of PARP cDNA in reticulocyte lysate (5% by volume) was incubated with (lanes 2 and 3) or without (lane 1) 100 μg apoptotic cytosol protein in a total volume of 20 μl, in the presence (lane 3) or absence (lanes 1 and 2) of 1 mM dATP, and equivalent portions were analyzed for the appearance of the 24-kD cleavage product of PARP (24K PARP) by 12% SDS PAGE and fluorography. (D) As in A, except that before import, mitochondria were treated with 0.4 mg/ml trypsin and 50-fold weight excess soybean trypsin inhibitor added either at the beginning (− Pre-Trypsin; columns 2 and 4) or at the end (+ Pre-Trypsin; columns 1 and 3) of the reaction. Mitochondria were collected by centrifugation and import conducted in the presence (columns 3 and 4) or absence (columns 1 and 2) of apoptotic cytosol (Extract), and the relative amount of alkali-insoluble full-length BAX was determined (maximum set at 100).
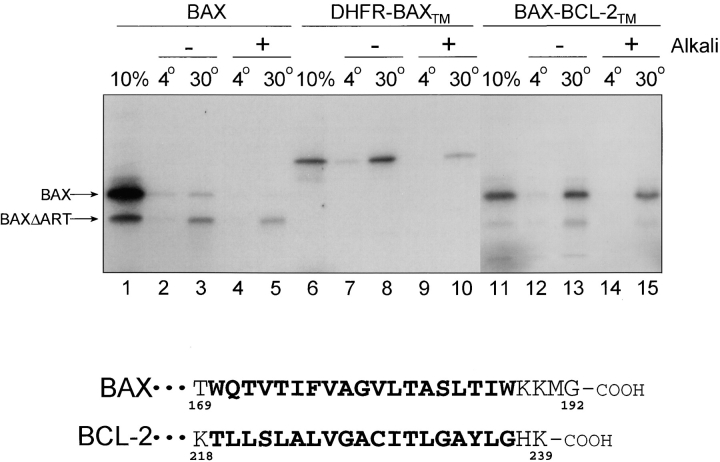
Figure 5.
Failure of BAX to target mitochondria in vitro depends on the BAX TM. cDNAs encoding BAX (lanes 2–5), cytosolic dihydrofolate reductase fused at its COOH terminus to amino acids 169– 192 of BAX (DHFR-BAX™, lanes 7–10), and BAX in which the COOH-terminal 24 amino acids have been replaced by the corresponding domain of BCL-2 (amino acids 218–239) (BAX-BCL-2™, lanes 12–15) were transcribed and translated in reticulocyte lysate and the 35S-labeled, alkali-insoluble mitochondrial import products were analyzed by SDS PAGE and fluorography. Lanes 1, 6, and 11 represent 10% of input translation product, as indicated. The sequence (single letter code) of the COOH-terminal 24 amino acids of BAX and 22 amino acids of BCL-2 are shown; bold letters designate the predicted TMs.
Importantly, the inability of full-length BAX to target mitochondria in vitro was overcome by supplementing the import reaction mixture with an apoptotic cell extract derived from KB epithelial cells. This extract was prepared according to Liu et al. (1996), and involved cycles of freeze/thaw and homogenization of cells in hypotonic medium, causing swelling of mitochondria and consequent release of cytochrome c as a result of a ruptured outer membrane. The resulting high-speed cytosolic supernatant contains endogenous procaspases whose activation can be achieved by adding dATP and incubating the extract at 37°C, resulting in diagnostic cleavage of the caspase-3 death substrate, poly(ADP ribosyl) polymerase (PARP; Liu et al., 1996). The presence of this extract in import reactions stimulated binding (Fig. 4 A, lanes 3 and 4) and alkaline-resistant membrane insertion of full-length BAX (lanes 6 and 7), but did not stimulate BAXΔART insertion (lanes 6 and 7). Membrane-integrated BAX was accessible to exogenous trypsin in these intact mitochondria (not shown), indicative of insertion into the outer membrane. Apoptotic activity of the cell extract was verified by demonstrating its capacity to generate the 24-kD apoptotic fragment of PARP in response to dATP (Liu et al., 1996) (Fig. 4 C). Nonapoptotic high speed cytosol did not stimulate import of BAX, and did not support cleavage of PARP (not shown). Also, BAX failed to target mitoplasts (mitochondria partially or wholly stripped of outer membrane) in the absence of apoptotic extract (not shown), indicating that failure to import cannot be explained by the fact that an intact outer membrane constitutes a barrier that prevents BAX from accessing the inner membrane. Finally, pretreatment of intact mitochondria with trypsin abolished subsequent membrane insertion of full-length BAX in response to apoptotic extract (Fig. 4 D), indicating that insertion is dependent on protein(s) exposed on the organelle surface.
