Abstract
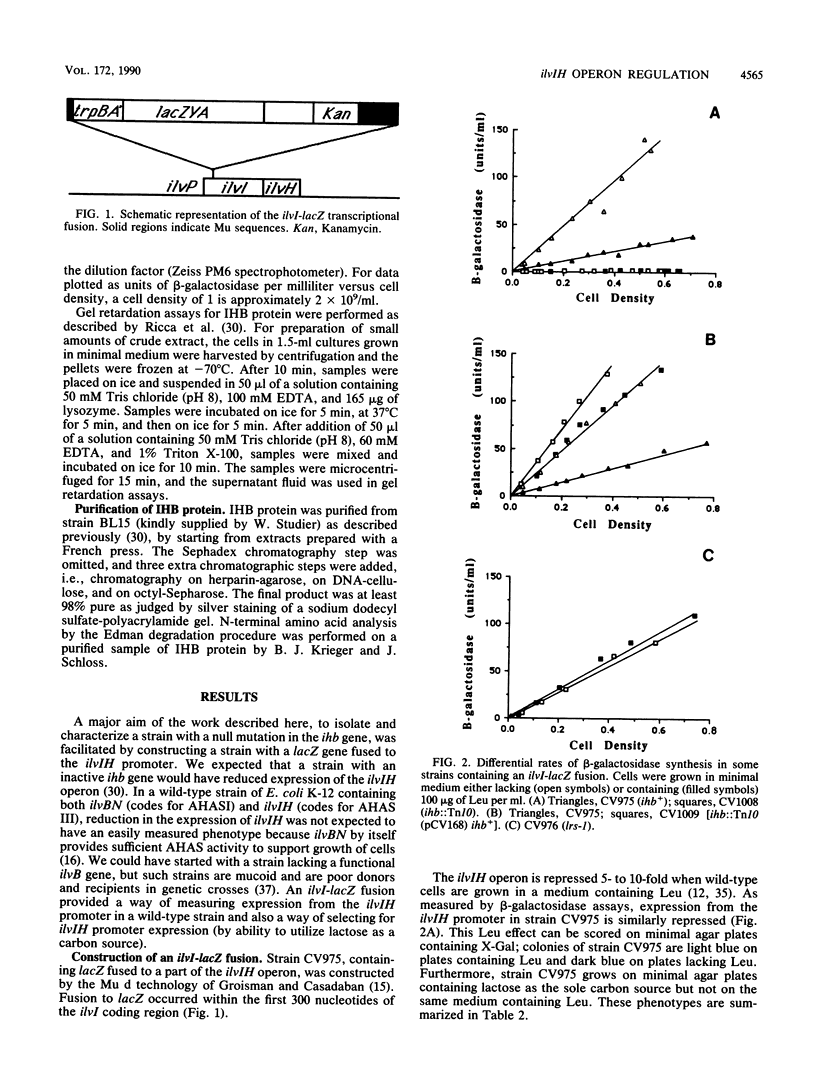
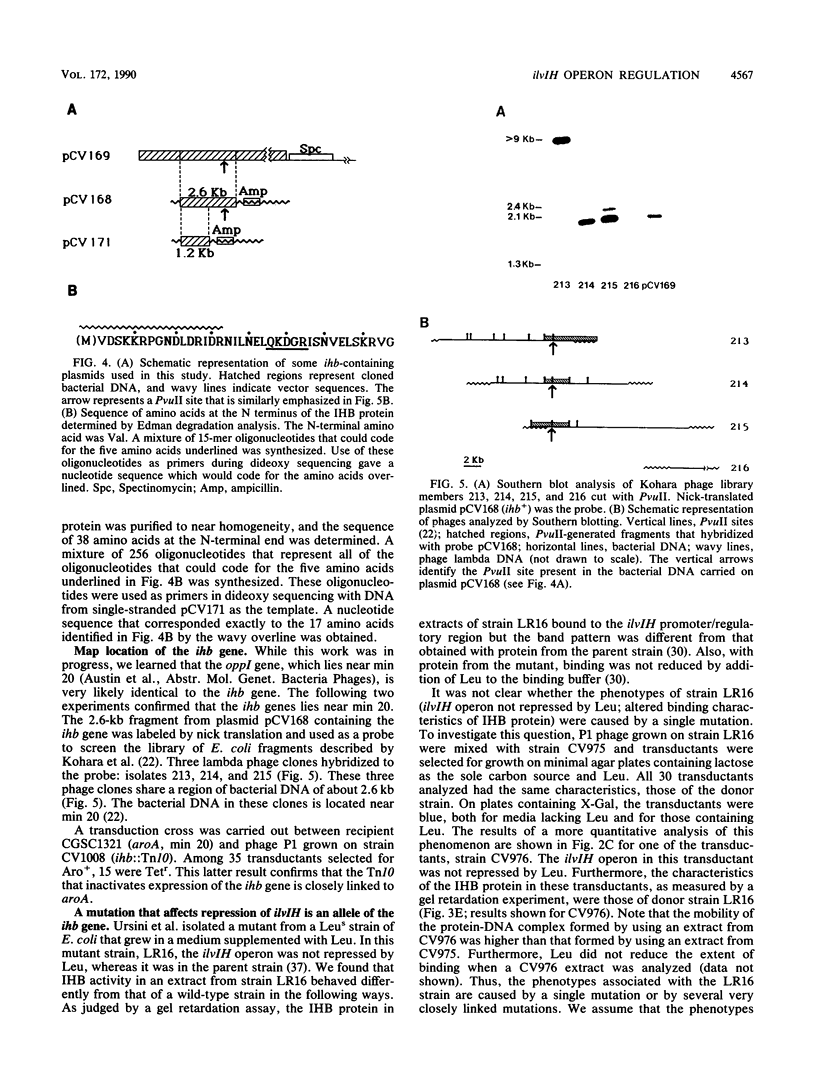
The ilvIH operon of Escherichia coli (located near min 2) encodes acetohydroxyacid synthase III, an isozyme involved in branched-chain amino acid biosynthesis. A strain with lacZ fused to the ilvIH promoter was constructed. Transposon Tn10 was introduced into this strain, and tetracycline-resistant derivatives were screened for those in which ilvIH promoter expression was markedly reduced. In one such derivative, strain CV1008, beta-galactosidase expression was reduced more than 30-fold. The transposon giving rise to this phenotype inserted near min 20 on the E. coli chromosome. Extract from a wild-type strain contains a protein, the IHB protein, that binds to two sites upstream of the ilvIH promoter (E. Ricca, D. A. Aker, and J. M. Calvo, J. Bacteriol. 171:1658-1664, 1989). Extract from strain CV1008 lacks IHB-binding activity. These results indicate that the IHB protein is a positive regulator of ilvIH operon expression. The gene that encodes the IHB protein, ihb, was cloned by complementing the transposon-induced mutation. Definitive evidence that the cloned DNA encodes the IHB protein was provided by determining the sequence of more than 17 amino acids at the N terminus of the IHB protein and comparing it with the nucleotide sequence. A mutation that prevents repression of the ilvIH operon by leucine in vivo and that alters the DNA-binding characteristics of the IHB protein in vitro was shown to be an allele of the ihb gene. The ihb gene is identical to oppI, a gene that regulates the oppABCDF operon (E. A. Austin, J. C. Andrews, and S. A. Short, Abstr. Mol. Genet. Bacteria Phages, p. 153, 1989). Thus, oppI/ihb encodes a protein that regulates both ilvIH, an operon that is repressed by leucine, and oppABCDF, an operon involved in peptide transport that is induced by leucine. We propose that the designation lrp be used in the future instead of oppI or ihb and that Lrp (leucine-responsive regulatory protein) be used in place of IHB.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Rosenberg M., Hatfield G. W. Analysis of in vivo RNA transcription products of the ilvGEDA attenuator region of Escherichia coli K12. J Biol Chem. 1985 Jul 15;260(14):8538–8544. [PubMed] [Google Scholar]
- Anderson J. J., Quay S. C., Oxender D. L. Mapping of two loci affecting the regulation of branched-chain amino acid transport in Escherichia coli K-12. J Bacteriol. 1976 Apr;126(1):80–90. doi: 10.1128/jb.126.1.80-90.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J. C., Blevins T. C., Short S. A. Regulation of peptide transport in Escherichia coli: induction of the trp-linked operon encoding the oligopeptide permease. J Bacteriol. 1986 Feb;165(2):428–433. doi: 10.1128/jb.165.2.428-433.