Abstract
A small GTPase Ran is a key regulator for active nuclear transport. In immunoblotting analysis, a monoclonal antibody against recombinant human Ran, designated ARAN1, was found to recognize an epitope in the COOH-terminal domain of Ran. In a solution binding assay, ARAN1 recognized Ran when complexed with importin β, transportin, and CAS, but not the Ran-GTP or the Ran-GDP alone, indicating that the COOH-terminal domain of Ran is exposed via its interaction with importin β–related proteins. In addition, ARAN1 suppressed the binding of RanBP1 to the Ran–importin β complex. When injected into the nucleus of BHK cells, ARAN1 was rapidly exported to the cytoplasm, indicating that the Ran–importin β–related protein complex is exported as a complex from the nucleus to the cytoplasm in living cells. Moreover, ARAN1, when injected into the cultured cells induces the accumulation of endogenous Ran in the cytoplasm and prevents the nuclear import of SV-40 T-antigen nuclear localization signal substrates. From these findings, we propose that the binding of RanBP1 to the Ran–importin β complex is required for the dissociation of the complex in the cytoplasm and that the released Ran is recycled to the nucleus, which is essential for the nuclear protein transport.
Keywords: nuclear protein import, Ran, monoclonal antibody, importin, microinjection
The intracellular transport of macromolecules between the nucleus and the cytoplasm takes place through the nuclear pore complexes (NPCs)1 which permit the passive diffusion of molecules smaller than 20– 40 kD and also support an active and receptor-mediated transport. The active protein import pathway which has been studied most intensively is mediated by a basic-type nuclear localization signal (NLS) which contains a short amino acid stretch rich in basic amino acids. The basic-type NLS of the import substrate binds to the heterodimeric importin α/β to form a nuclear pore-targeting complex. Importin α provides the NLS-binding site, whereas importin β accounts for the targeting of the complex to the NPC (for reviews see Görlich and Mattaj, 1996; Koepp and Silver, 1996; Görlich, 1997; Nigg, 1997; Yoneda, 1997). In addition to the basic-type NLS mediated import pathway, it is known that the import of heterogeneous nuclear RNA-binding protein A1 (hnRNP A1) is mediated by a signal comprised of 38 amino acids, which is referred to as M9, and which is rich in glycine and aromatic residues. M9 directly binds to an importin β–related transport factor, transportin (Nakielny et al., 1996; Pollard et al., 1996). It has also been demonstrated that the nuclear export of some proteins depend on nuclear export signal (NES), which is rich in hydrophobic residues (for review see Nakielny and Dreyfuss, 1997). Nuclear export of proteins containing NES is mediated by an importin β–related export receptor, CRM1 (Fornerod et al., 1997b; Fukuda et al., 1997; Ossareh et al., 1997; Stade et al., 1997). Although the export signal of importin α has not been identified, its export is also mediated by another importin β–related export receptor, cellular apoptosis susceptibility gene (CAS) (Kutay et al., 1997). These transport factors, importin β, transportin, CAS, and CRM1 constitute a protein superfamily which has a Ran-GTP– binding motif (Fornerod et al., 1997a; Görlich et al., 1997).
A small GTPase Ran is known to be essential for the active transport pathways including the import of the basic-type NLS and M9 containing proteins or snRNPs, or the export of importin α, NES-containing proteins, tRNA, UsnRNA, and several mRNAs (Melchior et al., 1993a; Moore and Blobel, 1993; Koepp et al., 1996; Nakielny et al., 1996; Izaurralde et al., 1997; Kutay et al., 1997; Richards et al., 1997; for reviews see Avis and Clarke, 1996; Koepp and Silver, 1996; Görlich, 1997; Goldfarb, 1997). Ran is predominantly, but not exclusively, a nuclear protein (Bischoff and Ponstingl, 1991a). The Ran GTPase cycle is regulated by the GDP–GTP exchange factor, RCC1, which charges Ran with GTP (Bischoff and Ponstingl, 1991b), the GTPase-activating protein, RanGAP1, which converts Ran-GTP into Ran-GDP (Bischoff et al., 1994; Becker et al., 1995; Corbett et al., 1995), and RanBP1 (or RanBP2) whose binding to Ran-GTP further stimulates the GTPase-activating activity of RanGAP1 (Coutavas et al., 1993; Bischoff et al., 1995; Richards et al., 1995). RCC1 is a chromatin-associated nuclear protein (Ohtsubo et al., 1989), whereas RanGAP1 and its coactivator, RanBP1, are exclusively cytoplasmic, and RanBP2 is located on the cytoplasmic fibrils of the NPC (Hopper et al., 1990; Melchior et al., 1993b; Bischoff et al., 1994, 1995; Wu et al., 1995; Yokoyama et al., 1995; Matunis et al., 1996; Richards et al., 1996; Mahajan et al., 1997). Because of this asymmetric distribution of RCC1, RanGAP1, and RanBP1/RanBP2, it has been predicted that a steep Ran-GTP gradient across the nuclear envelope exists (very low levels of cytoplasmic Ran-GTP and high levels inside the nucleus).
In the case of nuclear import of proteins which contain the basic-type classical NLS, it has been proposed that Ran is involved in several different steps in the process. At least a portion of the energy required for translocation through the NPC is supplied via GTP hydrolysis by Ran (Melchior et al., 1993a), although other GTPases may also be involved (Sweet and Gerace, 1996). In addition, nuclear Ran-GTP functions to terminate the import reaction. After the translocation of the trimeric complex, which is comprised of importin α/β and a karyophile with the classical NLS, through the nuclear envelope, the direct binding of nuclear Ran-GTP to importin β causes the dissociation of the trimeric complex thus releasing the cargo (Rexach and Blobel, 1995; Görlich et al., 1996; Moroianu et al., 1996). In the same manner, transportin binds to its cargo only in the absence of Ran-GTP, i.e., in the cytoplasm and releases it in the nucleus. For the next rounds of nuclear import, importin β and transportin are required to return to the cytoplasm and dissociate from Ran-GTP. In contrast, the binding of the export receptors such as CRM1 and CAS to their cargos is enhanced by the simultaneous binding of Ran-GTP, which occurs in the nucleus. The trimeric cargo-export receptor–Ran-GTP complex is then transferred to the cytoplasm. Thus, the binding of Ran-GTP to importin β–related transport factors regulates their interaction with cargos or adapter molecules. Therefore, it has been suggested that this Ran-GTP concentration gradient is a critical determinant in the directionality of nucleocytoplasmic transport, ensuring that the transport factors carry their cargoes unidirectionally (Görlich et al., 1996, 1997; Izaurralde et al., 1997).
It is also noteworthy that the Ran-GTP–importin β complex is very stable and resistant to the Ran GTPase activating activity of RanGAP1 (Floer and Blobel, 1996). Biochemical analyses have suggested that RanBP1 directly promotes the dissociation of Ran-GTP from importin β, CAS, and transportin (Bischoff and Görlich, 1997; Lounsbury et al., 1997). Nuclear injection of RanGAP1 significantly inhibited the export of injected importin α and β, transportin, and NES-containing proteins in Xenopus laevis oocyte (Izaurralde et al., 1997). As a result, it has been proposed that the importin β–related transport factors are probably exported to the cytoplasm as complexes with Ran-GTP, and that these complexes dissociate and Ran is then converted to the GDP-bound form by RanGAP1 in conjunction with RanBP1 in the cytoplasm (Bischoff and Görlich, 1997; Izaurralde et al., 1997). However, direct in vivo evidence for this proposal is currently lacking.
Unlike other small GTPases, Ran has negatively charged amino acid residues at the COOH terminus instead of consensus sequences for lipid modification. To better understand the biological significance of the COOH-terminal domain, the COOH terminus-deleted mutant Ran, referred to as ΔDE-Ran, has been used (Lounsbury et al., 1994, 1996a,b; Ren et al., 1995; Richards et al., 1995; Carey et al., 1996; Chi et al., 1997). This mutant is capable of supporting the nuclear import of basic-type NLS-containing substrates in digitonin-permeabilized semi-intact cells, whereas the ΔDE-Ran, which is expressed in cultured cells has a dominant-negative phenotype for both nuclear protein import and RNA export (Ren et al., 1995; Richards et al., 1995; Carey et al., 1996). This mutant protein has a lower affinity for RanBP1 than the wild-type (wt) Ran and binds to importin β with higher affinity than wild-type Ran in an overlay assay (Richards et al., 1995; Lounsbury et al., 1996b). These results suggest that the deletion of the COOH-terminal portion of Ran may be the cause of the defect in the Ran GTPase cycle. However, the issue of how the COOH-terminal domain is involved in the nucleocytoplasmic transport or the Ran GTPase cycle is not known with certainty, since the deletion in COOH-terminal domain may cause drastic conformational changes in Ran.
In this study, in order to identify functional domains of Ran including the COOH-terminal domain, we produced anti-Ran monoclonal antibodies (mAbs). By using one of the anti-Ran mAbs, we provide evidence that the COOH-terminal domain of Ran is not exposed to the surface of the molecule until Ran interacts with importin β or importin β–related transport factors, CAS and transportin. This observation suggests that the exposed COOH-terminal acidic sequence of Ran may be essential for the binding of RanBP1 to the Ran-GTP complexed with importin β–related transport factors. Furthermore, we show in vivo evidence that Ran/importin β can be exported in the form of a complex from the nucleus to the cytoplasm. Our results indicate that the binding of RanBP1 to the Ran/importin β complex in the cytoplasm, which appears to be blocked by injected mAb, is essential for the recycling of Ran and nuclear protein import.