ART and TM Domains Are Required for Regulated Targeting of BAX In Vitro
The results presented in Fig. 4 demonstrate that apoptotic regulation of BAX targeting in vitro can be bypassed by deleting the NH2-terminal ART domain, resulting in constitutive targeting of the protein. Similarly, replacement of the entire cytosolic portion of BAX (amino acids 1–168) with that of the monomeric reporter protein dihydrofolate reductase (DHFR-BAX™) permitted import into the outer membrane of intact mitochondria in the absence of apoptotic cell extract, as revealed by temperature-sensitive acquisition of resistance to extraction at alkaline pH (Fig. 5, lanes 6–10). This shows that the BAX TM can function as a signal-anchor sequence that, in the appropriate context, is independently active in targeting and membrane insertion. It also suggests that ART directly or indirectly prevents manifestation of this signal-anchor function of the BAX TM in the context of the full-length BAX molecule. Importantly, however, replacement of the COOH-terminal 22 amino acids of full-length BAX, which contains the TM, with the corresponding TM domain of BCL-2 now permitted targeting of the previously membrane insertion–incompetent BAX (Fig. 5, lanes 11–15). Thus, the mechanism underlying the inability of BAX to target mitochondria in vitro requires both the presence of the specific BAX TM within the molecule, as well as the NH2-terminal ART domain.
BAXΔART and BAX-BCL-2™ Exhibit Enhanced Cytotoxicity
In cotransfection experiments with a luciferase reporter as an index of survival of COS-7 and CHO cells (Ng et al., 1997), expression of hemagglutinin epitope (HA)-BAXΔART or HA-BAX-BCL-2™ resulted in luciferase activity three to five times lower than that obtained with HA-BAX 24 h after transfection (not shown). Moreover, when analyzed microscopically by immunofluorescence with anti-HA antibody, 7.5% of cells expressed HA-BAX at 24 h, whereas <1% expressed HA-BAXΔART weakly (not shown), indicating that most cells expressing HA-BAX ΔART had previously perished. Thus, the enhanced cytotoxicity of HA-BAXΔART compared with HA-BAX in vivo correlates with inhibition of BAX membrane integration by the ART domain in vitro.
Stimulation of BAX Targeting by Cell Extract Requires Cytochrome c and Is Inhibited by zVAD-fmk
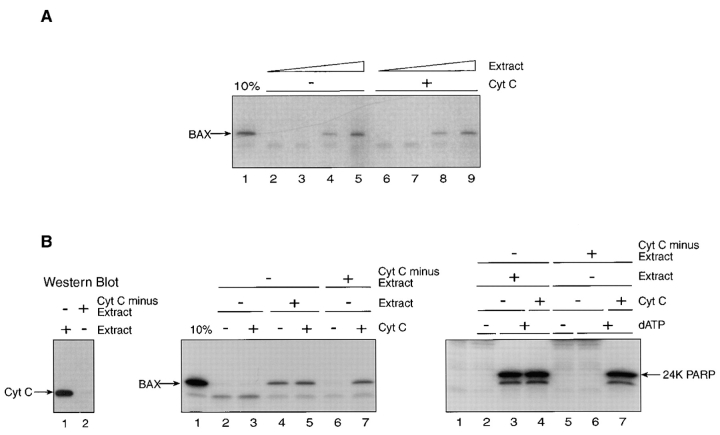
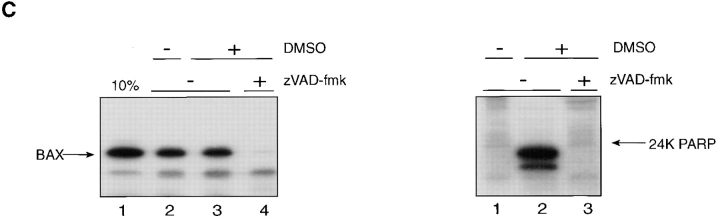
When high-speed apoptotic cytosol supplemented with dATP was examined for its ability to support targeting of full-length BAX to mitochondria in vitro, a marked dose-dependent stimulation was observed (Fig. 6 A). Of note, no improvement to mitochondrial membrane insertion of BAX was achieved by providing additional cytochrome c (lanes 6–9). The requirement for nucleotide co-factor in the apoptotic extract was not assessed because ATP, which can substitute for dATP in caspase activation, is normally required for mitochondrial protein import. Depleting cytochrome c from the high-speed cytosol by immunoadsorption before adding dATP and incubating at 37°C (Fig. 6 B, left), on the other hand, inhibited the ability of the extract to support BAX targeting (Fig. 6 B, middle, compare lanes 4 and 6) and PARP cleavage (Fig. 6 B, right, compare lanes 3 and 6); cytochrome c reinstated both events when added back to the extract (Fig. 6 B middle and right; compare lanes 6 and 7). Also, at least one other factor present in the extract was needed for cytochrome c–dependent regulation of BAX targeting to mitochondria since supplementation of reticulocyte lysate translation mixtures with cytochrome c alone failed to stimulate BAX targeting (not shown). Consistent with this and with the role of cytochrome c in initiating activation of a caspase cascade (Li et al., 1997), the wide spectrum inhibitor of caspases, zVAD-fmk (Zhu et al., 1995), was found to inhibit BAX targeting supported by apoptotic high-speed cytosol effectively (Fig. 6 C, left; compare lanes 3 and 4) at concentrations that abolished the ability of the extract to drive PARP cleavage (Fig. 6 C, right; compare lanes 2 and 3).
