Abstract
Cytosolic phospholipase A2 (cPLA2) mediates agonist-induced arachidonic acid release, the first step in eicosanoid production. cPLA2 is regulated by phosphorylation and by calcium, which binds to a C2 domain and induces its translocation to membrane. The functional roles of phosphorylation sites and the C2 domain of cPLA2 were investigated. In Sf9 insect cells expressing cPLA2, okadaic acid, and the calcium-mobilizing agonists A23187 and CryIC toxin induce arachidonic acid release and translocation of green fluorescent protein (GFP)-cPLA2 to the nuclear envelope. cPLA2 is phosphorylated on multiple sites in Sf9 cells; however, only S505 phosphorylation partially contributes to cPLA2 activation. Although okadaic acid does not increase calcium, mutating the calcium-binding residues D43 and D93 prevents arachidonic acid release and translocation of cPLA2, demonstrating the requirement for a functional C2 domain. However, the D93N mutant is fully functional with A23187, whereas the D43N mutant is nearly inactive. The C2 domain of cPLA2 linked to GFP translocates to the nuclear envelope with calcium-mobilizing agonists but not with okadaic acid. Consequently, the C2 domain is necessary and sufficient for translocation of cPLA2 to the nuclear envelope when calcium is increased; however, it is required but not sufficient with okadaic acid.
Keywords: phospholipase A2, calcium, phosphorylation, C2 domain, Sf9 cells
The 85-kD cytosolic phospholipase A2 (cPLA2)1 plays an important role in agonist-induced arachidonic acid release (Clark et al., 1995; Leslie, 1997). This is a tightly regulated process since the availability of free arachidonic acid controls the level of eicosanoid production. The oxygenated metabolites of arachidonic acid, including leukotrienes, prostaglandins, and thromboxanes, have important roles in normal homeostasis and acute inflammation. An essential role for cPLA2 in reproductive function and inflammation has been confirmed recently from studies using cPLA2-deficient mice (Bonventre et al., 1997; Uozumi et al., 1997). cPLA2 is regulated posttranslationally by phosphorylation and by calcium (Channon and Leslie, 1990; Clark et al., 1991, 1995; Lin et al., 1993; Qiu et al., 1993; Leslie, 1997). Phosphorylation of cPLA2 on S505 by mitogen-activated protein kinases has been shown to be required for agonist-induced arachidonic acid release in some cell models but not in others (Lin et al., 1993; Kramer et al., 1996; Qiu et al., 1998). A critical step in the regulation of cPLA2 is its translocation from cytosol to membrane for access to phospholipid (Channon and Leslie, 1990; Clark et al., 1990). In several cell models cPLA2 has been shown to translocate primarily to the nuclear envelope and endoplasmic reticulum, where 5-lipoxygenase and cyclooxygenase also localize (Glover et al., 1995; Schievella et al., 1995; Sierra-Honigmann et al., 1996; Hirabayashi et al., 1999). The amino terminus of cPLA2 contains a C2 domain, which mediates the calcium-dependent binding of cPLA2 to membrane and phospholipid vesicles (Nalefski et al., 1994). C2 domains were originally described in protein kinase C (PKC) and now have been identified in >60 proteins (Nalefski and Falke, 1996; Rizo and Südhof, 1998). They are involved in the calcium-dependent association of proteins to membrane or in membrane trafficking, although the function of all C2 domains is not known. The C2 domain of cPLA2 is a β-sandwich structure that binds two ions of calcium and preferentially interacts with phosphocholine headgroups (Hixon et al., 1998; Nalefski et al., 1998; Perisic et al., 1998; Xu et al., 1998; Bittova et al., 1999). An increase in intracellular calcium promotes translocation of cPLA2 to membrane, which is an important regulatory step necessary for association of the catalytic domain of cPLA2 with phospholipid substrate.
In macrophages, we have demonstrated that a transient increase in calcium and phosphorylation of cPLA2 on S505 by mitogen-activated protein kinase can act together to fully activate cPLA2 leading to arachidonic acid release (Qiu et al., 1998). However, neither of these events alone is sufficient for activation. There are also alternative pathways for regulating cPLA2 activation in the macrophage model since both PMA and okadaic acid induce arachidonic acid release without increasing intracellular calcium (Qiu et al., 1998). We have used the baculovirus expression system in insect cells to study regulation of cPLA2 (de Carvalho et al., 1996). This model is now being used not only as a source of recombinant proteins but also to study the function of proteins and their role in signal transduction (Stancato et al., 1993, 1997; Chefalo et al., 1994). Similar to macrophages, Spodoptera frugiperda (Sf9) cells expressing cPLA2 release arachidonic acid in response to A23187 and okadaic acid (de Carvalho et al., 1996). cPLA2 is phosphorylated on multiple sites when expressed in Sf9 cells, and phosphorylation on S727 is preferentially induced by okadaic acid (de Carvalho et al., 1996). Okadaic acid also induces phosphorylation of the S727-containing tryptic peptide in mouse peritoneal macrophages and human monocytes (de Carvalho et al., 1996; Qiu et al., 1998). Phosphorylation of cPLA2 on S505 and S727 also occurs in thrombin- and collagen-stimulated platelets and cytokine-stimulated HeLa cells (Börsch-Haubold et al., 1998). The mechanisms involved in regulating the translocation of cPLA2 to membrane in response to agonists that do not increase intracellular calcium, and the preferential targeting of cPLA2 to the nuclear envelope are not understood. The role of these novel phosphorylation sites and the C2 domain in regulating cPLA2 translocation and arachidonic acid release in Sf9 cells in response to okadaic acid and the calcium-mobilizing agonists was investigated.
Materials and Methods
Reagents
Sf9 cells, the transfer vector pVL1393, and the TA cloning kit were obtained from Invitrogen. Grace's insect cell culture medium was obtained from JRH Biosciences. The transfer vectors BioGreen-His and pAcGHLT-A, as well as baculovirus linearized DNA (BaculoGold), were from PharMingen. Yeastolate was obtained from Becton Dickinson. Lactalbumin hydrolysate, penicillin (10,000 U/ml)/streptomycin (10,000 μg/ ml)/l-glutamine (29.2 mg/ml) solution, and a human spleen SuperScript cDNA library were from Life Technologies. FBS was purchased from Gemini Bioproducts. Human serum albumin (HSA) was from Intergen Co. [5,6,8,9,11,12,14,15-3H]Arachidonic acid (sp act 100 Ci/mmol), 1-O-hexadecyl-2-[3H]arachidonyl-phosphatidylcholine (200 Ci/mmol), and l-1-[1-14C]palmitoyl-2-lysophosphatidylcholine (55 mCi/mmol) were from DuPont/NEN. Anti–rabbit horseradish peroxidase–linked F(ab′)2 IgG fragment, reagents for enhanced chemiluminescence detection on immunoblots, and glutathione–Sepharose beads were purchased from Amersham Pharmacia Biotech. Calcium ionophore A23187 and pluronic acid F-68 (10% solution) were obtained from Sigma Chemical Co. Ionomycin was from Calbiochem-Novabiochem. Okadaic acid (ammonium salt) was from Alexis Biochemicals. Fura-2 acetoxymethyl ester (AM) and the nuclear dye Hoechst 33342 were obtained from Molecular Probes. A donkey anti–rabbit IgG antibody conjugated to Cy3 was obtained from Jackson ImmunoResearch. Reagents for protein determination by the bicinchoninic acid method were from Pierce Chemical Co. Bacillus thuringiensis δ-endotoxin CryIC was generously provided by Dr. Jean-Louis Schwarz (National Research Council, Montreal, Canada) and Dr. Marianne Carey (Case Western Reserve University, Cleveland, OH).