Materials and Methods
Production of mAbs
mAbs were obtained essentially according to the procedure of Köhler and Milstein (1975). 50 μg of denatured recombinant human Ran was initially intraperitoneally administered with Freund's complete adjuvant to a 16-wk-old BDF1 mouse (Japan SLC), followed by three subsequent injections at 3-wk intervals with the same dose in Freund's incomplete adjuvant. 1 mo after the fourth injection, the mouse was given a booster injection of the same dose. 4 d later, spleen cells isolated from the mouse were fused with the mouse myeloma cell line P3U1 using standard methods. Screening was performed by ELISA and immunoblotting using the recombinant human Ran. Ran-specific mAbs were typed by using mouse monoclonal antibody isotyping kit (Amersham). Ascites fluid was produced from a BDF1 × BALB/3T3 mouse (Japan SLC) which had been implanted with the hybridoma. The IgG fraction was obtained by precipitation with 50% saturated ammonium sulfate followed by chromatography on a protein A affinity column.
Antibodies
Rabbit anti-Ran polyclonal antibodies were produced as described previously (Sekimoto et al., 1996). Chromatographically purified normal mouse IgG was purchased from Zymed. Rabbit anti–importin β polyclonal antibodies were prepared as described previously (Kose et al., 1997). Mouse anti–human CAS monoclonal antibody and mouse anti–human transportin monoclonal antibody were purchased from Transduction Laboratories.
Expression and Purification of Recombinant Proteins
Expression and purification of recombinant importin β were performed as described previously (Kose et al., 1997). The human CAS gene was amplified from a HeLa cDNA library via the polymerase chain reaction (PCR) using the synthetic oligonucleotide primers, 5′-TTTTTTGGATCCATGGAACTCAGCGATGCAAATCTGCAA-3′ and 5′-TTTTTTCTCGAGTTAAAGCAGTGTCACACTGGCTGCCTG-3′. The PCR product was inserted into BamHI and XhoI sites of pGEX-6P-2/hGFP vector, which was an expression vector of glutathione-S-transferase (GST)- fused green fluorescent protein (containing the S65A/Y145F mutation) (hGFP). This construct was transformed to express the recombinant GST–hGFP-CAS fusion protein in Escherichia coli strain BL21. The expressed GST–hGFP-CAS fusion protein was purified on glutathione– Sepharose (Pharmacia Biotech). After the digestion of GST portion with PreScission Protease (Pharmacia Biotech), hGFP–CAS protein was purified by ion-exchange chromatography on Hi-TrapQ (Pharmacia Biotech, Inc.), and then was dialyzed against 20 mM Hepes, pH 7.3, 110 mM potassium acetate, 2 mM DTT, and 1 μg/ml each of aprotinin, leupeptin, and pepstatin.
Human transportin/karyopherin β2 gene (Pollard et al., 1996; Bonifaci et al., 1997) was amplified from HeLa cell cDNA library by the PCR using the synthetic oligonucleotide primers (5′-CTCAGCGGATCCATGGAGTATGAGTGGAAACCTGAC-3′ and 5′-CTCAGCGGTACCTTAAACACCATAAAAAGCTGCAAG-3′). The PCR product was inserted into the BamHI and KpnI sites of a modified pGEX-6P-3 (Pharmacia) and verified by DNA sequencing. Recombinant GST-transportin was expressed and purified as described for hGFP–CAS.
E. coli strains expressing wild-type Ran were prepared as described previously (Sekimoto et al., 1996). Mutation (Q69L) within Ran was created using QuikChange site-directed mutagenesis kit (Strategene). RanQ69L fragment was inserted into NcoI and BamHI sites of pET3d. Recombinant RanQ69L protein was expressed in E. coli and purified in the same manner as wild-type Ran. Recombinant wild-type and Q69L Ran were expressed, purified, and then charged with GTP or GDP according to the method of Bischoff and Ponstingl (1995) and Melchior et al. (1995) with slight modifications. In brief, expression was induced by addition of 1 mM isopropyl-β d-thiogalactopyranoside (IPTG) and incubation for 14 h at 20°C. The E. coli cells were lysed in buffer C (50 mM Tris-HCl, pH 8.0, 75 mM NaCl, 1 mM MgCl2, 0.1 mM PMSF, 1 mM DTT, 1 mg/ml each of aprotinin, leupeptin, and pepstatin) by freeze-thaw. The clarified lysates were applied to DEAE-Sepharose FF column (Pharmacia) and flow through fractions were collected. After 60% saturate ammonium sulfate precipitation, 2 mM GTP or GDP was added and incubated for at least 1 h in buffer D (50 mM phosphate buffer, pH 7.0, 1 mM 2-mercaptoethanol, 10% glycerol, 2 mM GTP or GDP) on ice. The samples were applied to HiPrep Sephacryl S-200 HR FPLC column (Pharmacia) equilibrated with buffer D, and peak fractions containing Ran proteins were pooled. GTP- and GDP-form of Ran were further separated on Fractogel EMDSO3 −650 (s) column (Merck) with linear gradient of buffer D containing 50 mM phosphate to 500 mM phosphate. GDP-bound form of Ran was eluted between 200 and 250 mM phosphate. GTP-bound form was eluted between 350 and 400 mM phosphate. The purified GTP- or GDP-form of Ran was then desalted with PD10 column (Pharmacia) equilibrated with transport buffer (see below), concentrated with centricon 30 (Amicon), and then frozen in small aliquots.
The guanine nucleotides bound to purified Ran were determined by FPLC (Pharmacia) after denaturation. The Ran preparations were heated at 95°C for 2 min. After filtration, the solutions were applied to a monoQ HR5/5 (Pharmacia) column and then the guanine nucleotides were eluted with a linear gradient from 10 mM potassium phosphate buffer, pH 8.0, to 50 mM potassium phosphate buffer, pH 8.0, containing 250 mM NaCl. Nucleotides were detected at 254 nm and quantified against a standard GDP/ GTP solution. The bound nucleotides were found to be virtually 100% GDP for the GDP-bound preparations and 95% GTP for the GTP-bound preparations.
All GST-fused deletion mutants of human Ran were prepared using PCR with appropriate oligonucleotides. The PCR product was inserted into the BamHI/EcoRI site of pGEX-2T vector. For the production of the GST-fused COOH-terminal domain of Ran, the synthesized oligonucleotide, corresponding to the COOH-terminal region of human Ran, TALPDEDDDL, was inserted into the BamHI/EcoRI site of pGEX-2T vector (Pharmacia). These resultant plasmids were sequenced to confirm the fidelity of the region, which had been amplified by PCR and in-frame ligation of the fused region. Recombinant GST-fused proteins were expressed by incubation with 1 mM IPTG for 6 h at 37°C in E. coli strain BL21(DE3). The purification of the GST-fused COOH-terminal domain of Ran with glutathione–Sepharose was performed as described for importin β. The protein was then dialyzed against 20 mM Hepes, pH 7.3, 110 mM potassium acetate, and 2 mM DTT.
Mouse RanBP1 was expressed as GST fusion protein. A fragment which is encoding the entire amino acid sequence of this protein was amplified from mouse Ehrlich ascites tumor cell cDNA by PCR using synthetic oligonucleotides (5′-CCTACGGATCCATGGCGGCCGCCAAGGACA-3′ and 5′-CCACTGAATTCTCATTGTTTCTCCTCAGAC-3′) and cloned into the BamH1-EcoRI sites of pGEX-2T, to produce an in-frame fusion with GST. Expression in BL21(DE3), lysis of bacteria, and purification of the fusion protein with glutathione–Sepharose were performed as described for importin β. The GST portion of chimeras was cleaved off by a 2-h incubation at room temperature with 1 NIH U of thrombin (Sigma) per 100 μg of chimeras. GST and thrombin were separated from recombinant protein on a MonoQ column (Pharmacia Biotech) at flow rate of 0.5 ml/min with a linear gradient from 0.025 to 1.0 M NaCl in 20 mM Hepes, pH 7.3, 2 mM DTT, and 1 μg/ml each of aprotinin, leupeptin, and pepstatin. Free RanBP1 protein was dialyzed against 20 mM Hepes, pH 7.3, 110 mM potassium acetate, 2 mM DTT, and 1 μg/ml each of aprotinin, leupeptin, and pepstatin.
Preparation of Ehrlich Ascites Tumor Cells Cytosolic Extract
Cytosolic extract was prepared from Ehrlich ascites tumor cells as described previously (Imamoto et al., 1995).
Gel Electrophoresis and Immunoblotting
One-dimensional SDS-PAGE was based on the method of Laemmli (1970) and the gels were stained with Coomassie brilliant blue. For immunoblotting, after electrophoresis, proteins were electrophoretically transferred from gels to nitrocellulose sheets. After blocking with 3% skim milk in PBS, the sheets were then incubated with the first antibody followed by alkaline phosphatase-conjugated anti-mouse or anti-rabbit IgG (Bio-Rad).
Immunofluorescence
BHK cells, plated on coverslips, were washed twice in PBS and fixed with 3.7% formaldehyde in PBS (at room temperature for 20 min) followed by permeabilization with 0.5% Triton X-100 in PBS (at room temperature for 5 min). After blocking with 3% skim milk in PBS, the cells were incubated with ARAN1 (3 μg/ml) or affinity-purified rabbit anti-Ran polyclonal antibodies (3 μg/ml) overnight at 4°C and detected with Cy3-conjugated goat anti–mouse IgG (Amersham) or FITC-conjugated goat anti–rabbit IgG (Tago). The samples were examined using a Zeiss Axiophot fluorescent microscope (Carl Zeiss).
Immunoprecipitation Using Recombinant Proteins
50 pmol of Ran (GTP-bound or GDP-bound) and/or 50 pmol of importin β were incubated with 50 pmol of ARAN1 in total 300 μl of transport buffer (TB: 20 mM Hepes, pH 7.3, 100 mM potassium acetate, 2 mM magnesium acetate, 5 mM sodium acetate, 1 mM glycoletherdiaminetetraacetic acid [EGTA], 2 mM DTT, and 1 μg/ml each of aprotinin, leupeptin, and pepstatin) for 1 h at 4°C. Protein A–bound agarose beads (Calbiochem-Novabiochem) were then added and the mixture was incubated for 1 h at 4°C. The beads were collected by centrifugation and washed five times with TB. The bound fraction and unbound fraction were analyzed by SDS-PAGE and immunoblotting.
