Abstract
We developed the alkane and fatty-acid utilizing yeast Candida tropicalis as a host for DNA transformations. The system is based on an auxotrophic mutant host of C. tropicalis which is defective in orotidine monophosphate decarboxylase (ura3). The ura3 host was isolated by mutagenesis and a double-selection procedure that combined nystatin enrichment selection and 5-fluoro-orotic acid resistance selection. As a selectable marker, we isolated and characterized the C. tropicalis URA3 gene. Plasmid vectors that contained the C. tropicalis URA3 gene transformed the C. tropicalis mutant host at a frequency of 10(3) to 10(4) transformants per micrograms of plasmid DNA. Vectors that contained the Saccharomyces cerevisiae URA3 gene could not transform C. tropicalis. DNA transfer was accomplished by modified versions of either spheroplast generation (CaCl2-polyethylene glycol)-fusion or cation (LiCl) procedures developed for S. cerevisiae. Plasmid vectors that had been cut within the C. tropicalis URA3 fragment integrated by homologous recombination at the URA3 locus.
Full text
PDF

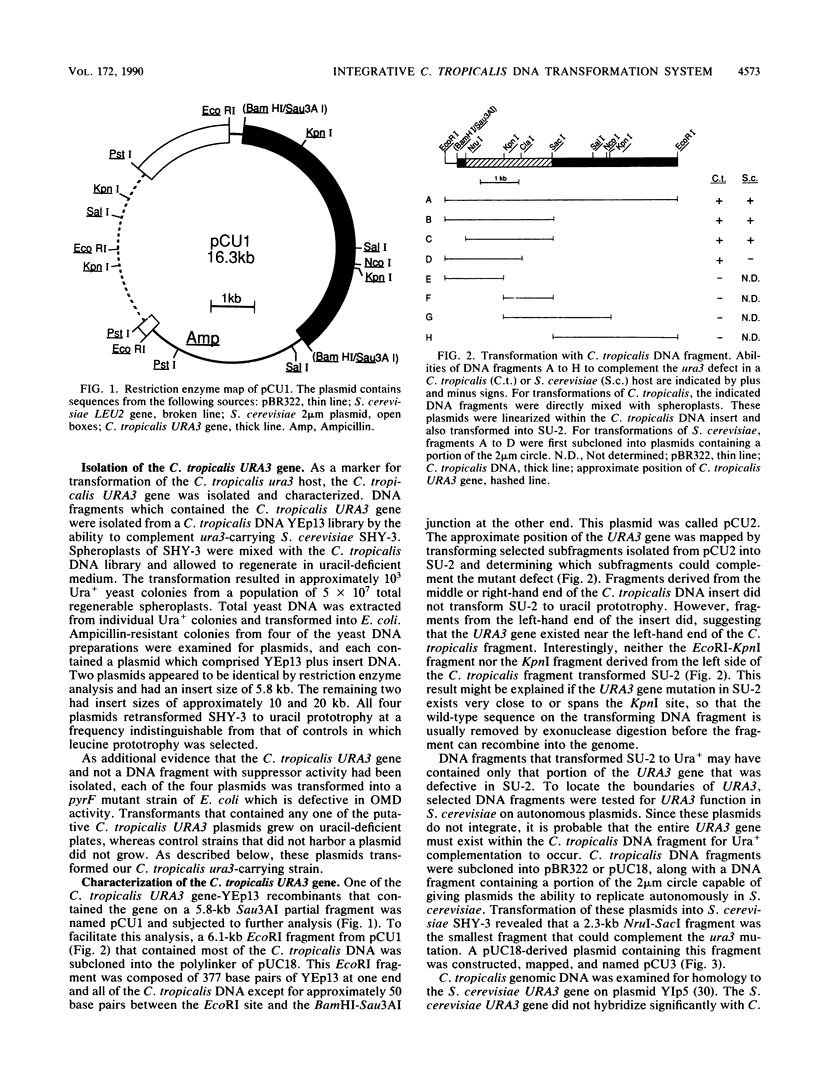
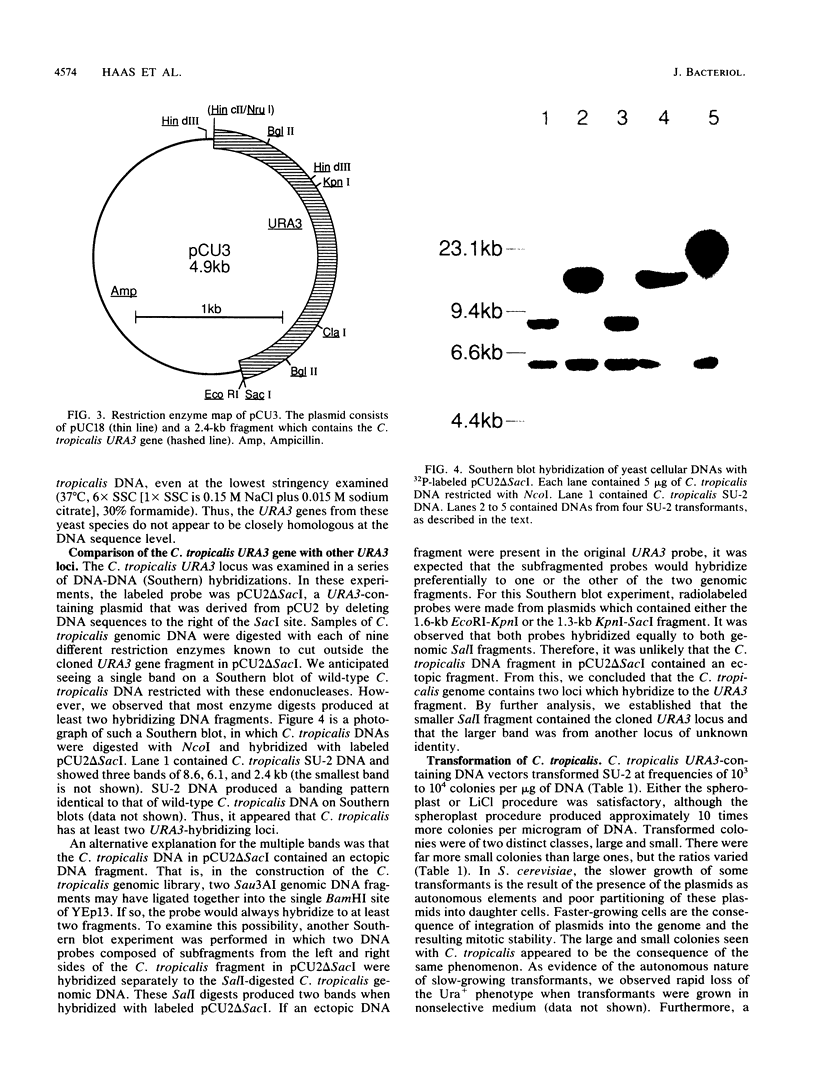



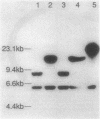
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alani E., Cao L., Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987 Aug;116(4):541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Trueheart J., Natsoulis G., Fink G. R. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Cregg J. M., Barringer K. J., Hessler A. Y., Madden K. R. Pichia pastoris as a host system for transformations. Mol Cell Biol. 1985 Dec;5(12):3376–3385. doi: 10.1128/mcb.5.12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz M. B., Cortelyou M. W., Kirsch D. R. Integrative transformation of Candida albicans, using a cloned Candida ADE2 gene. Mol Cell Biol. 1986 Jan;6(1):142–149. doi: 10.1128/mcb.6.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier-Carpentier F., Kiehn T. E., Armstrong D. Fungemia in the immunocompromised host. Changing patterns, antigenemia, high mortality. Am J Med. 1981 Sep;71(3):363–370. doi: 10.1016/0002-9343(81)90162-5. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Tan H., Fukui S., Kubota I., Kamiryo T. Peroxisomal acyl-coenzyme A oxidase multigene family of the yeast Candida tropicalis; nucleotide sequence of a third gene and its protein product. Gene. 1987;58(1):37–44. doi: 10.1016/0378-1119(87)90027-8. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Genetic applications of yeast transformation with linear and gapped plasmids. Methods Enzymol. 1983;101:228–245. doi: 10.1016/0076-6879(83)01017-4. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sandford G. R., Merz W. G., Wingard J. R., Charache P., Saral R. The value of fungal surveillance cultures as predictors of systemic fungal infections. J Infect Dis. 1980 Oct;142(4):503–509. doi: 10.1093/infdis/142.4.503. [DOI] [PubMed] [Google Scholar]
- Small G. M., Imanaka T., Shio H., Lazarow P. B. Efficient association of in vitro translation products with purified stable Candida tropicalis peroxisomes. Mol Cell Biol. 1987 May;7(5):1848–1855. doi: 10.1128/mcb.7.5.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small G. M., Szabo L. J., Lazarow P. B. Acyl-CoA oxidase contains two targeting sequences each of which can mediate protein import into peroxisomes. EMBO J. 1988 Apr;7(4):1167–1173. doi: 10.1002/j.1460-2075.1988.tb02927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. An enrichment method for auxotrophic yeast mutants using the antibiotic 'nystatin'. Nature. 1966 Jul 9;211(5045):206–207. doi: 10.1038/211206a0. [DOI] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T. J., Merz W. G. Pathologic features in the human alimentary tract associated with invasiveness of Candida tropicalis. Am J Clin Pathol. 1986 Apr;85(4):498–502. doi: 10.1093/ajcp/85.4.498. [DOI] [PubMed] [Google Scholar]
- Wingard J. R., Dick J. D., Merz W. G., Sandford G. R., Saral R., Burns W. H. Differences in virulence of clinical isolates of Candida tropicalis and Candida albicans in mice. Infect Immun. 1982 Aug;37(2):833–836. doi: 10.1128/iai.37.2.833-836.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard J. R., Dick J. D., Merz W. G., Sandford G. R., Saral R., Burns W. H. Pathogenicity of Candida tropicalis and Candida albicans after gastrointestinal inoculation in mice. Infect Immun. 1980 Aug;29(2):808–813. doi: 10.1128/iai.29.2.808-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard J. R., Merz W. G., Saral R. Candida tropicalis: a major pathogen in immunocompromised patients. Ann Intern Med. 1979 Oct;91(4):539–543. doi: 10.7326/0003-4819-91-4-539. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoshimoto A., Umezu K., Kobayashi K., Tomita K. Orotidylate decarboxylase (yeast). Methods Enzymol. 1978;51:74–79. doi: 10.1016/s0076-6879(78)51013-6. [DOI] [PubMed] [Google Scholar]