Preparation of DNA Constructs
The DNA encoding for human cPLA2 (provided by Dr. James Clark, Genetics Institute, Cambridge, MA) was cloned into the transfer vector pVL1393 as previously described (de Carvalho et al., 1993). For fusion-protein construction, the human cPLA2 coding region was obtained by PCR from a human spleen cDNA library and cloned into the pCR2.1 vector following the TA cloning method. The PCR primers used were 5′-GGATCCTGACTGAAAGCTAGAGGC-3′ (bases 22–45; GenBank accession number M72393) and 5′-CAGCCAGTCTCTCATGATCAGTACGAC-3′ (bases 2475–2501). Two in-frame codons present in the 5′ untranslated region were removed by PCR amplification using the primers 5′-GAGAGCGGGTACCCCGGTTTGAAGTGTGAAAACATTTCCTG-3′ (which contains a KpnI restriction site) and 5′-CCTGATTAGGATCCAAAATAAATTCAAAGGTCTC-3′ (which straddles a BamHI site). The PCR product was cut with KpnI and BamHI and cloned into pCR2.1 containing the cPLA2 gene. The modified cPLA2 sequence was cloned into the transfer vectors pAcGHLT (for expression of GST-cPLA2) and BioGreen-His (for expression of green fluorescent protein [GFP]-cPLA2) using the SacI and PstI sites. Different mutants of cPLA2 were obtained by site-directed mutagenesis using a kit from 5Prime→ 3Prime Inc., following the manufacturer's instructions. The mutagenic oligonucleotides used were 5′-CTTGATACTCCAAATCCCTATGTG-3′ (D43N), 5′-CGTTAATGAATGCCAATTATG-3′ (D93N), 5′-GATAGCTCGGACGCTGATGATGAATCAC-3′ (S437A), 5′-GAAGATGCTGGAGCTGACTATCAAAGTG-3′ (S454A), 5′-CTTATCCACTGGCTCCTTTGAG-3′ (S505A), and 5′-CCATCTCGTTGCGCTGTTTCCC-3′ (S727A). A truncated version of cPLA2 (Δ721–749) was generated by introducing a stop codon after amino acid 720 using the mutagenic oligonucleotide 5′-GAATATAGAAGATAGAATCCATCTCG-3′. A GFP-C2 construct was generated by using the oligonucleotide 5′-GCCCAGACCTATGATTTAGTATGGC-3′ to insert a stop codon after amino acid 144. All mutations were confirmed by sequencing of DNA from several bacterial colonies selected on agar plates.
Cell Culture and Generation of Recombinant Baculovirus Stocks
Sf9 cells were routinely cultured in 100-ml vented spinner flasks, at 27°C, in TNM-FH medium (Grace's medium supplemented with 3.3 g/liter yeastolate, 3.3 g/liter lactalbumin hydrolysate, and 1% penicillin/streptomycin/ glutamine solution) containing 10% FBS and 0.1% pluronic acid. Cell density was maintained at 0.2–1.5 × 106 cells/ml. Recombinant baculovirus was generated by cotransfection of Sf9 cells with the transfer vector pVL1393 containing the gene for cPLA2 and linearized baculovirus DNA (BaculoGold; PharMingen) following the manufacturer's instructions. Viral stocks derived from single virus particles were obtained by plaque purification and amplified (two cycles) following standard procedures. The titer of the working viral stocks was determined by infecting cells with serial dilutions of the stocks, overlaying them with agarose-containing TNM-FH medium, and counting the plaques after 4–6 d of incubation.
Measurement of Intracellular Calcium Concentration
Sf9 cells were plated on glass coverslips (13 mm diam) in 24-well plates (4 × 105 cells/well) and infected with baculovirus containing wild-type cPLA2. After infection (50–54 h), Sf9 cells were washed three times in buffer A (10 mM 2-[N-morpholino]ethanesulfonic acid, 10 mM NaCl, 60 mM KCl, 25 mM MgCl2, 4 mM d-glucose, 110 mM sucrose, adjusted to pH 6.2 with Trizma base) and incubated with 2 μM Fura-2 AM (in buffer A) for 60 min at room temperature protected from light. The cells were rinsed twice with buffer A, positioned in a fluorometer cuvette, and maintained at 27°C with continuous stirring. Calcium chloride or EGTA was added to buffer A to reach the final concentrations indicated in the figures. The effect of agonists on the intracellular concentration of calcium was determined by spectrofluorometry as previously described (Qiu et al., 1998).
Arachidonic Acid Release
Sf9 cells were plated in 24-well tissue culture plates (2.5 × 105 cells/well) and infected with baculovirus. After infection (30–35 h), they were labeled with 0.2 μCi/well [3H]arachidonic acid and incubated overnight (16–18 h). The cells were washed three times with TNM-FH medium containing 0.1% HSA to remove unincorporated arachidonic acid and then treated with agonists. After stimulation, the medium was centrifuged at 500 g for 10 min, and the amount of radioactivity in the supernatant was determined by scintillation counting. Cells were scraped in 0.5 ml of 0.1% Triton X-100 for determining the total cellular radioactivity. For immunoblot analysis of cPLA2, Sf9 cells were scraped in 100 μl of Laemmli buffer and the protein concentration was determined. DTT was added to a final concentration of 25 mM, and samples were boiled for 5 min before loading (0.5 μg/lane) on a SDS-polyacrylamide gel (Qiu et al., 1998). After electrophoresis, proteins were transferred to nitrocellulose and cPLA2 was detected by chemiluminescence using a 1:5,000 dilution of polyclonal antiserum to cPLA2 (de Carvalho et al., 1993; Qiu et al., 1998).
Phospholipase A2 Assay
Sf9 cells (1.25 × 106 cells/35 mm dish) were infected with recombinant baculovirus for 48–50 h. The monolayers were rinsed with 2 ml of PBS and scraped into 100 μl of ice-cold homogenization buffer (10 mM Hepes, pH 7.4, 1 mM EGTA, 0.34 M sucrose, 10% glycerol, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF). Cells were lysed with a probe sonicator (3 times for 5 s, then 2 times for 10 s) on ice, and the homogenates centrifuged at 100,000 g for 1 h. The protein concentration in the cytosols was determined and then DTT was added to a final concentration of 1 mM. The cytosols were kept on ice and assayed within 24 h, or stored at −20°C and thawed just before the assay. The PLA2 and lysophospholipase activities of cPLA2 were determined by using 1-O-hexadecyl-2-[3H]arachidonylphosphatidylcholine (final concentration 30 μM), or [1-14C]palmitoyl-2-lysophosphatidylcholine (50 μM), respectively, as previously described (de Carvalho et al., 1995). Reactions were started with the addition of cytosolic protein (10 μg for PLA2 assays, 5 μg for lysophospholipase assays), and proceeded for 5 min at 37°C. Equal expression of the mutant enzymes was verified by immunoblotting and quantitation of the cPLA2 bands using a Storm 840 system from Molecular Dynamics.
Affinity Purification of cPLA2 Antibody
Sf9 cells grown in suspension (6 × 108 cells in 600 ml medium) were infected with baculovirus encoding the fusion protein GST-cPLA2. Cells were lysed in 20 mM Hepes, pH 7.4, containing 2 mM EGTA, 1% Triton X-100, 10% glycerol, 1 mM PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. Lysates were incubated with 200 μl glutathione–Sepharose beads for 2 h at 4°C. The beads were washed sequentially with 2 ml each of 10 mM Tris (pH 7.5), 10 mM Tris (pH 8.8), 100 mM triethylamine (pH 11.5), and 10 mM Tris (pH 7.5). The beads were mixed with rabbit anti-cPLA2 antiserum (500 μl, diluted 1:9 in 10 mM Tris, pH 7.5), and then washed with 2 ml each of 10 mM Tris (pH 7.5), 0.5 M NaCl in 10 mM Tris (pH 7.5), and 10 mM Tris (pH 8.8). Antibody was eluted with 2 ml of 100 mM triethylamine (pH 11.5) into tubes containing 1 M Tris (pH 8.0) to adjust the pH.
Microscopy
For microscopy experiments, 1.25 × 106 cells were plated on 35-mm tissue culture dishes and infected with baculovirus containing the genes for the different GFP-containing fusion constructs at multiplicities of infection ranging from 2 to 10 colony forming units per cell. After infection (40–50 h), cells were washed twice with TNM-FH medium and observed at room temperature using an Olympus Vanox-T Microscope equipped with an Achroplan 63X Zeiss lens and a FITC filter. Digital confocal images were obtained with a SpectraSource Orbis 16 camera, using Slidebook software from Intelligent Imaging Innovations, Inc. Images shown in the figures are representative of at least three independent experiments.
For immunofluorescence, Sf9 cells (2.5 × 105) were plated on 13-mm glass coverslips in 24-well culture plates and infected with baculovirus containing cPLA2. After infection (40–50 h), cells were rinsed twice with 0.5 ml PBS, pH 7.5, and then fixed for 15 min with 0.5 ml 3% paraformaldehyde in PBS containing 3% sucrose. After rinsing with PBS, cells were permeabilized with 0.2% Triton X-100 for 15 min, and then incubated for 30 min with HBSS containing 10% FBS (blocking solution). Cells were incubated for 2 h with affinity-purified anti-cPLA2 antibody diluted 1:20 in blocking solution. After extensive rinsing with PBS, cells were incubated for 1 h with donkey anti–rabbit IgG antibody conjugated to Cy3 (10 μg/ml in blocking solution). Coverslips were mounted on microscope slides in 0.1 M Tris-HCl, pH 8.5, containing 90% glycerol and 2 mg/ml orthophenylenediamine. Cells were observed by digital confocal microscopy using a Cy3 filter.