150 pmol of transportin or hGFP-CAS and 150 pmol of Ran-GTP were incubated with 150 pmol of ARAN1 in the presence or absence of 150 pmol of importin α in TB for 1 h at 4°C. The proteins which bound to ARAN1 were then analyzed using the same procedure as above.
Immunoprecipitation Using Cytosolic Extract
To preclear the extract, anti-mouse IgG-conjugated agarose beads (American Qualex) were added to the Ehrlich ascetics tumor cell cytosolic extract and rotated for 1 h at 4°C. The beads were then removed by centrifugation. 300 pmol of ARAN1 and 300 pmol of Q69L Ran-GTP was added to 200 μl of the precleared cytosolic extract followed by 1 h of incubation at 4°C. 15 μl of protein A–agarose beads were then added to the mixture, which was then incubated for 1 h. After washing five times with TB, the proteins bound to the protein A–conjugated agarose beads were resuspended in SDS-PAGE sample buffer containing DTT and analyzed by immunoblotting using antibodies indicated in the figure legend.
Solution Binding Assay
Solution binding assays were performed in TB. 50 pmol of GST-importin β and 50 pmol of Ran-GTP was incubated with 10 μl of a packed volume of glutathione–Sepharose for 1 h at 4°C. After washing the beads twice with TB, 250 pmol or 2,500 pmol of ARAN1 was added to the mixture followed by a 30-min incubation, 50 pmol of RanBP1 was then added and the mixture incubated for an additional 30 min at 4°C. The beads were washed with TB and proteins bound to the beads were resuspended in SDS-PAGE sample buffer containing DTT and analyzed by SDS-PAGE.
50 pmol of GST–importin β, 50 pmol of Ran-GDP, and 250 pmol of ARAN1 was incubated with 10 μl of a packed volume of glutathione– Sepharose for 1 h at 4°C. After washing the beads twice with TB, 50 pmol of RanBP1 was added to the beads and the mixture incubated for 1 h. For a control, 50 pmol of GST–importin β, Ran-GDP, and RanBP1 were incubated with glutathione–Sepharose for 1 h. The beads were then washed with TB and proteins bound to the beads were resuspended in SDS-PAGE sample buffer containing DTT, and then analyzed by SDS-PAGE.
Preparation of Fluorescein-labeled NLS-containing Transport Substrates
BSA was fluorescein-labeled and then chemically conjugated to the synthetic peptides which contained the amino acid sequence of SV-40 large T-antigen NLS (CYGGPKKKRKVEDP), for the transport substrates termed FITC–T-BSA, as described previously (Tachibana et al., 1994).
Microinjection
BHK cells were grown on coverslips. Microinjection of proteins was performed as described previously (Yoneda et al., 1987). After incubation for the indicated time in each figure at 37°C, the cells were fixed with 3.7% formaldehyde in PBS. The injected fluorescently labeled proteins were detected by fluorescent microscopy (model Axiophot 2; Carl Zeiss).
Results
A Monoclonal Antibody Specific for Ran
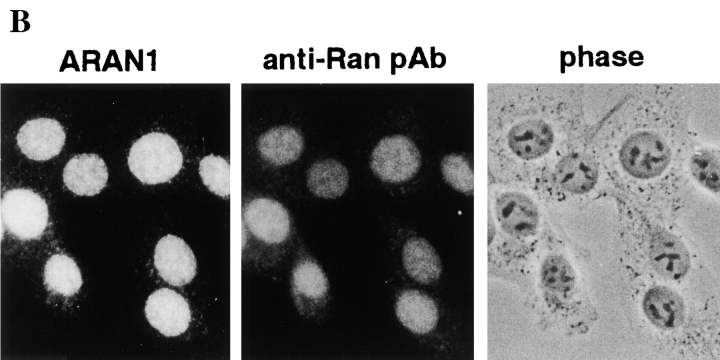
To study the functional domain(s) of Ran, monoclonal antibodies were produced against recombinant human Ran. The screening of the monoclonal antibodies was based on two criteria: (a) that they react with only Ran protein in immunoblotting; and (b) that they show the same staining pattern with polyclonal anti-Ran antibodies in immunofluorescence microscopy. Through this screening procedure, we isolated only one clone, designated ARAN1, for further characterization. ARAN1 was typed using a mouse monoclonal antibody isotyping kit as an immunoglobulin kappa G2b. On an immunoblot, ARAN1 detected recombinant human Ran and specifically reacted with a single 25-kD band in cell extracts from mouse Ehrlich ascites tumor cells, BHK21 cells, human embryonic lung (HEL) cells (Fig. 1 A), Madin-Darby bovine kidney-derived epithelial cells (MDBK), and Xenopus laevis oocytes (data not shown), which was also detected by affinity-purified rabbit anti-Ran polyclonal antibodies. Furthermore, ARAN1 recognized a single spot by immunoblotting analysis of HeLa total cell extracts after two-dimensional electrophoresis, which was also detected with the anti-Ran polyclonal antibodies (data not shown). Immunofluorescent image with ARAN1 in BHK cells showed the same typical staining pattern as that with the anti-Ran polyclonal antibodies (Fig. 1 B). From these findings, it was concluded that the monoclonal antibody ARAN1 specifically binds to Ran.
Figure 1.
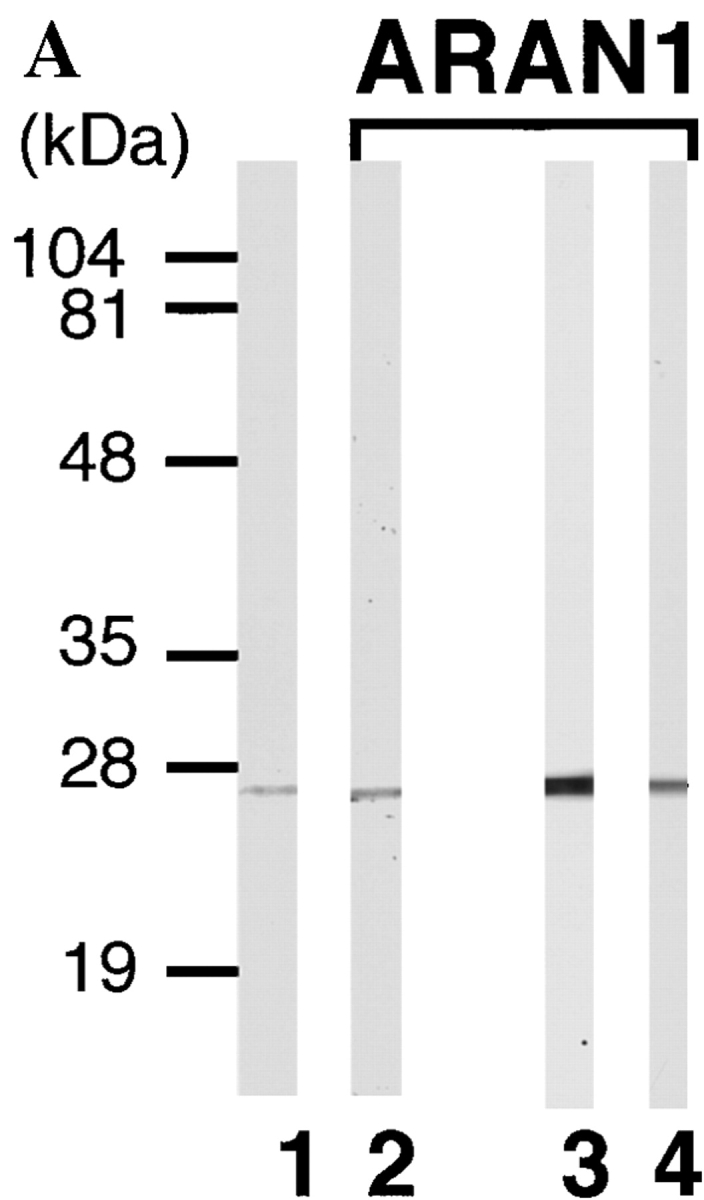
Anti-Ran monoclonal antibody, ARAN1, mono-specifically recognizes Ran molecule. (A) Cytosolic extract from mouse Ehrlich ascites tumor cells (lanes 1 and 2), and total extracts of HEL cells (lane 3) and BHK21 cells (lane 4) were electrophoresed on 12.5% polyacrylamide gels, transferred to nitrocellulose, and then probed with anti-Ran polyclonal antibodies (lane 1) and ARAN1 (lanes 2, 3, and 4). (B) BHK21 cells were double stained with ARAN1 and rabbit anti-Ran polyclonal antibodies. ARAN1 and rabbit anti-Ran polyclonal antibodies were detected with goat Cy3-conjugated anti–mouse IgG and goat FITC-conjugated anti–rabbit IgG, respectively. Phase–contrast microscopy is also shown.