Figure 6.
The ability of apoptotic high-speed cytosol to support BAX targeting to mitochondria in vitro depends on cytochrome c, and is inhibited by zVAD-fmk. (A) The 35S-labeled transcription-translation products of BAX cDNA were subjected to mitochondrial import in vitro in the presence of 0 (lanes 2 and 6), 13 (lanes 3 and 7), 65 (lanes 4 and 8), or 260 (lanes 5 and 9) μg apoptotic high-speed cytrosolic protein (Extract) with (lanes 6–9) or without (lanes 2–5) added cytochrome c (Cyt C; 75 μg/ml), and the alkali-resistant products were visualized after SDS PAGE and fluorography. Lane 1, 10% of input translation product. (B; left) Cytochrome c was removed from apoptotic cell extract by immunoadsorption (Cyt C minus extract) using mouse monoclonal 2G8.B6 antibody (Mueller and Jemmerson, 1996) as described (Liu et al., 1996). Equivalent aliquots of the extract before (lane 1) and after (lane 2) immunoadsorption were analyzed by Western immunoblot using mouse monoclonal 7H8.2C12 antibody against cytochrome c (Liu et al., 1996), and were visualized by enhanced chemiluminescence. (Middle) Membrane insertion of BAX translation products, determined by resistance to extraction at pH 11.5, was conducted and analyzed as in A in the presence or absence of apoptotic cytosol, which had or had not been subjected to immunoadsorption with anti-cytochrome c or supplemented with added cytochrome c before the addition of dATP and incubation at 37°C, as indicated. (Right) The ability of this same apoptotic cytosol to generate the 24-kD apoptotic cleavage product of PARP was determined under the conditions indicated, as described in Fig. 4. (C) The ability of the apoptotic high-speed cytosol to support membrane insertion of BAX (alkaline-resistant product, lanes 2–4; left) and PARP cleavage (right) was assayed as in A in the presence or absence of 50 μM tetrapeptide zVAD-fmk that was delivered from a 100× concentrated stock dissolved in dimethylsulfoxide before adding dATP and incubating at 37°C. An equivalent volume of this solvent had no effect on BAX import (left, lane 3).
Discussion
In vivo, BAX is restrained from inserting into target membranes, including mitochondria, until the cell receives a death signal. Similarly, BAX synthesized in a rabbit reticulocyte translation system fails to target mitochondria in vitro. Failure to target in this reconstituted mitochondrial import reaction depends on two regions within the BAX polypeptide: the NH2-terminal ART domain and the COOH-terminal TM. Significantly, the TM functions as a signal anchor that is required for targeting and insertion into the mitochondrial outer membrane. Under normal conditions, however, manifestation of this signal-anchor activity is repressed, and depends not only on the nature of the signal-anchor itself but on the NH2-terminal ART domain as well. As expected, deletion of the ART domain enhances the cytotoxic properties of BAX in vivo, which correlates with stimulation of membrane integration in vitro. Although the underlying mechanism for stimulating BAX targeting remains to be elucidated, it is noteworthy that overexpression of the protein in vivo induces cell death (Xiang et al., 1996; Rossé et al., 1998). This is consistent, for example, with the existence of a saturable inhibitor of BAX targeting. It is interesting in this regard that the NH2 terminus, including the ART domain, is further exposed after membrane insertion of BAX in vivo (Fig. 2 B).