Results
Arachidonic Acid Release and Calcium Mobilization in Sf9 Cells Expressing cPLA2
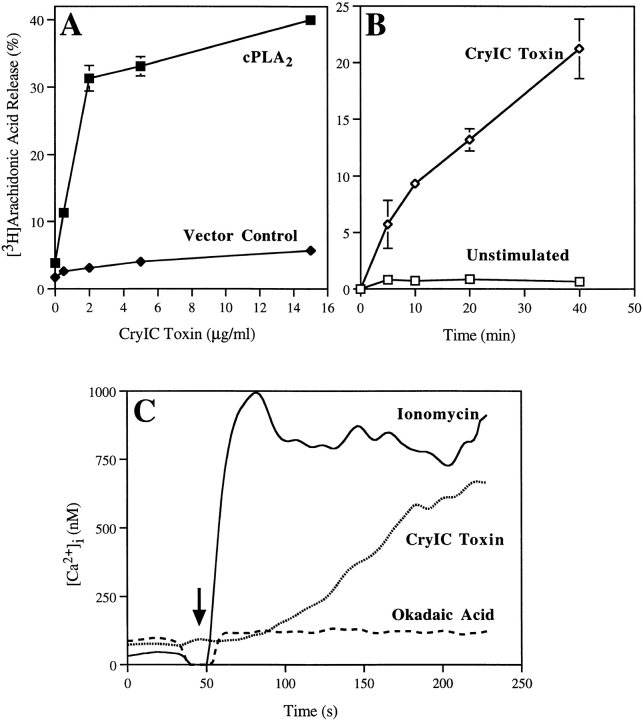
Sf9 cells expressing cPLA2 release arachidonic acid in response to A23187 and okadaic acid (de Carvalho et al., 1996). We have found that CryIC toxin from Bacillus thuringiensis also stimulates arachidonic acid release from Sf9 cells expressing cPLA2, but not from cells infected with baculovirus containing the vector alone (Fig. 1 A). The crystal (Cry) protein toxins are inclusions formed by the gram-positive soil bacterium during sporulation. CryIC toxin has been shown to induce an increase in intracellular calcium in Sf9 cells, probably in a receptor-mediated fashion (Schwartz et al., 1991). A 40-kD binding protein for CryIC toxin has been identified recently (Kwa et al., 1998). Arachidonic acid release in response to CryIC toxin was near maximal at 2 μg/ml, a concentration well below the ED50 for toxicity (15 μg/ml) for Sf9 cells (Monette et al., 1997). Rapid and prolonged release of arachidonic acid was induced by CryIC toxin (Fig. 1 B).
Figure 1.
CryIC toxin–induced arachidonic acid release and calcium mobilization in Sf9 cells expressing cPLA2. Sf9 cells were infected with recombinant baculovirus containing transfer vector pVL1393 alone (Vector Control) or the gene for human cPLA2. After infection (50–54 h), cells were prelabeled with [3H]arachidonic acid and then incubated for 20 min with the indicated concentrations of CryIC toxin (A) or with 2 μg/ml CryIC toxin or vehicle (Unstimulated), for the times indicated (B). The amount of [3H]arachidonic acid released into the medium is expressed as a percentage of the total radioactivity incorporated (cell-associated plus medium). Data represent the average ± SD of a representative experiment and were verified in at least three independent experiments performed in triplicate. (C) Calcium mobilization was measured in Fura-2 AM–loaded Sf9 cells expressing cPLA2 after addition (indicated by the arrow) of ionomycin (1 μM), okadaic acid (1 μM), or CryIC toxin (2 μg/ml). The intracellular calcium concentration was monitored over time by recording the excitation spectra at 340 and 380 nm. The extracellular calcium concentration was 9 mM. Results shown are from a representative experiment and were verified in at least three independent experiments.
An increase in intracellular calcium is an important mechanism for regulating cPLA2 by promoting its translocation and binding to membrane. However, we demonstrated previously that okadaic acid induces arachidonic acid release in macrophages without increasing intracellular calcium (Qiu et al., 1998). Experiments were carried out to determine the effect of okadaic acid on intracellular calcium levels in the Sf9 model in comparison to ionomycin and CryIC, which are known to increase intracellular calcium. As shown in Fig. 1 C, okadaic acid did not promote an increase in intracellular calcium in Sf9 cells expressing cPLA2, and no effect was seen when the incubation was prolonged for as long as 30 min. In contrast, CryIC toxin induced an increase in intracellular calcium that did not occur as rapidly as with ionomycin, but did reach a similar level. As previously reported, the increase in intracellular calcium induced by CryIC was sustained for at least 15 min (not shown) (Schwartz et al., 1991).
Agonist-induced Translocation of GFP-cPLA2 to the Nuclear Envelope in Sf9 Cells
GFP was linked to the amino terminus of cPLA2 and its subcellular localization was visualized in living Sf9 cells. GFP-cPLA2, like GFP alone, was uniformly distributed throughout unstimulated cells when observed in different planes of the cell by confocal analysis (data not shown). In Sf9 cells treated with the calcium-mobilizing agonists A23187 and CryIC toxin, as well as okadaic acid, the GFP-cPLA2 fusion protein exhibited time-dependent translocation to the nuclear envelope (Fig. 2). Translocation was evident in at least 80–85% of the cells observed in each experiment. Diffuse fluorescence emanating from the nuclear envelope could also be observed and may represent cPLA2 bound to endoplasmic reticulum. cPLA2 that is present both inside the nucleus and in the cytosol in unstimulated cells translocated to the nuclear envelope. Importantly, GFP alone did not translocate in response to agonist treatment and remained uniformly distributed. Translocation of GFP-cPLA2 to the nuclear envelope was evident by 5–10 min after treatment with the agonists and was nearly complete by 20–30 min (Fig. 2). The time course of translocation was not identical from cell to cell in the population and could vary by minutes. As previously reported the agonists induce significant arachidonic acid release by 15 min, which is consistent with the time course of translocation (Fig. 1 B) (de Carvalho et al., 1996).
Figure 2.
Agonist-induced translocation of GFP-cPLA2, but not GFP alone, to the nuclear envelope in living Sf9 cells. Sf9 cells expressing GFP-cPLA2 were incubated with 2 μg/ml A23187, 2 μg/ml CryIC toxin, or 1 μM okadaic acid. Digital confocal images were obtained at the times indicated (in minutes). The bottom two series show images of Sf9 cells expressing GFP alone at different times after addition of CryIC toxin (in minutes), or at 30 min after addition of A23187 (bottom left) or okadaic acid (bottom right).
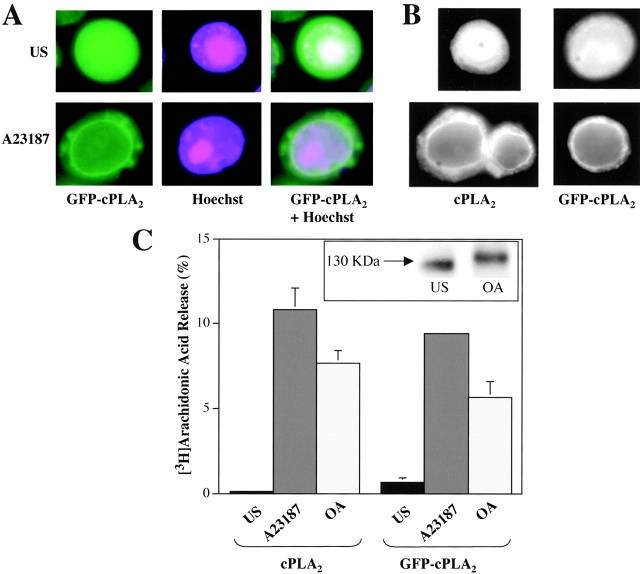
To further substantiate that GFP-cPLA2 localizes at the nuclear envelope, Sf9 cells expressing GFP-cPLA2 were treated with the nuclear dye Hoechst 33342. As shown in Fig. 3 A, GFP-cPLA2 was uniformly distributed throughout the cell, whereas the Hoechst localized to the nucleus. When GFP-cPLA2 and Hoechst colocalized, as seen inside the nucleus of the unstimulated cell, the purple color was diminished and the fluorescence appears white. As shown in the cell treated with A23187, GFP-cPLA2 localized to the nuclear envelope and the perinuclear region, whereas the purple Hoechst stain remained distributed throughout the nucleus. When the GFP-cPLA2 and Hoechst fluorescence were visualized together a bright ring of GFP-cPLA2 was seen surrounding the purple nucleus in the stimulated cell.