ARAN1 Recognizes an Epitope at the COOH-terminal Domain of Ran
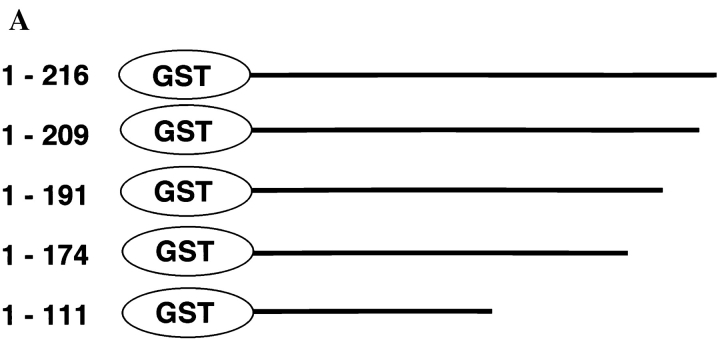
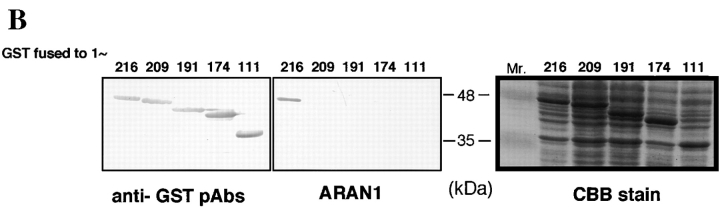
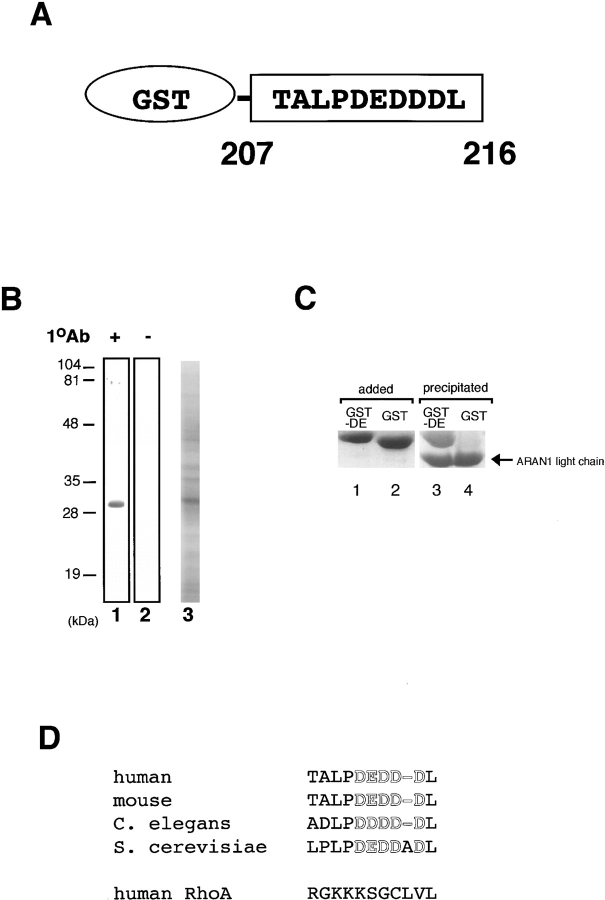
To determine the specific epitope recognized by ARAN1, we prepared a series of truncated forms of recombinant Ran fused with GST (Fig. 2 A) and analyzed the interaction of these with ARAN1 by immunoblotting. Although anti-GST polyclonal antibodies showed that all of the Ran mutants were appropriately expressed and transferred to nitrocellulose (Fig. 2 B), ARAN1 recognized only the full-length of GST-Ran. Removal of the COOH-terminal seven amino acids (210–216) of Ran completely abolished the reactivity with ARAN1 in immunoblotting, suggesting that the COOH-terminal domain, which consists of seven amino acid residues in Ran, represents the epitope for ARAN1. To confirm this, we prepared the 10-mer peptide of the COOH-terminal domain, 207–216, of Ran fused to GST (Fig. 3 A). As shown in Fig. 3, B and C, ARAN1 clearly reacted with the residues between 207 and 216 of human Ran not only in immunoblotting but also in a solution binding assay. From these findings, it was concluded that the epitope of ARAN1 is located in the COOH-terminal portion of Ran, which is highly negatively charged (−DEDDDL) and conserved among species (Fig. 3 D).
Figure 2.
Mapping of the ARAN1 binding domain in the Ran molecule. (A) Schematic presentation of Ran and a series of truncated forms of Ran, which were expressed in E. coli as recombinant GST fusion proteins. Numbers at left, amino acid positions of the Ran sequence. A full length of human Ran consists of 216 amino acid residues. (B) Immunoblotting analysis of full and mutant Ran proteins using ARAN1 and rabbit anti-GST polyclonal antibodies. E. coli was harvested after 6 h of induction with IPTG and lysed in SDS-PAGE sample buffer. Samples were electrophoresed on 12.5% polyacrylamide gels, transferred to nitrocellulose, and then probed with the ARAN1 and anti-GST polyclonal antibodies. Right, a loading control stained with Coomassie brilliant blue.
Figure 3.
ARAN1 recognizes an epitope located within 10 amino acid residues of the COOH terminus of Ran. (A) Schematic drawing of the recombinant fusion protein of the COOH-terminal tail fragment of Ran expressed in E. coli. (B) Immunoblotting analysis of the E. coli extract which expresses the GST fused Ran COOH-terminal tail, 207–216 with ARAN1 (lane 1). Lane 2 shows negative control without ARAN1. The right panel shows an SDS-PAGE profile of E. coli extract expressing GST-fused COOH-terminal tail fragment of Ran, applied in the same amount as was used for immunoblotting. (C) Recombinant GST-fused COOH-terminal portion (207–216) of Ran (lane 1) or GST (lane 2) is incubated with ARAN1 and protein A–bound agarose beads for 1 h. The beads were then washed and the bound proteins were analyzed by SDS-PAGE followed by Coomassie brilliant blue staining. (D) Amino acid comparison of the COOH- terminal domain of Ran in human, mouse, C. elegans and S. cerevisiae. The COOH-terminal domain of human RhoA is also shown. Negatively charged residues are shown in boldface.
ARAN1 Binds to the Ran–Importin β Complex But Not the Ran Molecule Alone in Solution Binding Assay
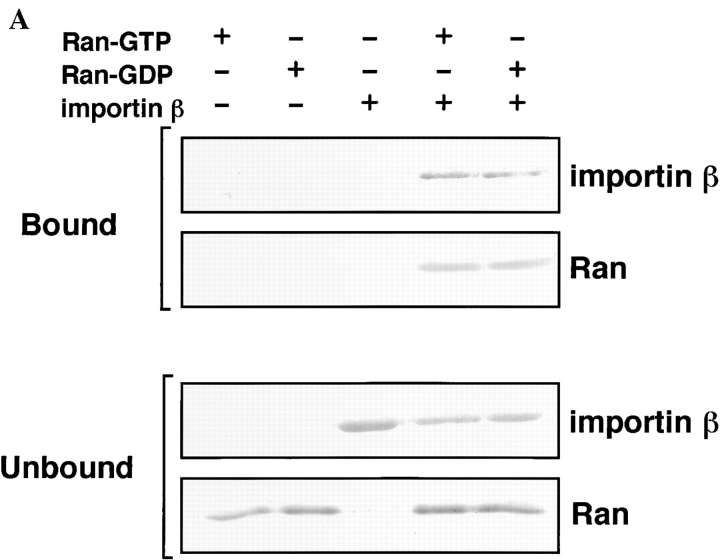
It is known that the stable Ran-GTP–importin β complex causes the inhibition of GTP hydrolysis on Ran stimulated by RanGAP1. In contrast, Ran-GDP shows a much lower affinity to importin β than Ran-GTP. To determine which form of Ran is recognized by ARAN1 in solution, immunoprecipitation analysis of ARAN1 using recombinant proteins was performed. Purified recombinant human Ran-GTP or Ran-GDP was incubated with ARAN1 in the presence or absence of importin β followed by the precipitation with protein A–conjugated agarose beads. The results showed that, whereas ARAN1 precipitated neither Ran-GTP nor Ran-GDP alone, it reacted with both Ran-GTP and Ran-GDP only in the presence of importin β and precipitated both Ran and importin β. To clearly confirm that ARAN1 can't recognize Ran molecule alone, precipitated proteins were analyzed by immunoblotting with anti-Ran polyclonal antibodies (Fig. 4 A). The molar ratio of the Ran and importin β precipitated with ARAN1 was estimated to be ∼1:1 (data not shown). Since ARAN1 did not bind to importin β directly in this assay, these results indicate that ARAN1 reacts only with Ran when bound to importin β, and suggest that Ran changes conformation when bound to importin β which, in turn, exposes its COOH-terminal portion.
Figure 4.
ARAN1 recognizes the Ran–importin β–related transport factors complex but not the Ran molecule alone in solution. (A) Recombinant Ran-GTP, Ran-GDP, and/or importin β were incubated with ARAN1 for 1 h at 4°C and protein A–agarose was then added. After a 1-h incubation, the bound proteins were analyzed by SDS-PAGE followed by Coomassie blue staining for importin β and by immunoblotting for Ran using rabbit anti-Ran polyclonal antibodies. Top and bottom panels show the bound and unbound fractions, respectively. ARAN1 failed to precipitate Ran-GTP, Ran-GDP, and importin β alone, and precipitated only the Ran-GTP–importin β complex and Ran-GDP–importin β complex. (B) ARAN1 also recognizes the Ran/transportin complex and Ran/CAS–importin α complex. Recombinant transportin or CAS and importin β were incubated with Ran-GTP and ARAN1, then precipitated by protein A–agarose and analyzed by SDS-PAGE.
ARAN1 Also Binds to the Ran–Transportin Complex and the Ran–CAS–Importin α Complex
It is well known that Ran interacts with some import and export receptors, e.g., transportin, CAS, and CRM1, which are members of the superfamily of Ran-GTP-binding protein/importin β (Kutay et al., 1997; Görlich et al., 1997; Fornerod et al., 1997a). Tranportin forms a complex with Ran-GTP and CAS simultaneously binds to both Ran-GTP and importin α (Kutay et al., 1997). Judging from the conservation of the Ran-binding domain in this family, it is likely that the COOH-terminal tail of Ran may also be exposed as the result of the interaction with these molecules. To confirm this, we performed the solution binding assay with different importin β–related transport factors, transportin, and CAS. The results clearly showed that ARAN1 binds to both Ran-GTP/transportin complex and Ran-GTP–CAS–importin α complex (Fig. 4 B), suggesting that importin β–related transport factors might induce a general conformational change in Ran through their interaction. In the case of CAS, it should be noted that a slight recognition of a complex between CAS and Ran-GTP by ARAN1 can also be observed (Fig. 4 B). This suggests that CAS directly binds to Ran-GTP in the absence of importin α, although the affinity is very low.