After delivery of diverse death signals to cells in culture (Hsu et al., 1997; Wolter et al., 1997), BAX moves to mitochondria and other membrane sites, and triggers a catastrophic transformation of mitochondrial function. This transformation includes release of cytochrome c to the surrounding cytosol, production of reactive oxygen species, loss of transmembrane potential, and induction of mitochondrial permeability transition (Xiang et al., 1996; Rossé et al., 1998), events that result in apoptotic cell death (Reed, 1997; Kroemer, 1997). Of note, BCL-2 proteins have the potential to intercede and block these BAX-induced events at several levels, including prevention of BAX redistribution after a death signal (Gross et al., 1998), heterodimerization with mitochondrial BAX (Oltvai et al., 1993), and inhibition of cytochrome c release (Kluck et al., 1997; Yang et al., 1997), and interception of cytochrome c–mediated downstream pathways (Li et al., 1997; Rossé et al., 1998; Zhivotovsky et al., 1998; Pan et al., 1998).
In view of the fact that zVAD-fmk partly blocked BAX insertion into mitochondrial membrane in response to a death signal in vivo (Fig. 2 C), we examined apoptotic cytosolic extracts (Liu et al., 1996) with the potential to activate a caspase cascade, and its influence on BAX targeting to mitochondria in vitro. Previous analyses of such an extract led to the discovery of a core caspase-activating complex in mammalian cells in which cytochrome c and the nucleotide cofactor dATP/ATP (Liu et al., 1996) interact with the Ced-4 homologue Apaf-1 (Zou et al., 1997), causing it to recruit and activate initiator procaspase-9. Caspase-9, in turn, processes procaspase-3 (Li et al., 1997). When such an extract was added in vitro to a reticulocyte lysate harboring BAX, targeting and mitochondrial membrane integration was stimulated. This stimulating activity depended on cytochrome c being present in these extracts during incubations to activate caspases, and was blocked by the wide spectrum caspase inhibitor, zVAD-fmk. In view of current models suggesting that BAX initiates cytochrome c release from mitochondria (see Jürgensmeier et al., 1998), however, these findings were unexpected. One possibility, therefore, is that the in vitro reconstitution sytem described here reflects a mechanism for amplifying BAX targeting. In this scenario, BAX targeting to mitochondria might be initiated by a signal transduction event; BAX, as a consequence of its pore-forming properties (Schlesinger et al., 1997), then triggers release of cytochrome c and activation of a caspase, which in turn cleaves a protein that regulates BAX targeting (e.g., it inactivates an inhibitor or activates an inducer of BAX targeting). This model is consistent with the partial inhibitory influence of zVAD-fmk on stimulated membrane integration of BAX in vivo.
Alternatively, release of low levels of cytochrome c might be initiated by a BAX-independent event, perhaps involving controlled swelling of mitochondria and localized rupture of the outer membrane (Vander Heiden et al., 1997). This release in turn would result in activation of caspases, stimulation of BAX targeting, and consequent amplification of cytochrome c release via a BAX-mediated process. One consequence of the possibility that limited rupturing of the outer membrane may precede BAX targeting to mitochondria relates to the options for final destination of the protein. In this scenario, BAX might gain access to and directly influence both the outer and inner membranes. However, failure to access the inner membrane does not explain the failure of BAX to target mitochondria in the absence of a death signal, since mitoplasts do not insert BAX in the absence of apoptotic cytosol (not shown).
In conclusion, we have found that activation of one or more zVAD-sensitive caspases stimulate BAX targeting to mitochondria, possibly by influencing, directly or indirectly the activity of a regulator that controls membrane insertion of BAX. Regulated targeting of BAX depends on both the NH2-terminal ART domain and the COOH-terminal TM within the BAX molecule. It remains to be determined, however, if these domains mediate physical association of BAX with cytosolic or membrane proteins that control targeting, or if they are susceptible to other forms of regulation.