Figure 3.
Translocation of cPLA2 and GFP-cPLA2 to the nuclear envelope in living and fixed Sf9 cells. (A) Sf9 cells expressing GFP-cPLA2 were incubated for 20 min with the nuclear dye Hoechst 33342 (10 μg/ml), and then treated with vehicle (0.1% DMSO; US) or 2 μg/ml A23187 for 15 min. Digital confocal images of living Sf9 cells were obtained with a FITC filter to visualize the fluorescence from GFP (shown in green, left) or a UV filter for Hoechst (shown in purple, middle). A combined image showing the signals from both fluorophores is shown on the right. (B) Sf9 cells expressing cPLA2 or GFP-cPLA2 were treated with vehicle or 2 μg/ml A23187 for 20 min. Proteins were visualized by immunofluorescence in fixed cells using an affinity-purified anti-cPLA2 antibody, and images were obtained by digital confocal microscopy. (C) [3H]Arachidonic acid release from Sf9 cells expressing cPLA2 or GFP-cPLA2 was measured after treatment with vehicle, 2 μg/ml A23187, or 1 μM okadaic acid for 90 min at room temperature. The data shown are average ± SD from a representative experiment of at least three performed in triplicate. The inset shows an immunoblot of cPLA2 from unstimulated and okadaic acid–treated Sf9 cells.
To verify that the translocation of cPLA2 was not influenced by the GFP moiety, cPLA2 was expressed alone and as a GFP fusion protein in Sf9 cells and visualized by immunofluorescence using affinity-purified polyclonal anti-cPLA2 antibody. Localization of cPLA2 was similar to GFP-cPLA2 in fixed Sf9 cells (Fig. 3 B). In unstimulated cells, the enzyme was uniformly distributed throughout the cell. In response to A23187, cPLA2 and GFP-cPLA2 similarly translocated to the nuclear envelope and perinuclear region. Experiments were also carried out to verify that the GFP-cPLA2 fusion protein was catalytically active in Sf9 cells and could mediate agonist-induced arachidonic acid release. As shown in Fig. 3 C, cells expressing either cPLA2 or GFP-cPLA2 released arachidonic acid to a similar extent in response to A23187 and okadaic acid. GFP-cPLA2 exhibited a decrease in electrophoretic mobility (gel shift) in response to okadaic acid, as occurs with the native enzyme (Fig. 3 C) (de Carvalho et al., 1996).
Arachidonic Acid Release in Sf9 Cells Expressing Phosphorylation Site Mutants of cPLA2
We reported previously that treatment of Sf9 cells with okadaic acid increases phosphorylation of cPLA2 on S727 and to a lesser extent on S437, S454, and S505 (de Carvalho et al., 1996). To determine if phosphorylation of these sites was functionally important for okadaic acid– induced arachidonic acid release, the serines were mutated to alanines and the mutant cPLA2 constructs expressed in Sf9 cells. A double mutant construct, S505A/S727A, was also generated since both sites may act cooperatively in regulating cPLA2. In addition, a carboxy-terminal deletion (Δ721–749) was produced to avoid the possibility that serines near S727 (S724, S729, and S731) may become phosphorylated when S727 is converted to an alanine. Sf9 cells expressing the S727A, S437A, or S454A mutant constructs exhibited the same magnitude of arachidonic acid release in response to A23187, CryIC toxin, and okadaic acid as Sf9 cells expressing wild-type cPLA2 (Fig. 4). These results demonstrate that phosphorylation of these sites does not play a functional role in cPLA2 activation in this model. cPLA2 containing the S505A substitution was the only phosphorylation site mutant that was less effective (by 50%) at mediating arachidonic acid release. The results with cells expressing cPLA2 containing either the S505A/S727A double mutation or the S505A single mutation were not significantly different. Arachidonic acid release from Sf9 cells expressing the carboxy-terminal deletion mutant was no different from the response of Sf9 cells expressing wild-type cPLA2, confirming that phosphorylation of S727 plays no functional role in this model and that the extreme carboxy terminus of cPLA2 is not essential. Importantly, the level of expression of wild-type and mutant forms of cPLA2 was similar (Fig. 4). All the mutant cPLA2 enzymes except S505A were able to gel-shift in response to okadaic acid, demonstrating that the characteristic retardation of the electrophoretic mobility is only due to phosphorylation of S505, and not the other sites. A23187 and CryIC toxin have no effect on cPLA2 electrophoretic mobility in Sf9 cells (de Carvalho et al., 1996).
Figure 4.
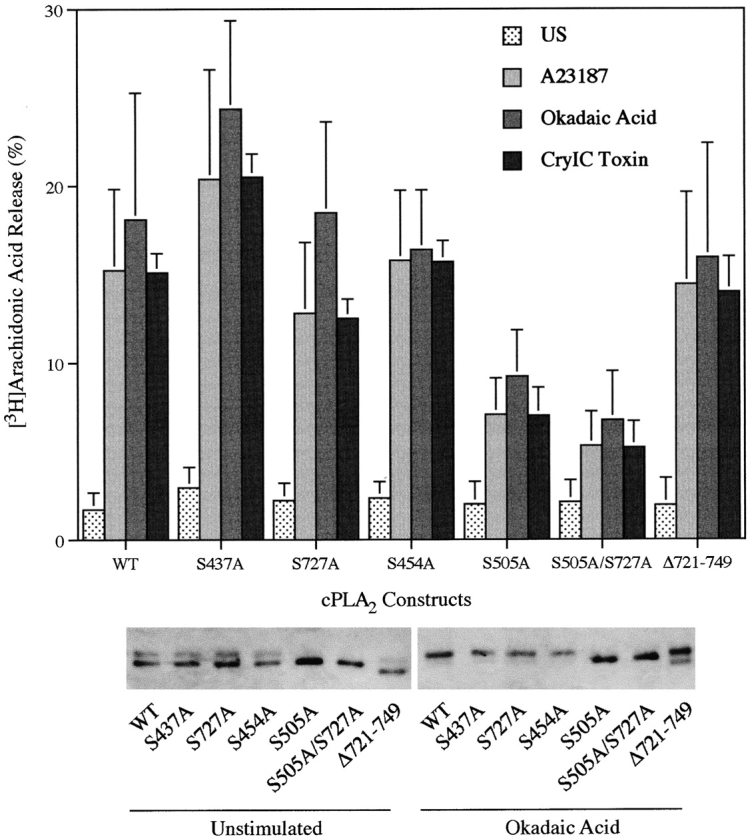
Agonist-induced arachidonic acid release from Sf9 cells expressing wild-type or phosphorylation-site mutants of cPLA2. [3H]Arachidonic acid release from Sf9 cells was measured after treatment with A23187 (2 μg/ml) or okadaic acid (1 μM) for 90 min, or with CryIC toxin (2 μg/ml) for 20 min. The amount of label released into the medium is expressed as a percentage of the total radioactivity incorporated (cell-associated plus medium). Data represent the mean ± SEM of three experiments performed in triplicate. The bottom panel shows a representative immunoblot of cPLA2.