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J. C., Short S. A. Genetic analysis of Escherichia coli oligopeptide transport mutants. J Bacteriol. 1985 Feb;161(2):484–492. doi: 10.1128/jb.161.2.484-492.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J. C., Short S. A. opp-lac Operon fusions and transcriptional regulation of the Escherichia coli trp-linked oligopeptide permease. J Bacteriol. 1986 Feb;165(2):434–442. doi: 10.1128/jb.165.2.434-442.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. S., West R., Hilderman R. H., Bayliss F. T., Klines E. L. A new locus (leuK) affecting the regulation of branched-chain amino acid, histidine, and tryptophan biosynthetic enzymes. J Bacteriol. 1978 Aug;135(2):542–550. doi: 10.1128/jb.135.2.542-550.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M., Levinthal M. The acetohydroxy acid synthase III isoenzyme of Escherichia coli K-12: regulation of synthesis by leucine. Biochem Biophys Res Commun. 1977 Nov 7;79(1):82–87. doi: 10.1016/0006-291x(77)90063-8. [DOI] [PubMed] [Google Scholar]
- Fraser J., Newman E. B. Derivation of glycine from threonine in Escherichia coli K-12 mutants. J Bacteriol. 1975 Jun;122(3):810–817. doi: 10.1128/jb.122.3.810-817.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden P., Newman T., Freundlich M. Nucleotide sequence of the ilvB promoter-regulatory region: a biosynthetic operon controlled by attenuation and cyclic AMP. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6156–6160. doi: 10.1073/pnas.79.20.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman E. A., Casadaban M. J. Mini-mu bacteriophage with plasmid replicons for in vivo cloning and lac gene fusing. J Bacteriol. 1986 Oct;168(1):357–364. doi: 10.1128/jb.168.1.357-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Iaccarino M. Mutant of Escherichia coli K-12 missing acetolactate synthase activity. J Bacteriol. 1974 Oct;120(1):536–538. doi: 10.1128/jb.120.1.536-538.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H., Shimamoto T., Tsuda M., Tsuchiya T. Characterization of a novel L-serine transport system in Escherichia coli. J Bacteriol. 1988 May;170(5):2236–2239. doi: 10.1128/jb.170.5.2236-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughn G. W., Squires C. H., De Felice M., Largo C. T., Calvo J. M. Unusual organization of the ilvIH promoter of Escherichia coli. J Bacteriol. 1985 Jul;163(1):186–198. doi: 10.1128/jb.163.1.186-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C. A., Hatfield G. W. Nucleotide sequence of the ilvB multivalent attenuator region of Escherichia coli K12. Nucleic Acids Res. 1983 Jan 11;11(1):127–139. doi: 10.1093/nar/11.