Acknowledgments
This work was supported by operating grants from the National Cancer Institute and the Medical Research Council of Canada, and by CA49712. J.N. Lavoie and A. Gross are supported by postdoctoral fellowships from the Medical Research Council and the European Molecular Biology Organization, respectively.
Abbreviations used in this paper
- HA
hemagglutinin epitope
- PARP
poly(ADP ribosyl) polymerase
- TM
transmembrane segment
Footnotes
Address all correspondence to Gordon C. Shore, Department of Biochemistry, McIntyre Medical Sciences Building, McGill University, Montreal, Quebec, Canada H3G 1Y6. Tel.: (514) 398-7282. Fax: (514) 398-7384. E-mail: shore@med.mcgill.ca
References
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J-J, Mazzei G, et al. Inhibition of Bax channel-forming activity by BCL-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Aja T, Xiang J, Gaur S, Krebs JF, Hoang K, Bai X, Korsmeyer SJ, Karanewsky DS, Fritz LC, Tomaselli KJ. Fas-induced activation of the cell death-related protease CPP32 is inhibited by BCL-2 and by ICE family protease inhibitors. J Biol Chem. 1996;271:16850–16855. doi: 10.1074/jbc.271.28.16850. [DOI] [PubMed] [Google Scholar]
- Boulakia CA, Chen G, Ng FWH, Teodoro JG, Branton PE, Nicholson DW, Poirier GG, Shore GC. BCL-2 and adenovirus E1B 19-kDa protein prevent E1A-induced processing of CPP32 and cleavage of poly(ADP-ribose) polymerase. Oncogene. 1996;12:529–535. [PubMed] [Google Scholar]
- Cheng EH-Y, Levine B, Boise LH, Thompson CG, Hardwick JM. Bax-independent inhibition of apoptosis by BCL-XL . Nature. 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, Orth K, O'Rourke K, Duan H, Poirier GG, Dixit VM. Molecular ordering of the cell death pathway. BCL-2 and BCL-XLfunction upstream of the CED-3-like apoptotic proteases. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, O'Rourke K, Lane BR, Dixit VM. Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science. 1997;275:1122–1126. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- Fujiki Y, Fowler S, Shio H, Hubbard AL, Lazarow P. Polypeptide and phospholipid composition of the membrane of rat liver peroxisomes: comparison with endoplasmic reticulum and mitochondrial membranes. J Cell Biol. 1982;93:103–110. doi: 10.1083/jcb.93.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO (Eur Mol Biol Organ) J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MO. Death cycle and Swiss army knives. Nature. 1998;391:441–442. doi: 10.1038/35036. [DOI] [PubMed] [Google Scholar]
- Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Hsu Y-T, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-XLduring apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James C, Gschmeissner S, Fraser A, Evan GI. CED-4 induces chromatin condensation in Schizosaccharomyces pombe and is inhibited by direct physical association with CED-9. Curr Biol. 1997;7:246–252. doi: 10.1016/s0960-9822(06)00120-5. [DOI] [PubMed] [Google Scholar]
- Jürgensmeier JM, Xie Z, Devereaux Q, Ellerby L, Breseden D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Knudson CM, Korsmeyer SJ. Bcl-2 and Baxfunction independently to regulate cell death. Nat Genet. 1997;16:358–363. doi: 10.1038/ng0897-358. [DOI] [PubMed] [Google Scholar]
- Krajewski S, Tanaka S, Takayama S, Schibler M, Fenton W, Reed JC. Investigation of the subcellular distribution of the Bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum and outer mitochondrial membranes. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- Li J-M, Shore GC. Reversal of the orientation of an integral protein of the mitochondrial outer membrane. Science. 1992;256:1815–1817. doi: 10.1126/science.1615327. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan C, Budihardjo I, Srinivasula S, Ahmad M, Alnemri E, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/ caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- McBride HM, Millar DG, Li J-M, Shore GC. A signal-anchor sequence selective for the mitochondrial outer membrane. J Cell Biol. 1992;119:1451–1457. doi: 10.1083/jcb.119.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride HM, Silvius JR, Shore GC. Insertion of an uncharged polypeptide into the mitochondrial inner membrane does not require a trans-bilayer electrochemical potential: effects of positive charges. Biochim Biophys Acta. 1995;1237:162–168. doi: 10.1016/0005-2736(95)00088-k. [DOI] [PubMed] [Google Scholar]