Arachidonic Acid Release in Sf9 Cells Expressing C2 Domain Mutants of cPLA2
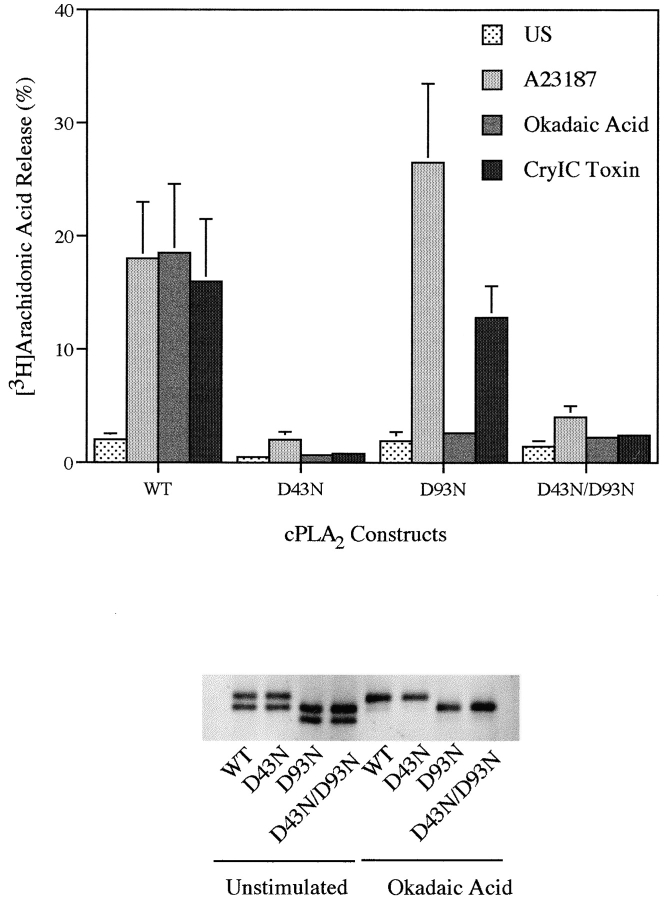
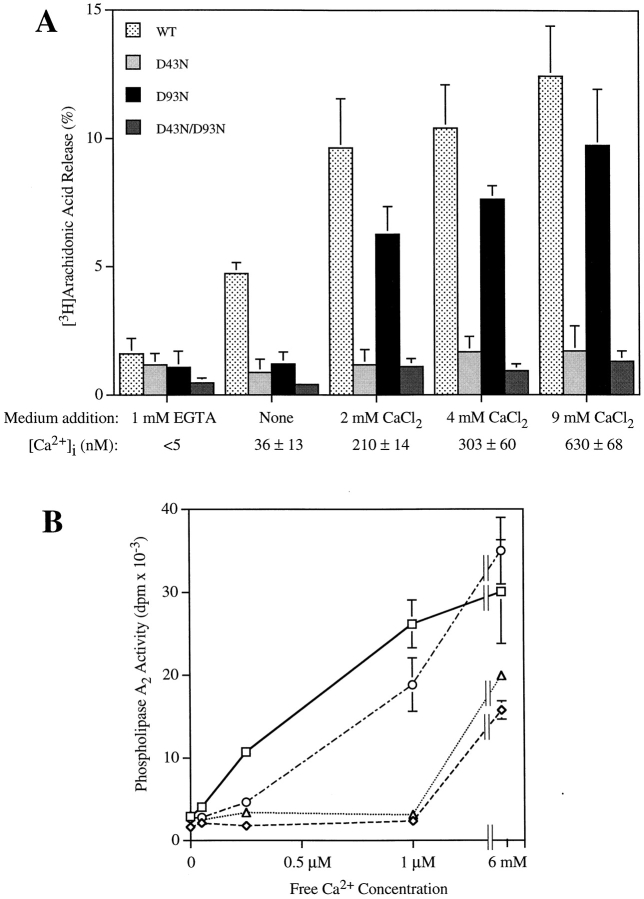
Although okadaic acid induces arachidonic acid release without increasing intracellular calcium, our previous work with macrophages has shown that resting levels of calcium are required (Qiu et al., 1998). This suggested that the C2 domain of cPLA2 is functionally important for regulation of the enzyme in response to okadaic acid. The crystal structure of the cPLA2 C2 domain has revealed several amino acid residues (D40, T41, D43, N65, D93, A94, and N95) that participate in binding calcium, and it has been shown recently that mutation of these residues impairs PLA2 activity and binding of cPLA2 to phospholipids (Perisic et al., 1998; Bittova et al., 1999). To determine if a functional C2 domain was required for cPLA2 to mediate arachidonic acid release in response to okadaic acid compared with the calcium-mobilizing agonists, two calcium binding residues (D43 and D93) were mutated to asparagine residues. cPLA2 containing the D43N, D93N, or D43N/D93N mutations was unable to mediate okadaic acid–induced arachidonic acid release in Sf9 cells (Fig. 5). Arachidonic acid release induced by A23187 or CryIC toxin was also dramatically suppressed in Sf9 cells expressing D43N or D43N/D93N cPLA2, whereas the D93N mutant was fully functional. In fact, the D93N mutant exhibited a modest but reproducible increased response to A23187, as compared with wild-type cPLA2. As shown in the Western blot, the C2 domain mutants of cPLA2 are expressed at similar levels and gel-shifted in response to okadaic acid. Mutation of D93N to an asparagine resulted in an increased electrophoretic mobility of cPLA2 (Fig. 5).
Figure 5.
Arachidonic acid release from Sf9 cells expressing wild-type or C2 domain mutants of cPLA2. [3H]Arachidonic acid release from Sf9 cells was measured after treatment with A23187 (2 μg/ml) or okadaic acid (1 μM) for 90 min, or with CryIC toxin for 20 min. Data represent the mean ± SEM of three experiments performed in triplicate. The bottom panel is a representative immunoblot of cPLA2 and the results were verified in at least three independent experiments.
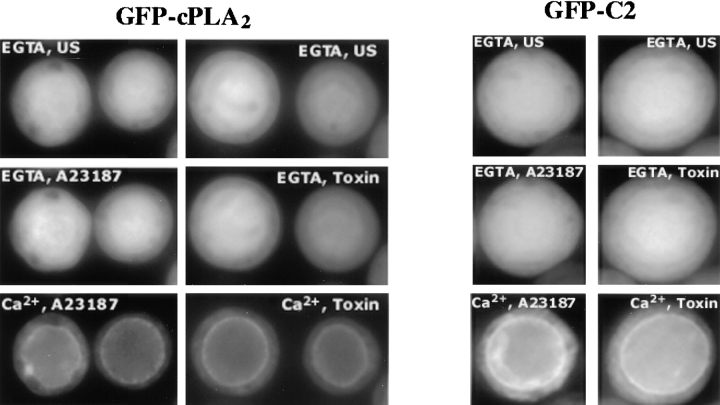
The effect of the C2 domain mutations on translocation of GFP-cPLA2 in response to okadaic acid and the calcium-mobilizing agonists was evaluated. The D43N or D43N/D93N mutant enzymes were unable to translocate to the nuclear envelope in living Sf9 cells treated with A23187, whereas GFP-cPLA2 containing the D93N mutation was fully functional and translocated in response to the calcium ionophore (Fig. 6). Translocation was evident in at least 85% of the cells observed in each experiment. Similar results were also obtained with CryIC toxin (not shown). In contrast, the D93N mutant did not translocate to the nuclear envelope in response to okadaic acid (Fig. 6). The D43N and D43N/D93N constructs were also unable to translocate in response to okadaic acid (not shown).
Figure 6.
Effect of D43N and D93N mutations on A23187- and okadaic acid–induced translocation of GFP-cPLA2. Sf9 cells expressing the indicated mutant constructs of GFP-cPLA2 were treated with A23187 or okadaic acid, and the intracellular distribution of GFP-cPLA2 was visualized by digital confocal microscopy.
Role of Extracellular Calcium in Regulating Arachidonic Acid Release by Wild-Type and C2 Mutants of cPLA2
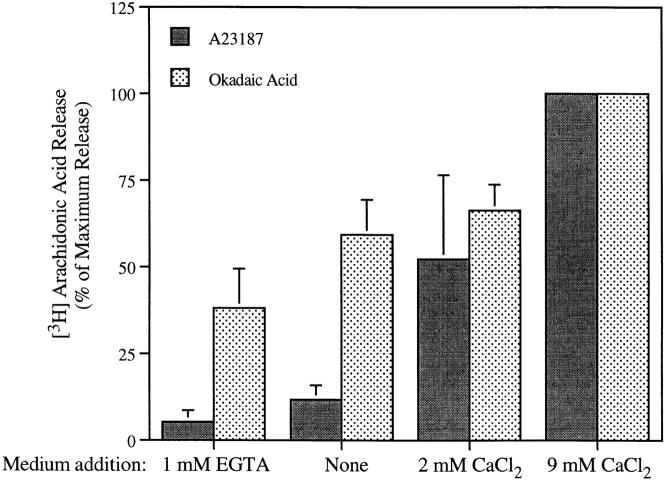
The implication of the above results is that arachidonic acid release induced by okadaic acid in Sf9 cells would show less dependency on extracellular calcium than the response to A23187 or CryIC toxin, but would be dependent on resting levels of intracellular calcium. As shown in Fig. 7, depleting extracellular calcium dramatically reduced A23187-induced arachidonic acid release from Sf9 cells expressing wild-type cPLA2 but had less effect on cells treated with okadaic acid. The significant effect of depleting extracellular calcium on okadaic acid–induced arachidonic acid release may be due to the observed decrease in basal levels of intracellular calcium that occurs when extracellular calcium levels are decreased. In normal Sf9 medium containing 9 mM calcium the intracellular calcium concentration was 68 ± 16 nM (mean ± SEM, n = 4), and the level decreased to 17 ± 8 nM when no calcium was added to the medium. With EGTA in the medium, intracellular calcium was below the level of detection (≤5 nM).