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth B. G., Higgins C. F. Genetic organization of the oligopeptide permease (opp) locus of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1983 Mar;153(3):1548–1551. doi: 10.1128/jb.153.3.1548-1551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg S., Newman E. B. Studies on L-serine deaminase in Escherichia coli K-12. J Bacteriol. 1974 Apr;118(1):53–58. doi: 10.1128/jb.118.1.53-58.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Lenny A. B., Margolin P. Locations of the opp and supX genes of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1980 Aug;143(2):747–752. doi: 10.1128/jb.143.2.747-752.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargang F. E., Subrahmanyam C. S., Umbarger H. E. Nucleotide sequence of ilvGEDA operon attenuator region of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1823–1827. doi: 10.1073/pnas.77.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. B., Kapoor V., Potter R. Role of L-threonine dehydrogenase in the catabolism of threonine and synthesis of glycine by Escherichia coli. J Bacteriol. 1976 Jun;126(3):1245–1249. doi: 10.1128/jb.126.3.1245-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Induced formation of serine and threonine deaminases by Escherichia coli. J Bacteriol. 1955 Dec;70(6):667–674. doi: 10.1128/jb.70.6.667-674.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca E., Aker D. A., Calvo J. M. A protein that binds to the regulatory region of the Escherichia coli ilvIH operon. J Bacteriol. 1989 Mar;171(3):1658–1664. doi: 10.1128/jb.171.3.1658-1664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal E. R., Calvo J. M. Transcription termination sites at the distal end of the leu operon of Salmonella typhimurium. J Mol Biol. 1987 Apr 5;194(3):443–452. doi: 10.1016/0022-2836(87)90673-5. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searles L. L., Jones J. W., Fournier M. J., Grambow N., Tyler B., Calvo J. M. Escherichia coli B/r leuK mutant lacking pseudouridine synthase I activity. J Bacteriol. 1986 Apr;166(1):341–345. doi: 10.1128/jb.166.1.341-345.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Wessler S. R., Calvo J. M. Physical characterization of the ilvHI operon of Escherichia coli K-12. J Bacteriol. 1981 Sep;147(3):797–804. doi: 10.1128/jb.147.3.797-804.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini M. V., Arcari P., De Felice M. Acetohydroxy acid synthase isoenzymes of Escherichia coli K-12: a trans-acting regulatory locus of ilvHI gene expression. Mol Gen Genet. 1981;181(4):491–496. doi: 10.1007/BF00428741. [DOI] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felice M., Lago C. T., Squires C. H., Calvo J. M. Acetohydroxy acid synthase isoenzymes of Escherichia coli K12 and Salmonella typhimurium. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):251–256. [PubMed] [Google Scholar]