- McCarthy NJ, Whyte MKB, Gilbert CS, Evan GI. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Vélez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M, Thompson CB. Bcl-XLforms an ion channel in synthetic lipid membranes. Nature. 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- Mueller CM, Jemmerson R. Maturation of the antibody reponse to the major epitope on the self antigen mouse cytochrome c. Restricted V gene usage, selected mutations, and increased affinity. J Immunol. 1996;157:5329–5338. [PubMed] [Google Scholar]
- Ng FWH, Nguyen M, Kwan T, Branton PE, Nicholson DW, Cromlish JA, Shore GC. p28 Bap31, a Bcl-2/Bcl-X-L- and procaspase-8-associated protein in the endoplasmic reticulum. J Cell Biol. 1997;139:327–338. doi: 10.1083/jcb.139.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng FWH, Shore GC. Bcl-XLcooperatively associates with the Bap31 complex in the endoplasmic reticulum, dependent on procaspase-8 and Ced-4 adaptor. J Biol Chem. 1998;273:3140–3143. doi: 10.1074/jbc.273.6.3140. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Millar DG, Yong VW, Korsmeyer SJ, Shore GC. Targeting of Bcl-2 to the mitochonrial outer membrane by a COOH-terminal signal anchor sequence. J Biol Chem. 1993;268:25265–25268. [PubMed] [Google Scholar]
- Nguyen M, Branton PE, Walton PA, Oltavai ZN, Korsmeyer SJ, Shore GC. Role of membrane anchor domain of Bcl-2 in suppression of apoptosis caused by E1B-defective adenovirus. J Biol Chem. 1994;269:16521–16524. [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homologue, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Pan G, O'Rourke K, Dixit VM. Caspase-9, Bcl-XL, and Apaf-1 form a ternary complex. J Biol Chem. 1998;273:5841–5845. doi: 10.1074/jbc.273.10.5841. [DOI] [PubMed] [Google Scholar]
- Reed JC. Cytochrome c: can't live with it-can't live without it. Cell. 1997;91:559–562. doi: 10.1016/s0092-8674(00)80442-0. [DOI] [PubMed] [Google Scholar]
- Rossé T, Olivier R, Monney L, Roger M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- Schendel SL, Xie Z, Montal MO, Matsuyama S, Montal M, Reed JC. Channel formation by anti-apoptotic protein Bcl-2. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger PH, Gross A, Yin X-M, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc Natl Acad Sci USA. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham S, Horvitz JR. Developing Caenorhabditis elegansneurons may contain both cell-death protective and killer activities. Genes Dev. 1996;10:578–591. doi: 10.1101/gad.10.5.578. [DOI] [PubMed] [Google Scholar]
- Shibasaki F, Kondo E, Akagi T, McKeon F. Suppression of signaling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature. 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- Spector MS, Desnoyers S, Hoeppner DJ, Hengartner MO. Interaction between the C. eleganscell-death regulators CED-9 and CED-4. Nature. 1997;385:653–656. doi: 10.1038/385653a0. [DOI] [PubMed] [Google Scholar]
- Vander Heiden, M.G., N.S. Chandel, E.K. Williamson, P.T. Schumacker, and C.B. Thompson. Bcl-XLregulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- Wang H-G, Rapp UR, Reed JC. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;86:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu Y-T, Smith CL, Nechushtan A, Xi X-G, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Wallen HD, Nuñez G. Interaction and regulation of subcellular localization of Ced-4 by Ced-9. Science. 1997;275:1126–1129. doi: 10.1126/science.275.5303.1126. [DOI] [PubMed] [Google Scholar]
- Xiang J, Chao DT, Korsmeyer SJ. BAX-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Korsmeyer SJ. Molecular Thanatopsis: a discourse on the Bcl-2 family and cell death. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- Yang J, Siu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T-I, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B, Orrenius S, Brustugun OT, Døskeland SO. Injected cytochrome c induces apoptosis. Nature. 1998;39:449–450. doi: 10.1038/35060. [DOI] [PubMed] [Google Scholar]
- Zhu H, Fearnhead HO, Cohen GM. An ICE-like protease is a common mediator of apoptosis induced by diverse stimuli in human monocytic THP.1 cells. FEBS Lett. 1995;374:303–308. doi: 10.1016/0014-5793(95)01116-v. [DOI] [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf 1, a human protein homologous to C. elegansCED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]