Figure 7.
Role of extracellular calcium in regulating A23187- and okadaic acid–induced arachidonic acid release. Sf9 cells expressing cPLA2 were labeled with [3H]arachidonic acid and then rinsed in calcium-free TNM-FH medium containing 0.1% HSA. The cells were incubated in TNM-FH medium containing 0.1% HSA with the indicated concentrations of EGTA or calcium chloride and then treated with vehicle, A23187 (2 μg/ml), or okadaic acid (1 μM) for 90 min. The data (mean ± SEM of four experiments in triplicate) are expressed as a percentage of the maximum [3H]arachidonic acid release, which was 19.5 ± 8.2% for A23187-treated cells and 15.0 ± 2.9% for okadaic acid–treated cells. The measured percent release from unstimulated cells was 3.0%.
The agonist-induced level of intracellular calcium can be manipulated by incubating Sf9 cells in medium containing varying levels of extracellular calcium. The effect of extracellular calcium concentration on the ability of CryIC toxin to induce arachidonic acid release in Sf9 cells expressing the C2 mutant constructs of cPLA2 was evaluated and correlated with the intracellular levels of calcium (Fig. 8 A). Arachidonic acid release induced by CryIC toxin was dependent on extracellular calcium and decreased to near baseline levels when it was chelated with EGTA. Sf9 cells expressing cPLA2 containing the D43N or D43N/ D93N mutations exhibited little arachidonic acid release above baseline levels in response to CryIC toxin. The ability of cPLA2 containing the D93N mutation to mediate arachidonic acid release when extracellular calcium was decreased from 9 to 2 mM paralleled the response observed with wild-type cPLA2, both exhibiting an ∼30–35% decrease in responsiveness. In contrast, when no calcium was added to the medium, the D93N mutant enzyme was unable to mediate arachidonic acid release, whereas there was still a significant response in cells expressing wild-type cPLA2. These results demonstrate that the D43N and D43N/D93N mutations dramatically reduce the ability of the enzyme to mediate arachidonic acid release even at the highest levels of intracellular calcium achieved (630 nM) with CryIC toxin–treated Sf9 cells in medium containing 9 mM calcium. However, cPLA2 containing the D93N mutation is functional at concentrations of intracellular calcium in the 0.2–0.6 μM range but not at 36 nM intracellular calcium.
Figure 8.
Differential sensitivity of D43N and D93N C2 domain mutant cPLA2 enzymes to calcium. (A) Correlation of CryIC toxin–induced arachidonic acid release from Sf9 cells by C2 domain mutants of cPLA2 and intracellular calcium levels. [3H]Arachidonic acid–labeled Sf9 cells expressing wild-type and C2 domain mutants of cPLA2 were incubated in TNM-FH medium containing 0.1% HSA with the indicated concentrations of EGTA or calcium chloride, and then treated with vehicle or 2 μg/ml CryIC toxin for 20 min. The amount of [3H]arachidonic acid released into the medium is expressed as a percentage of the total incorporated (cell-associated plus medium). Results shown are average ± SD of a representative experiment and were verified in at least three independent experiments. The levels of intracellular calcium in Sf9 cells treated with CryIC toxin are shown below the abscissa. Fura-2 AM–loaded Sf9 cells expressing cPLA2 were incubated in medium containing the indicated concentrations of EGTA or calcium and then treated with 2 μg/ml CryIC toxin for 5 min. Data shown are the mean ± SEM of three independent experiments. (B) Effect of calcium concentration on the enzymatic activity of wild-type and C2 domain mutants of cPLA2. Cytosols were prepared from Sf9 cells expressing wild-type (□), D43N (⋄), D93N (○), or D43N/D93N (▵) cPLA2 enzymes and assayed for PLA2 activity at various calcium concentrations. Results shown are average ± SD of a representative experiment and were verified in three independent experiments in triplicate. Background radioactivity (typically <1,000 dpm) was determined by incubating the substrates in the absence of enzyme, and was subtracted from the data.
Calcium Differentially Affects Enzymatic Activity of C2 Domain Mutants of cPLA2
The results of expressing the C2 domain mutants of cPLA2 in Sf9 cells suggested that mutating D43 and D93 differentially affected the affinity of the enzyme for calcium. This was verified in vitro by measuring the effect of calcium concentration on cPLA2 activity of the mutant enzymes in the cytosolic fraction of Sf9 cells in which equal expression of cPLA2 was confirmed by densitometry (Fig. 8 B). Wild-type cPLA2 exhibited significant activity at nanomolar calcium that continued to increase substantially up to 1 μM calcium with little additional increase in the millimolar range. Consistent with the results of expressing the C2 mutants in Sf9 cells, mutating D43 had a more profound effect on the calcium sensitivity of cPLA2 than mutating D93. cPLA2 containing the D43N or D43N/D93N mutations exhibited very little activity at concentrations of calcium ≤1 μM but exhibited significant activity at millimolar calcium. Unlike wild-type cPLA2, the D93N mutant did not exhibit an increase in activity at 50 nM calcium, but did have significant activity in the 0.25–1 μM range. At 1 mM calcium the wild-type and D93N mutant enzymes exhibited similar activities. As previously reported, cPLA2 exhibits lysophospholipase activity, which is calcium independent (Leslie, 1991; de Carvalho et al., 1995). The lysophospholipase activity of cPLA2 containing the mutations in the C2 domain was not significantly different from wild-type cPLA2 (data not shown).
Translocation of the C2 Domain of cPLA2 to the Nuclear Envelope
Experiments were undertaken to determine if the C2 domain of cPLA2 played a role in the preferential targeting of cPLA2 to the nuclear envelope. GFP was linked to the amino terminus of the C2 domain and expressed in Sf9 cells. GFP-C2 was uniformly distributed throughout unstimulated cells as observed for GFP-cPLA2 (Fig. 9). A23187 induced translocation of the C2 domain to the nuclear envelope in Sf9 cells with a similar time course as the full-length enzyme. The same results were observed in response to the CryIC toxin (not shown). In contrast to the calcium-mobilizing agonists, okadaic acid did not induce translocation of GFP-C2. Translocation of GFP-C2 to the nuclear envelope in response to A23187 and CryIC toxin required extracellular calcium as observed for full-length cPLA2 (Fig. 10). The calcium-mobilizing agonists did not induce translocation of GFP-C2 in Sf9 cells incubated in medium containing EGTA. However, the subsequent addition of calcium to the medium resulted in translocation of GFP-C2 to the nuclear envelope.
Figure 9.

GFP-C2 translocates to the nuclear envelope in Sf9 cells treated with A23187 but not okadaic acid. Sf9 cells expressing a GFP-C2 domain fusion protein were incubated with 2 μg/ml A23187 (left) or 1 μM okadaic acid (right) for the times indicated (in minutes). Images were obtained with a digital confocal microscopy system.
Figure 10.
Extracellular calcium is required for translocation of GFP-C2 and GFP-cPLA2 in Sf9 cells. Sf9 cells expressing GFP-cPLA2 (left series) or GFP-C2 (right series) were treated with 2 μg/ml A23187 or 2 μg/ml CryIC toxin in TNM-FH medium containing 10 mM EGTA. After a 15-min incubation, calcium chloride was added (final concentration of 9 mM), and cells were incubated for an additional 10 min. Images were obtained with a digital confocal microscopy system.
Discussion
Sf9 Cells as a Model for cPLA2 Structure–Function Studies
The baculovirus expression system in Sf9 insect cells is a useful model to study the regulation of cPLA2. This system has been used extensively to study the function of expressed proteins and their roles in signal transduction (Mancini and Evans, 1993; Stancato et al., 1993, 1997; Chefalo et al., 1994). The effect of cPLA2 mutations on arachidonic acid release and translocation can be directly compared in intact, living cells. Importantly, the stimulated arachidonic acid release is due to cPLA2 since there is little contribution of endogenous PLA2 enzymes. The responses of Sf9 cells expressing cPLA2, such as arachidonic acid release and phosphorylation, are similar to responses that we have observed in murine macrophages and human monocytes (Qiu et al., 1993; de Carvalho et al., 1996; Qiu et al., 1998). In addition, GFP-cPLA2 is targeted to the nuclear envelope in response to the calcium-mobilizing agonists and okadaic acid. Using the GFP fusion protein allows translocation to be followed in real time in living cells, and the GFP moiety attached to the amino terminus of cPLA2 does not appear to influence the function of the enzyme. It should be noted that the mechanisms involved in the regulation of cPLA2 such as the importance of phosphorylation, calcium, and even the site of subcellular localization, appear cell type– and agonist-dependent. Sf9 cells are ovarian cells and may lack regulatory components present in certain mammalian cells. However, there are also considerable differences in the regulation of cPLA2 activation among different mammalian cell types. For example, phorbol esters or calcium ionophores alone are poor inducers of cPLA2 activation (and arachidonic acid release) in many cell types, but can act synergistically. However, in macrophages and certain hemopoietic cells these are potent agonists. Similar to macrophages, Sf9 cells expressing cPLA2 respond to A23187 and okadaic acid but, unlike macrophages, not to PMA.