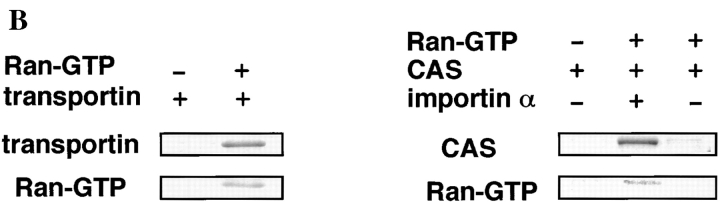
ARAN1 Suppresses the Ternary Complex Formation of Ran, Importin β, and RanBP1 in a Solution Binding Assay
Both Ran-GTP and Ran-GDP form a ternary complex with RanBP1 and importin β in a solution binding assay. Although it has been assumed that RanBP1 plays a role in the dissociation of the Ran-GTP–importin β complex, the biological significance of the ternary complex has not yet been established. The RanBP1–Ran-GDP–importin β complex has been proposed to play a role in nuclear protein import as a ternary complex (Chi et al., 1997), although it has not yet demonstrated how the complex functions in the nuclear protein import. We therefore examined the effects of ARAN1 on the interaction of RanBP1 with both Ran-GTP– importin β and Ran-GDP–importin β complexes in the solution binding assay. In the absence of ARAN1, GST-tagged importin β efficiently precipitated both Ran-GTP and Ran-GDP together with RanBP1 in a molar ratio of 1:1 (Fig. 5, A and B, lane 1). In contrast, in the presence of ARAN1, the amount of RanBP1 precipitated with GST-tagged importin β apparently decreased, although the Ran was efficiently precipitated. These results indicate that RanBP1 is not able to interact with the Ran-GTP–importin β and Ran-GDP– importin β complexes, when bound to ARAN1. That is, the binding of ARAN1 to the Ran–importin β complex suppresses the ternary complex formation of both RanBP1– Ran-GTP–importin β and RanBP1–Ran-GDP–importin β in solution.
Figure 5.
ARAN1 prevents the formation of Ran/RanBP1– importin β complex. (A) ARAN1 was added to the immobilized importin β–Ran-GTP complex and incubated for 30 min. RanBP1 was then added to the mixture. After 30 min of incubation, the proteins bound to importin β were precipitated and analyzed by SDS-PAGE followed by Coomassie brilliant blue staining. (B) GST-tagged importin β was mixed with the Ran-GDP and ARAN1 and then incubated with glutathione–Sepharose beads for 1 h. After washing the beads, RanBP1 was added the mixture and incubated for 1 h. The proteins bound to importin β were precipitated and analyzed by SDS-PAGE followed by Coomassie brilliant blue staining.
Ran–Importin β–related Transport Factor Complex Is Exported from the Nucleus to the Cytoplasm
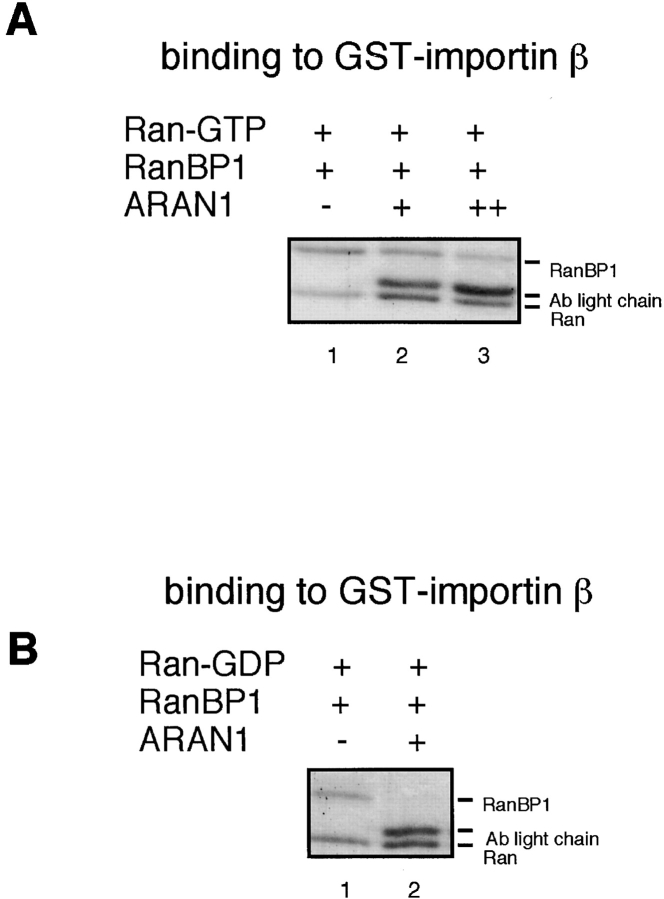
As described above, it has been proposed that the Ran-GTP– importin β complex and Ran-GTP–importin β–related transport factor (such as transportin and CAS) complex are formed in the nucleus and exported to the cytoplasm. To determine whether these complexes are actually formed in the nucleus and migrate to the cytoplasm as a single entity in living cells, ARAN1, which binds to only the Ran complexed with importin β and importin β–related transport factors in solution, was injected into the cytoplasm or nucleus of cultured BHK cells. It was found that nuclear-injected ARAN1 was efficiently exported to the cytoplasm within 30 min of incubation (Fig. 6 A), whereas control mouse IgG was retained in the injected nucleus (data not shown). Since due to its size (M r = 160,000), mouse IgG cannot passively traverse the nuclear envelope (Wen et al., 1995), these results indicate that ARAN1 migrates to the cytoplasm in a piggyback fashion. These findings suggest that it is most likely that ARAN1 binds to the Ran-GTP complexed with importin β–related transport factors in the nucleus, in which Ran would exist as the GTP-bound form, and is transported to the cytoplasm as a complex with them. That is, these results suggest that the Ran-GTP–importin β–related transport factor complex moves to the cytoplasm as a single entity without dissociation. On the other hand, cytoplasmically injected ARAN1 remained in the cytoplasm even after 3 h of incubation (data not shown).
Figure 6.
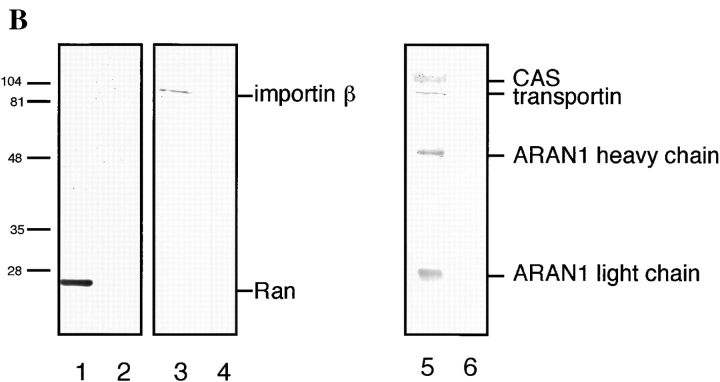
ARAN1 is exported from the nucleus to the cytoplasm. ARAN1 (5 mg/ml) was injected into the cytoplasm (top) or nucleus (bottom) of BHK21 cells with FITC-BSA. After incubation at 37°C for 30 min, cells were fixed and stained with Cy3-conjugated goat anti–mouse IgG antibodies (right). The localization of FITC-BSA shows the injected site in the cells. (B) ARAN1 recognizes the Ran–importin β complex in Ehrlich ascites tumor cell cytosolic extract. ARAN1 was added (lanes 1 and 3) or not (lanes 2 and 4) into the cytosolic extract and incubated for 1 h in the presence of Q69L Ran-GTP. The proteins which were bound to ARAN1 were precipitated with protein A–agarose beads and analyzed by immunoblotting with rabbit anti-Ran polyclonal antibodies (lanes 1 and 2), rabbit anti–importin β polyclonal antibodies (lanes 3 and 4), mouse anti-transportin monoclonal antibody, and mouse anti-CAS monoclonal antibody (lanes 5 and 6, respectively).
To determine whether ARAN1 actually recognizes the Ran-GTP–importin β–related transport factor complex in a crude cell extract, we performed immunoprecipitation analysis with mouse Ehrlich ascites tumor cell cytosolic extract in the presence of Q69L Ran-GTP, which should stabilize the complexes of importin β–related transport factors and Ran-GTP in the crude lysate. As shown in Fig. 6 B, importin β, CAS, and transportin were coprecipitated with the Ran from the cell lysate by ARAN1. This result supports the view that nuclear-injected ARAN1 binds to Ran-GTP–importin β–related factors and is exported from the nucleus to the cytoplasm in vivo.