In unstimulated Sf9 cells, the GFP-cPLA2 fusion protein was uniformly distributed both inside the nucleus and in the cytosol. This distribution was not influenced by the level of GFP-cPLA2 expression, which can be varied by decreasing the multiplicity of infection and by decreasing the infection time. In several unstimulated mammalian cells, cPLA2 has been observed inside the nucleus, in the cytosol, and in both locations depending on the cell type and growth conditions (Glover et al., 1995; Schievella et al., 1995; Peters-Golden et al., 1996; Sierra-Honigmann et al., 1996; Hirabayashi et al., 1999). In many of these cell models cPLA2 translocates to the nuclear envelope and endoplasmic reticulum in response to cell stimulation, although exceptions have been observed. In confluent endothelial cells treated with histamine, cPLA2 translocates to both the nuclear envelope and to the plasma membrane at intercellular junctions (Sierra-Honigmann et al., 1996). A punctate clustering of cPLA2 has been observed in the cytoplasm of mouse 3T3 fibroblasts overexpressing the epidermal growth factor receptor, and this distribution is not influenced by epidermal growth factor or calcium ionophore (Bunt et al., 1997). A punctate cytoplasmic localization of cPLA2 occurs in U937 cells and appears to be due to association with cytoplasmic lipid bodies (Yu et al., 1998).
The Role of Phosphorylation in cPLA2 Activation in Sf9 Cells
Using a variety of agonists that act on cells by diverse mechanisms, it has become clear that there are alternative regulatory pathways that can lead to activation of cPLA2 and arachidonic acid release (Leslie, 1997). The ability of okadaic acid to activate cPLA2 and induce arachidonic acid release in macrophages and Sf9 cells without increasing intracellular calcium provides a useful tool to elucidate novel mechanisms involved in cPLA2 regulation. Previous work in macrophages and other cell models has demonstrated that phosphorylation on S505 is not sufficient for activation of cPLA2 and arachidonic acid release in the absence of an increase in intracellular calcium (Lin et al., 1992; Qiu et al., 1998). This is also evident in Sf9 cells since a significant portion of cPLA2 is constitutively phosphorylated on S505 when expressed in Sf9 cells, yet there is no arachidonic acid release unless the cells are treated with okadaic acid or agonists that increase intracellular calcium (Abdullah et al., 1995; de Carvalho et al., 1996). This implicates an alternative mechanism in the regulation of cPLA2 in response to okadaic acid. One possibility was a role for S727 phosphorylation which is preferentially induced in okadaic acid–treated Sf9 cells (de Carvalho et al., 1996). S505, S437, and S454 are constitutively phosphorylated on cPLA2 in unstimulated Sf9 cells, and their phosphorylation state is increased only modestly in response to agonist treatment. These four residues represent the only major phosphorylation sites on cPLA2 in okadaic acid– treated Sf9 cells. Whether phosphorylation of these novel sites, particularly S727, plays a functional role in cPLA2 activation has been an important question. The mutagenesis data demonstrate that okadaic acid–induced phosphorylation of cPLA2 on S727, S437, or S454 plays no functional role in cPLA2-mediated arachidonic acid release in this model. Whether phosphorylation of S727 plays a functional role in cPLA2 regulation in other cells where it has been identified remains to be established (Börsch-Haubold et al., 1998). Phosphorylation of S505 does play a role in augmenting arachidonic acid release by both okadaic acid and the calcium-mobilizing agonists. However, it is not absolutely essential since Sf9 cells expressing cPLA2 containing the S505A mutation still release arachidonic acid to ∼50% of the level of Sf9 cells expressing wild-type cPLA2. The role played by S505 phosphorylation in the regulation of cPLA2 appears to be cell type– and agonist-dependent. In macrophages, S505 phosphorylation is not essential for arachidonic acid release in response to agonists that induce a sustained increase in intracellular calcium but does appear to be required when there is only a transient increase in calcium (Qiu et al., 1998). In CHO cells overexpressing cPLA2, S505 phosphorylation is essential for agonist-induced arachidonic acid release (Schievella et al., 1995). However, S505 phosphorylation of cPLA2 is not required for arachidonic acid release in thrombin-stimulated platelets (Kramer et al., 1996).
The Role of the C2 Domain in cPLA2 Regulation
Our translocation results show a steady accumulation of GFP-cPLA2 in the perinuclear region of Sf9 cells after addition of A23187 or CryIC toxin. This observation is consistent with recent data showing that a sustained increase in the intracellular calcium concentration is required for the stable association of cPLA2-GFP to the nuclear envelope of CHO cells, which correlates with agonist-induced arachidonic acid release (Hirabayashi et al., 1999). Although okadaic acid does not increase intracellular calcium, our results demonstrate a requirement for a functional C2 domain. Mutagenesis of calcium-binding residues D43 and D93 in the C2 domain quantitatively suppress okadaic acid–induced arachidonic acid release and translocation of cPLA2 to the nuclear envelope. In addition, lowering the levels of intracellular calcium by including EGTA in the medium diminished okadaic acid– induced arachidonic acid release, suggesting a requirement for the resting levels of calcium. Attempts to completely chelate intracellular calcium by also including BAPTA or Quin-2 were unsuccessful due to adverse effects on Sf9 cells. The differences in the functional responses of Sf9 cells expressing the D43N and D93N mutant enzymes suggest that these mutations differentially affect the affinity of the enzyme for calcium. This is consistent with the structural analysis of the cPLA2 C2 domain, which binds two calcium ions with different affinities (Nalefski et al., 1997; Perisic et al., 1998; Xu et al., 1998; Bittova et al., 1999). D43 forms one interaction with each of the two calcium ions, whereas both side chain oxygens of D93 participate in binding only one of the calcium ions at site II. It has been shown recently that purified cPLA2 containing D43N and D93N mutations exhibits greatly diminished PLA2 activity and binding to phospholipids as compared with the wild-type enzyme (Bittova et al., 1999). The calcium requirements are higher for cPLA2 containing the D43N mutation than D93N, consistent with the functional differences we observe in the Sf9 model. Differential roles for calcium binding residues in the C2 domain of PKCα have also been reported (Medkova and Cho, 1998). D246 plays the most critical role for PKCα activation and calcium-dependent binding to lipid vesicles in vitro, and is thought to be due to its ability to coordinate two Ca2+ ions tightly. Our results demonstrate a pivotal role for D43 in mediating cPLA2 translocation in intact cells. cPLA2 containing the D43N mutation is unable to translocate to the nuclear envelope or to mediate arachidonic acid release in response to okadaic acid, which acts at resting levels of calcium (60–70 nM), but also in response to calcium-mobilizing agonists A23187 and CryIC toxin, which increase intracellular calcium to ∼0.6 μM calcium. In contrast, cPLA2 containing the D93N mutation translocates and releases arachidonic acid at intracellular calcium in the 0.2–0.6 μM range but not at resting calcium. This difference in affinity of the mutant cPLA2 enzymes is also evident when assayed in vitro. However, the mutations do not adversely affect the conformation of the active site since they all have significant enzymatic activity when assayed in vitro at millimolar calcium, and they exhibit similar lysophospholipase activity as the wild-type enzyme.