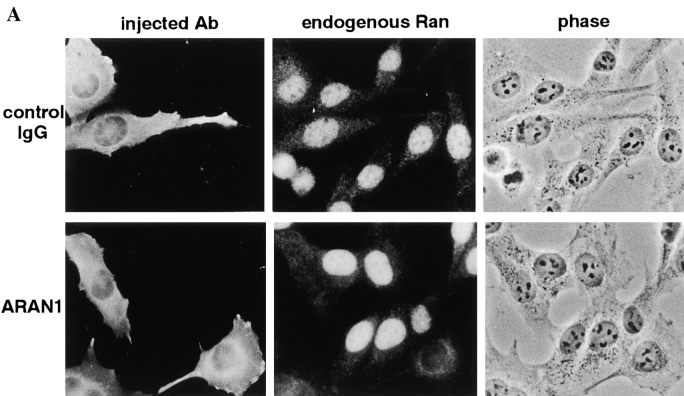
ARAN1 Affects the Distribution of Endogenous Ran
Ran is localized predominantly in the nucleus. If Ran continuously shuttles between two compartments, the nucleus and cytoplasm, and the binding of RanBP1 to the Ran-GTP–importin β complex in the cytoplasm is required for the recycling of Ran to the nucleus, ARAN1 would be expected to affect the distribution of Ran. To examine this hypothesis, ARAN1 was introduced into the cell and the distribution of endogenous Ran was observed. As shown in Fig. 6, 30 min after injection into either the nucleus or the cytoplasm, injected ARAN1 localized in the cytoplasm. 30 min after injection of ARAN1 into either the nucleus or the cytoplasm, the subcellular localization of endogenous Ran became apparently dominant in the cytoplasm of the ARAN1-injected cells, whereas control mouse IgG had no effect on this distribution (Fig. 7 A for cytoplasmic injection and data not shown for nuclear injection). The relocalization of Ran was caused irrespective of the injection sites of ARAN1.
Figure 7.
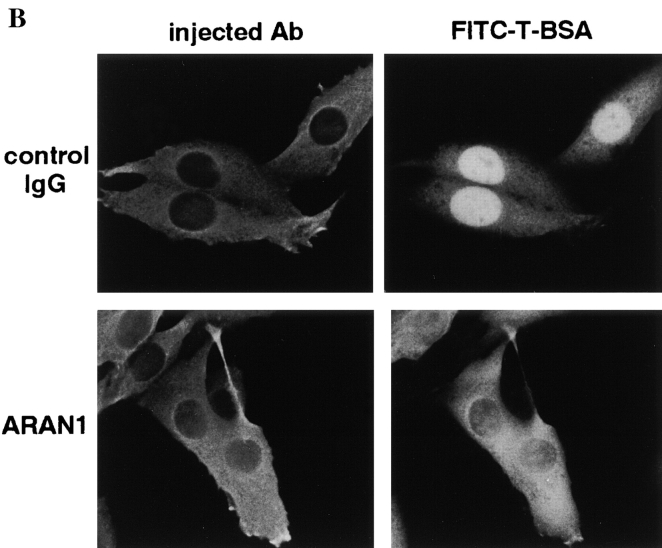
ARAN1 induces the intracellular localization rearrangement of Ran and inhibits the basic type NLS-dependent nuclear protein import. (A) ARAN1 (bottom) or control mouse IgG (top, both 50 mg/ml) was injected into the cytoplasm of the cultured BHK21 cells and incubated at 37°C for 30 min. The cells were fixed and probed with rabbit anti-Ran antibodies at 4°C overnight, and then stained with Cy3-conjugated goat anti–rabbit antibodies. Injected ARAN1 and control mouse IgG was detected with FITC-conjugated goat anti–mouse IgG antibodies. Phase–contrast microscopy is shown in the right panels. (B) ARAN1 or control mouse IgG (50 mg/ml) was injected into the cytoplasm of cultured BHK21. After a 30-min incubation at 37°C, FITC-labeled T-BSA was injected into the cytoplasm of the same cells. After incubation at 37°C for 20 min, the cells were fixed and observed by fluorescent microscopy. Injected IgG was detected with Cy3-labeled anti-mouse IgG.
ARAN1 Inhibits the Nuclear Import of Proteins Containing the Classical NLS
Lastly, we examined the effect of ARAN1 on the nuclear protein import in living cells. For this, ARAN1 or control mouse IgG was injected into nucleus or cytoplasm before the cytoplasmic injection of FITC-labeled BSA conjugated with the NLS peptide of SV-40 T antigen (FITC–T-BSA). In both cases, in which ARAN1 was injected into the cytoplasm or the nucleus, the nuclear import of FITC–T-BSA, which was injected afterwards was clearly inhibited (Fig. 7 B for cytoplasmic injection and data not shown for nuclear injection). In both cases, the injected ARAN1 was confirmed to be localized in the cytoplasm as expected from the results of Fig. 6 (data not shown). In contrast, the simultaneous cytoplasmic injection of ARAN1 with FITC– T-BSA failed to inhibit the nuclear import of the FITC– T-BSA (data not shown). These results suggest that the injected ARAN1 interrupts the Ran cycle in the cytoplasm probably by preventing RanBP1 from interacting with the Ran–importin β complex, which causes an abnormal distribution of Ran and further the suppression of nuclear protein import.
Discussion
The COOH-terminal Acidic Domain of Ran Is Exposed to the Molecular Surface as the Result of Its Interaction with Importin β or Importin β Families
In this study we obtained a monoclonal anti-Ran antibody, ARAN1, which recognizes mono-specific Ran as confirmed by immunoblotting of total cell extract and immunofluorescent analysis of cultured cells. An epitope of ARAN1 was found to lie in the acidic COOH-terminal domain of Ran. A solution binding assay revealed that ARAN1 interacts only with Ran, when it is complexed with importin β, transportin, or CAS–importin α but not Ran alone. These results indicate that the COOH-terminal domain of Ran is exposed to the molecular surface probably as the result of a conformational change only when importin β–related transport factors bind to Ran.
Although ARAN1 recognizes only Ran complexed with importin β or its related transport factors in solution, it shows the same immunofluorescence pattern as a polyclonal anti-Ran antibodies (Fig. 1). Since it was previously reported that importin β is located not only in the cytoplasm but also within the nucleus (Kose et al., 1997), it is likely that the apparent nucleoplasmic staining by ARAN1 means the complex formation of Ran with importin β and importin β–related transport factors in the nucleus. Alternatively, this can be explained by a similar degree of Ran denaturation during cell fixation as in immunoblotting.
It was found that ARAN1 binds to Ran-GDP–importin β complex as well as Ran-GTP–importin β complex, whereas it is known that importin β binds to Ran-GDP with much lower affinity than to Ran-GTP. Chi et al. (1996) demonstrated that the addition of RanBP1 with Ran-GDP increased the affinity of Ran-GDP for importin β. By analogy, it is speculated that ARAN1 may stabilize the interaction of Ran-GDP with importin β like RanBP1. In both cases, whereas ARAN 1 cannot reach the COOH-terminal domain of Ran-GTP or Ran-GDP alone, the interaction of Ran with importin β appears to induce a conformational change of Ran so that ARAN1 is able to recognize the epitope domain. Richards et al. (1995) demonstrated that a polyclonal antibody against a peptide corresponding to residues 196–207 of Ran, which is adjacent to the acidic 211DEDDDL-COOH216 domain, preferentially recognizes the GTP-bound form of Ran. They therefore proposed that the COOH-terminal domain is structurally flexible and undergoes a nucleotide-dependent conformational change, which is not inconsistent with the results herein.
Due to the characteristic amino acid sequences and conformational flexibility, it is likely that the COOH-terminal domain has some functions. Richards et al. (1995) also proposed from their biochemical analysis using ΔDE-Ran that the DEDDDL sequence stabilizes the GDP form of Ran, possibly by folding into the guanine nucleotide binding pocket and mimicking the negative charge on the γ-phosphate of GTP. In addition, it was suggested that the COOH terminus of Ran is implicated in the regulation of interaction between Ran and RanBP1 (Richards et al., 1995). Consistent with their suggestion, we showed that the binding of ARAN1 to the COOH-terminal tail suppresses the interaction of RanBP1 with the Ran–importin β complex. Although ΔDE-Ran is able to bind to RanBP1, its affinity to RanBP1 is lower than wild-type Ran (Richards et al., 1995). Therefore, we speculate that exposure of the COOH-terminal tail may increase the accessibility of RanBP1 not only to the Ran–importin β complex but also to the Ran– importin β–related transport factors.
A similar sequence in importin β (335DENDDDW342) with the COOH-terminal domain of Ran is immediately adjacent to sequences required for binding Ran-GDP/ RanBP1, but not Ran-GTP (Chi et al., 1997). They proposed that this sequence in importin β substitutes for the COOH-terminal Ran sequence in the nucleotide binding pocket of Ran when GDP is bound and that this rearrangement would expose the COOH terminus of Ran for binding to RanBP1, stabilizing the trimeric complex. Their proposal is consistent with our result that the COOH-terminal tail of Ran-GDP was also exposed by the binding of importin β.
The Export of Ran–Importin β–related Transport Factor Complexes from the Nucleus
Although it has been proposed that the Ran–importin β–related transport factor complexes form in the nucleus, are exported to the cytoplasm, and then dissociate in order to recycle (Bischoff and Görlich, 1997; Izaurralde et al., 1997), this proposal has not been experimentally verified in vivo. Recently, Izaurralde et al. (1997) showed that the depletion of nuclear Ran-GTP inhibits the export of importin α and β, transportin and NES-containing proteins in Xenopus laevis oocyte, suggesting that nuclear Ran-GTP is required for the export of importin α and β, transportin, and NES-containing proteins from the nucleus. However, their results do not necessarily mean that the Ran–importin β–related transport factor complexes are translocated from the nucleus to the cytoplasm as a complex. Thus, in regard to the dynamic behavior of Ran through the nuclear envelope, little concrete experimental data exist. In this study, we found that a monoclonal anti-Ran antibody ARAN1 failed to inhibit the export of Ran from the nucleus and inhibited reimport into the nucleus, and that nuclearly injected ARAN1 was efficiently exported from the nucleus. Our results indicate that the Ran–importin β–related transport factor complexes efficiently migrate from the nucleus to the cytoplasm as a complex in living cells, even when complexed with ARAN1. Although it can not be excluded that the nuclear export of ARAN1 injected into the nucleus could be due to complex formation with Ran-GDP–importin β–related factors rather than Ran-GTP–importin β–related factors, the current model of a steep Ran-GTP gradient across the nuclear membrane (see introduction) and the immunoprecipitation experiments with ARAN1 in the presence of Q69L Ran-GTP (Fig. 6 B) support that ARAN1 is exported from the nucleus as a complex with Ran-GTP and importin β–related factors.
The Role of RanBP1 in the Recycling of Ran
After the translocation of the Ran–importin β complex into the cytoplasm, it is predicted that the complex is disassembled for the next round of nuclear import and the recycling of Ran. The Ran-GTP–importin β complex is kinetically very stable (the dissociation constant is ∼1 nM, when nucleotide exchange and GTP hydrolysis are blocked) (Görlich et al., 1996; Izaurralde et al., 1997). Injected ARAN1 induced the relocalization of endogenous Ran in living cells. That is, endogenous Ran was found predominantly in the cytoplasm within 30 min after injection (Fig. 7). Since the injected ARAN1 was also localized in the cytoplasm, irrespective of the injection site (Fig. 6), it is concluded that ARAN1 prevented the event(s) from occurring in the cytoplasm but not in the nucleus. Our in vitro biochemical experiments (Figs. 4 and 5) strongly suggest that the injected ARAN1 efficiently interacts with the Ran–importin β complex in the nucleus or the cytoplasm and prevents the binding of RanBP1 to the Ran–importin β complex in the cytoplasm. Furthermore, it was found that the COOH-terminal tail of Ran is also exposed via interaction with other importin β family molecules. Therefore, we conceive that Ran probably remains in the cytoplasm complexed with importin β or its related transport factors due to the inhibition by ARAN1 on the binding of RanBP1 to Ran–importin β or its related transport factor complexes in the cytoplasm, although we did not examine in this study that ARAN1 actually inhibits the binding of RanBP1 to the complex of Ran with importin β–related transport factors other than importin β. At least part of our conclusion is consistent with the recent biochemical analysis by Bischoff and Görlich (1997). They showed that the importin α and RanBP1 cooperatively relieved the GAP resistance of the Ran-GTP–importin β complex, suggesting that RanBP1 is critical for the release of Ran-GTP from importin β. Moreover, in our study, it was found that ARAN1 competes much better for RanBP1 complexed to Ran-GDP–importin β than to Ran-GTP–importin β, which means that RanBP1 has much higher affinity for Ran-GTP– importin β complex than for Ran-GDP–importin β complex. This is consistent with the idea that RanBP1 efficiently binds to Ran-GTP–importin β complex in the cytoplasm to promote the dissociation of the complex.
RanBP2 contains four Ran-binding domains which are functionally equivalent to RanBP1 (Lounsbury et al., 1994; Yokoyama et al., 1995). It is located at the cytoplasmic filaments of the NPC (Wu et al., 1995; Yokoyama et al., 1995) and a fraction of RanGAP which is modified by the addition of a small ubiquitin-like peptide binds to RanBP2 (Matunis et al., 1996; Mahajan et al., 1997; Saitoh et al., 1997). Therefore, RanBP2 may play the same role as RanBP1 in the recycling of Ran, although further experiments will be required to better understand the role(s) of RanBP2 on the nucleocytoplasmic transport of macromolecules as well as to understand the effect of ARAN1 on RanBP2.
Nuclear Protein Import and Recycling of Ran and Importin β
This study showed that ARAN1, when injected into cultured cells inhibits nuclear protein import mediated by basic-type classical NLS irrespective of the injection site, i.e., nucleoplasm or cytoplasm. It has been recently reported that RanBP1 is required for nuclear protein import and RNA export in vivo (Schlenstedt et al., 1995; Richards et al., 1996) and stimulates the protein import in a permeabilized cell-free assay (Chi et al., 1996). Furthermore, it has been shown that the COOH terminus deleted Ran (ΔDE-Ran) represents the dominant-negative effect on the nuclear protein import. In this study, we found that ARAN1 inhibits the interaction of RanBP1 (and probably RanBP2) with the Ran–importin β complex. There are two possible explanations for the inhibitory effect of ARAN1 on the nuclear import of the classical NLS substrates. One possibility is that ARAN1 may inhibit the interaction of RanBP1 to Ran-GTP–importin β complex, which leads to the suppression of the disassembly of the complex. Alternatively, consistent with the suggestion of Chi et al. (1996) that RanBP1 promotes nuclear protein import by stabilizing the interaction of Ran-GDP with importin β, ARAN1 may block the function of RanBP1 on the interaction of Ran-GDP with importin β by binding to the Ran-GDP–importin β complex stably and, as a result, affect nuclear protein import.
Thus, it seems most likely that the inhibition of the function of RanBP1 (and probably RanBP2) blocks the disassembly of Ran–importin β complex in the cytoplasm, which causes the depletion of nuclear Ran, an inhibition in the recruitment of importin β, and further, the decline of the recycling of importin α/β to the cytoplasm by the suppression of the dissociation of the nuclear pore-targeting complex (importin α/β bound to a karyophile) in the nucleus following the depletion of nuclear Ran. It appears that such an imbalance in the import factors totally inhibits the nuclear protein import. Further studies will be necessary in order to understand whether ARAN1 also affects import and export pathways other than the classical NLS import pathway.
Acknowledgments
We thank S. Kuroda (Institute of Scientific and Industrial Research, Osaka University, Japan) for the gift of pGEX-6P-2–hGFP vector.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Area (07282103), a Grant-in-Aid for Scientific Research (B) (08458229), and a Grant-in-Aid for COE Research (07CE2006) from the Japanese Ministry of Education, Sciences, Sports and Culture, the Nissan Science Foundation, and the Naito Foundation. M. Hieda, T. Tachibana, F. Yokoya, and S. Kose are Research Fellows of the Japanese Society for the Promotion of Science.
Abbreviations used in this paper
- BSA
bovine serum albumin
- CAS
cellular apoptosis susceptibility gene
- GFP
green fluorescent protein
- GST
glutathione-S-transferase
- NES
nuclear export signal
- NLS
nuclear localization signal
- NPC
nuclear pore complex
- RanBP
Ran-binding protein
- TB
transport buffer
Footnotes
M. Hieda and T. Tachibana contributed equally to this work.
T. Tachibana's present address is Department of Neurochemistry and Neuropharmacology, Biomedical Research Center, Osaka University Medical School, 2-2 Yamada-oka, Suita, Osaka 565-0871, Japan.
References
- Avis JM, Clarke PR. Ran, a GTPase involved in nuclear processes: its regulators and effectors. J Cell Sci. 1996;109:2423–2427. doi: 10.1242/jcs.109.10.2423. [DOI] [PubMed] [Google Scholar]
- Bischoff FR, Ponstingl H. Mitotic regulator protein RCC1 is complexed with a nuclear ras-related polypeptide. Proc Natl Acad Sci USA. 1991a;88:10830–10834. doi: 10.1073/pnas.88.23.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff FR, Ponstingl H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 1991b;354:80–82. doi: 10.1038/354080a0. [DOI] [PubMed] [Google Scholar]
- Bischoff FR, Klebe C, Kretschmer J, Wittinghofer A, Ponstingl H. RanGAP1 induces GTPase activity of nuclear ras-related Ran. Proc Natl Acad Sci USA. 1994;91:2587–2591. doi: 10.1073/pnas.91.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff FR, Krebber H, Smirnova E, Dong W, Ponstingl H. Co-activation of Ran GTPase and inhibition of GTP dissociation by Ran-GTP binding protein RanBP1. EMBO (Eur Mol Biol Organ) J. 1995;14:705–715. doi: 10.1002/j.1460-2075.1995.tb07049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff FR, Ponstingl H. Catalysis of guanine nucleotide exchange of Ran by RCC1 and stimulation of hydrolysis of Ran-bound GTP by Ran-GAP1. Methods Enzymol. 1995;257:135–144. doi: 10.1016/s0076-6879(95)57019-5. [DOI] [PubMed] [Google Scholar]
- Bischoff FR, Görlich D. RanBP1 is crucial for the release of RanGTP from importin β-related nuclear transport factors. FEBS (Fed Eur Biochem Soc) Lett. 1997;419:249–254. doi: 10.1016/s0014-5793(97)01467-1. [DOI] [PubMed] [Google Scholar]
- Becker J, Melchior F, Gerke V, Bischoff FR, Ponstingl H, Wittinghofer A. RNA1 encodes a GTPase-activating protein specific for Gsp1p, the Ran/TC4 homologue of Saccharomyces cerevisiae. . J Biol Chem. 1995;270:11860–11865. doi: 10.1074/jbc.270.20.11860. [DOI] [PubMed] [Google Scholar]
- Bonifaci N, Moroianu J, Radu A, Blobel G. Karyopherin β2 mediates nuclear import of a mRNA binding protein. Proc Natl Acad Sci USA. 1997;94:5055–5060. doi: 10.1073/pnas.94.10.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KL, Richards SA, Lounsbury KM, Macara IG. Evidence using a green fluorescent protein-glucocorticoid receptor chimera that the Ran/TC4 GTPase mediates an essential function independent of nuclear protein import. J Cell Biol. 1996;133:985–996. doi: 10.1083/jcb.133.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Adam EJH, Visser GD, Adam SA. RanBP1 stabilizes the interaction of Ran with p97 in nuclear protein import. J Cell Biol. 1996;135:559–569. doi: 10.1083/jcb.135.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Adam EJH, Adam SA. Different binding domains for Ran-GTP and Ran-GDP/RanBP1 on nuclear import factor p97. J Biol Chem. 1997;272:6818–6822. doi: 10.1074/jbc.272.10.6818. [DOI] [PubMed] [Google Scholar]
- Corbett AH, Koepp DM, Schlenstedt G, Lee MS, Hopper AK, Silver PA. Rna1p, a Ran/TC4 GTPase activating protein, is required for nuclear import. J Cell Biol. 1995;130:1017–1026. doi: 10.1083/jcb.130.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutavas E, Ren M, Oppenheim JD, D'Eustachio P, Rush MG. Characterization of proteins that interact with the cell-cycle regulatory protein Ran/TC4. Nature. 1993;366:585–587. doi: 10.1038/366585a0. [DOI] [PubMed] [Google Scholar]
- Floer M, Blobel G. The nuclear transport factor karyopherin β binds stoichiometrically to Ran-GTP and inhibits the Ran GTPase activating protein. J Biol Chem. 1996;271:5313–5316. doi: 10.1074/jbc.271.10.5313. [DOI] [PubMed] [Google Scholar]
- Fornerod M, van Deursen J, van Bassl S, Reynolds A, Davis D. The human homologue of yeast Crm1 is in a dynamic subcomplex with Can/ Nup214 and a novel nuclear pore component Nup88. EMBO (Eur Mol Biol Organ) J. 1997a;16:807–816. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997b;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- Goldfarb DS. Whose finger is on the switch? . Science. 1997;276:1814–1816. doi: 10.1126/science.276.5320.1814. [DOI] [PubMed] [Google Scholar]
- Görlich D. Identification of different roles of for RanGTP and RanGDP in nuclear protein import. EMBO (Eur Mol Biol Organ) J. 1996;15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Mattaj IW. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- Görlich D. Nuclear protein import. Curr Opin Cell Biol. 1997;9:412–419. doi: 10.1016/s0955-0674(97)80015-4. [DOI] [PubMed] [Google Scholar]
- Görlich D, Dabrowski M, Bischoff FR, Kutay U, Bork P, Hartmann E, Prehn S, Izaurralde E. A novel class of RanGTP binding proteins. J Cell Biol. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper AK, Traglia HM, Dunst RW. The yeast Rna1 gene product necessary for RNA processing is located in the cytosol and apparently excluded from the nucleus. J Cell Biol. 1990;111:309–321. doi: 10.1083/jcb.111.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto N, Tachibana T, Matsubae M, Yoneda Y. A karyophilic protein forms a stable complex with cytoplasmic components prior to nuclear pore binding. J Biol Chem. 1995;270:8559–8565. doi: 10.1074/jbc.270.15.8559. [DOI] [PubMed] [Google Scholar]
- Izaurralde E, Kutay U, von Kobbe C, Mattaj IW, Görlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO (Eur Mol Biol Organ) J. 1997;1997:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp DM, Wong D, Corbett A, Silver P. Dynamic localization of the nuclear import receptor and its interactions with transport factors. J Cell Biol. 1996;133:1163–1176. doi: 10.1083/jcb.133.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp DM, Silver PA. A GTPase controlling nuclear trafficking: running the right way or walking RANdomly? . Cell. 1996;87:1–4. doi: 10.1016/s0092-8674(00)81315-x. [DOI] [PubMed] [Google Scholar]
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kose S, Imamoto N, Tachibana T, Shimamoto T, Yoneda Y. Ran-unassisted nuclear migration of a 97-kD component of nuclear pore-targeting complex. J Cell Biol. 1997;139:841–849. doi: 10.1083/jcb.139.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Bischoff FR, Kostka S, Kraft R, Görlich D. Export of importin α from the nucleus is mediated by a specific nuclear transport factor. Cell. 1997;90:1061–1071. doi: 10.1016/s0092-8674(00)80372-4. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lounsbury KM, Beddow AL, Macara IG. A family of proteins that stabilize the Ran/TC4 GTPase in its GTP-bound conformation. J Biol Chem. 1994;269:11285–11290. [PubMed] [Google Scholar]
- Lounsbury KM, Richards SA, Carey KL, Macara IG. Mutations within the Ran/TC4 GTPase. J Biol Chem. 1996a;271:32834–32841. doi: 10.1074/jbc.271.51.32834. [DOI] [PubMed] [Google Scholar]
- Lounsbury KM, Richards SA, Perlungher RR, Macara IG. Ran binding domain promote the interaction of Ran with p97/β-karyopherin, linking the docking and translocation steps of nuclear import. J Biol Chem. 1996b;271:2357–2360. doi: 10.1074/jbc.271.5.2357. [DOI] [PubMed] [Google Scholar]
- Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran GTPase-activating protein, RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol. 1996;135:1457–1470. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior F, Paschal B, Evans J, Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993a;123:1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior F, Weber K, Gerke V. A functional homologue of the RNA1 gene product in Schizosaccharomyces pombe: purification, biochemical characterization, and identification of a leucine-rich repeat motif. Mol Biol Cell. 1993b;4:569–581. doi: 10.1091/mbc.4.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior F, Sweet DJ, Gerace L. Analysis of Ran/TC4 function in nuclear protein import. Methods Enzymol. 1995;257:279–291. doi: 10.1016/s0076-6879(95)57032-2. [DOI] [PubMed] [Google Scholar]
- Moore MS, Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993;365:661–663. doi: 10.1038/365661a0. [DOI] [PubMed] [Google Scholar]
- Moroianu J, Blobel G, Radu A. Nuclear protein import: Ran-GTP dissociates the karyopherin α/β heterodimer by displacing α from an overlapping binding site on β. Proc Natl Acad Sci USA. 1996;93:7059–7062. doi: 10.1073/pnas.93.14.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny S, Siomi MC, Siomi H, Michael WM, Pollard V, Dreyfuss G. Transportin: nuclear transport receptor of a novel nuclear protein import pathway. Exp Cell Res. 1996;229:261–266. doi: 10.1006/excr.1996.0369. [DOI] [PubMed] [Google Scholar]
- Nakielny S, Dreyfuss G. Nuclear export of proteins and RNAs. Curr Biol. 1997;9:420–429. doi: 10.1016/s0955-0674(97)80016-6. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Nucleocytoplasmic transport: signals mechanism and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M, Okazaki H, Nishimoto T. The RCC1 protein, a regulator for the onset of chromosome condensation locates in the nucleus and binds to DNA. J Cell Biol. 1989;109:1389–1397. doi: 10.1083/jcb.109.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossareh NB, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- Pollard VW, Michael WM, Nakielny S, Siomi MC, Wang F, Dreyfuss G. A novel receptor mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Ren M, Villamarin A, Shin A, Coutavas E, Moore MS, Locurcio M, Clarke V, Oppenheim JD, Deustachio P, Rush MG. Separate domain of the Ran GTPase interact with different factors to regulate nuclear protein import and RNA processing. Mol Cell Biol. 1995;15:2117–2124. doi: 10.1128/mcb.15.4.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- Richards SA, Lounsbury KM, Macara IG. The C terminus of the nuclear Ran/TC4 GTPase stabilizes the GDP-bound state and mediates interaction with RCC1, RanGAP. J Biol Chem. 1995;270:14405–14411. doi: 10.1074/jbc.270.24.14405. [DOI] [PubMed] [Google Scholar]
- Richards SA, Lounsbury KM, Carey KL, Macara IG. A nuclear export signal is essential for the cytosolic localization of the Ran binding protein, RanBP1. J Cell Biol. 1996;134:1157–1168. doi: 10.1083/jcb.134.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SA, Carey KL, Macara IG. Requirement of guanosine triphosphate-bound Ran for signal-mediated nuclear protein export. Science. 1997;276:1842–1844. doi: 10.1126/science.276.5320.1842. [DOI] [PubMed] [Google Scholar]
- Saitoh H, Pu R, Cavenagh M, Dasso M. RanBP2 associates with Ubc9p and a modified form of RanGAP1. Proc Natl Acad Sci USA. 1997;94:3736–3741. doi: 10.1073/pnas.94.8.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenstedt G, Wong DH, Koepp DM, Silver PA. Mutants in yeast Ran binding protein are defective in nuclear transport. EMBO (Eur Mol Biol Organ) J. 1995;14:5367–5378. doi: 10.1002/j.1460-2075.1995.tb00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimoto T, Nakajima K, Tachibana T, Hirano T, Yoneda Y. Interferon- γ dependent nuclear import of Stat1 is mediated by the GTPase activity of Ran/TC4. J Biol Chem. 1996;271:31017–31020. doi: 10.1074/jbc.271.49.31017. [DOI] [PubMed] [Google Scholar]
- Stade K, Ford CS, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Sweet DJ, Gerace L. A GTPase distinct from Ran is involved in nuclear protein import. J Cell Biol. 1996;133:971–983. doi: 10.1083/jcb.133.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana, T., N. Imamoto, H. Seino, T. Nishimoto, and Y. Yoneda. 1994. Loss of RCC1 leads to suppression of nuclear protein import in living cells. J. Biol. Chem. 24542–24545. [PubMed]
- Wen W, Meinkoth JL, Tsien YR, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- Wu J, Matunis MJ, Kraemer D, Blobel G, Coutavas E. Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin A homologous domain, and a leucine-rich region. J Biol Chem. 1995;270:14209–14213. doi: 10.1074/jbc.270.23.14209. [DOI] [PubMed] [Google Scholar]
- Yokoyama N, Hayashi N, Seki T, Pante N, Ohba T, Nishi K, Hayashida T, Miyata T, Aebi U, Fukui M, Nishimoto T. A giant nucleopore protein that binds Ran/TC4. Nature. 1995;376:184–188. doi: 10.1038/376184a0. [DOI] [PubMed] [Google Scholar]
- Yoneda Y, Imamoto-Sonobe N, Yamaizumi M, Uchida T. Reversible inhibition of protein import into the nucleus by wheat germ agglutinin injected into cultured cells. Exp Cell Res. 1987;173:586–595. doi: 10.1016/0014-4827(87)90297-7. [DOI] [PubMed] [Google Scholar]
- Yoneda Y. How proteins are transported from cytoplasm to the nucleus. J Biochem. 1997;121:811–817. doi: 10.1093/oxfordjournals.jbchem.a021657. [DOI] [PubMed] [Google Scholar]