The Role of the C2 Domain in Nuclear Localization of cPLA2
The mechanisms involved in the preferential targeting of cPLA2 to the nuclear envelope are not known. However, an important observation from this study is that the C2 domain of cPLA2 translocates to the nuclear envelope in response to the calcium-mobilizing agonists. This suggests that the sequence determinants for this preferential localization reside in the C2 domain. The C2 domain of cPLA2 has been shown to preferentially bind to phosphatidylcholine headgroups (Nalefski et al., 1998). However, this would not be expected to be responsible for nuclear targeting since phosphatidylcholine is found in all cellular membranes. The C2 domain of Nedd4 also exhibits preferential binding to phosphatidylcholine but has been shown recently to translocate to the plasma membrane in cells treated with calcium ionophore (Plant et al., 1997). Although the C2 domains of Nedd4 and cPLA2 have similar properties including the same topology (topology II), similar affinities for calcium, and preference for binding amphipathic phospholipid, they exhibit considerable sequence diversity that may be responsible for their differences in subcellular localization. The C2 domain of PKCα, which binds phosphatidylserine, has been shown recently to bind to the plasma membrane when expressed in COS cells (Corbalán-García et al., 1999). The membrane binding of the PKCα C2 domain occurs at resting levels of calcium, consistent with its higher affinity for calcium for binding to phospholipid vesicles as compared with the cPLA2 or the Nedd4 C2 domains (Nalefski et al., 1997; Hixon et al., 1998; Corbalán-García et al., 1999).
Results of several recent in vitro studies have begun to shed light on the mechanisms involved in interaction of the C2 domain and full-length cPLA2 with phospholipid vesicles. Experiments have shown that the three calcium-binding loops are involved in the binding of the isolated C2 domain to phosphatidylcholine vesicles (Nalefski and Falke, 1998). These results are consistent with the observation that the two Ca2+ ions bound to the C2 domain become occluded, and dissociate more slowly upon binding of the C2 domain to phospholipid vesicles (Nalefski et al., 1997). Regions of the cPLA2 C2 domain that interact with phospholipid micelles have also been identified by 15N-HSQC spectroscopy revealing a role for the calcium-binding loops, proximal ends of attached β strands, and portions of β strands 2 and 3 (Xu et al., 1998). It has been shown recently that calcium induces not only binding but penetration of cPLA2 and the C2 domain into artificial lipid membranes (Davletov et al., 1998; Lichtenbergova et al., 1998). An important role for hydrophobic residues in the interaction of the cPLA2 C2 domain with phospholipid has been implicated from several studies (Davletov et al., 1998; Lichtenbergova et al., 1998; Nalefski and Falke, 1998; Nalefski et al., 1998; Bittova et al., 1999). The residues F35, L39, Y96, and V97 in the calcium binding loops 1 and 3 appear to be particularly important for phospholipid binding and membrane penetration of the C2 domain of cPLA2 (Nalefski and Falke, 1998; Bittova et al., 1999). Two tryptophan residues in the calcium-binding region 3 of PKCα C2 domain also have been shown recently to be essential for binding phospholipid vesicles and membrane penetration in vitro (Medkova and Cho, 1998). Mutagenesis studies of the C2 domain of PKCβII have shown that the calcium-induced interaction of PKC with membrane is not due to charge neutralization of the acidic residues (Edwards and Newton, 1997). However, unlike cPLA2 and PKC in which hydrophobic interaction with phospholipid plays a predominant role, the binding of the C2A domain of synaptotagmin I to phospholipid is largely electrostatic (Davletov et al., 1998; Zhang et al., 1998).
Contribution of Regions Other Than the C2 Domain of cPLA2 in Membrane Binding
Recent studies have demonstrated that the binding properties of the isolated C2 domain of cPLA2 do not necessarily predict the binding properties of the full-length enzyme (Hixon et al., 1998). Binding of the C2 domain to anionic and zwitterionic phospholipid vesicles occurs with similar affinities and requires calcium. However, full-length cPLA2 binds much tighter to anionic (phosphatidylmethanol) than zwitterionic (phosphatidylcholine) vesicles and, surprisingly, the binding to anionic vesicles does not require calcium. Consequently, this implicates regions downstream of the C2 domain in interacting with membranes containing anionic phospholipids. There is evidence that anionic phospholipids, particularly phosphatidylinositol-4,5-bisphosphate (PIP2), enhance enzymatic activity and promote high affinity binding of cPLA2 to phospholipid vesicles (Leslie and Channon, 1990; Buckland and Wilton, 1997; Mosior et al., 1998). Interestingly, PIP2 enhances both calcium-dependent and -independent binding and activity of cPLA2 and may represent a physiological equivalent of phosphatidylmethanol (Hixon et al., 1998; Mosior et al., 1998).
Our results demonstrate that the C2 domain of cPLA2 can translocate to the nuclear envelope in response to calcium-mobilizing agonists but not in response to okadaic acid. This provides evidence in the intact cell that another region of cPLA2 may also be involved in membrane targeting induced by agonists that do not increase intracellular calcium. However, mutating calcium-binding residues in the C2 domain of cPLA2 and depletion of intracellular calcium suppress okadaic acid–induced arachidonic acid release indicating a requirement for the C2 domain and resting levels of calcium for translocation. These data suggest that cPLA2 translocation to membrane induced by okadaic acid may be coordinately regulated by the C2 domain and another region of the enzyme. The cooperative regulation of membrane association by two domains has been observed in numerous proteins including cytohesin-1, Tiam1, Ras-GRF, and phospholipase C, to name a few (Buchsbaum et al., 1996; Williams and Katan, 1996; Stam et al., 1997; Nagel et al., 1998). Membrane binding of phospholipase Cδ1 has been studied extensively, and appears to involve a cooperative role for the PH, C2, and catalytic domains (Williams and Katan, 1996).
Our results demonstrate that the effects of the C2 domain mutations on the ability of cPLA2 to mediate arachidonic acid release correlate directly with their ability to translocate to the nuclear envelope. In addition, a differential functional role is shown for specific calcium-binding residues in the C2 domain in cPLA2 regulation in intact cells. A functional C2 domain is required for agonist-induced translocation of cPLA2 and arachidonic acid release in the Sf9 model even with okadaic acid, which activates cPLA2 without increasing intracellular calcium. This suggests that binding of the C2 domain to calcium, even at resting levels, may be required for stable association of the enzyme with the membrane. Interestingly, okadaic acid induces translocation of the full-length cPLA2, but not the C2 domain. Our results indicate that the C2 domain is necessary and sufficient for cPLA2 translocation to the nuclear envelope in response to calcium-mobilizing agonists. However, a functional C2 domain is necessary but not sufficient for translocation in response to okadaic acid, suggesting that additional regions of the enzyme are also involved.
Acknowledgments
This work was supported by National Institutes of Health grants HL34303 and HL61378.
Abbreviations used in this paper
- cPLA2
cytosolic phospholipase A2
- GFP
green fluorescent protein
- HSA
human serum albumin
- PKC
protein kinase C
- Sf9
Spodoptera frugiperda
References
- Abdullah K, Cromlish WA, Payette P, Laliberté F, Huang Z, Street I, Kennedy BP. Human cytosolic phospholipase A2expressed in insect cells is extensively phosphorylated on Ser-505. Biochem Biophys Acta. 1995;1244:157–164. doi: 10.1016/0304-4165(94)00218-m. [DOI] [PubMed] [Google Scholar]
- Bittova L, Sumandea M, Cho W. A structure-function study of the C2 domain of cytosolic phospholipase A2 . J Biol Chem. 1999;274:9665–9672. doi: 10.1074/jbc.274.14.9665. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, Sapirstein A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2 . Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- Börsch-Haubold AG, Bartoli F, Asselin J, Dudler T, Kramer RM, Apitz-Castro R, Watson SP, Gelb MH. Identification of the phosphorylation sites of cytosolic phospholipase A2in agonist-stimulated human platelets and HeLa cells. J Biol Chem. 1998;273:4449–4458. doi: 10.1074/jbc.273.8.4449. [DOI] [PubMed] [Google Scholar]
- Buchsbaum R, Telliez J-B, Goonesekera S, Feig LA. The N-terminal pleckstrin, coiled-coil, and IQ domains of the exchange factor Ras-GRF act cooperatively to facilitate activation by calcium. Mol Cell Biol. 1996;16:4888–4896. doi: 10.1128/mcb.16.9.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland AG, Wilton DC. The effect of phosphatidylinositols on cytosolic phospholipase A2activity using a continuous fluorescent displacement assay. Biochem Soc Trans. 1997;25:S599. doi: 10.1042/bst025s599. [DOI] [PubMed] [Google Scholar]
- Bunt G, de Wit J, van den Bosch H, Verkleij AJ, Boonstra J. Ultrastructural localization of cPLA2in unstimulated and EGF/A23187-stimulated fibroblasts. J Cell Sci. 1997;110:2449–2459. doi: 10.1242/jcs.110.19.2449. [DOI] [PubMed] [Google Scholar]
- Channon J, Leslie CC. A calcium-dependent mechanism for associating a soluble arachidonoyl-hydrolyzing phospholipase A2with membrane in the macrophage cell line, RAW 264.7. J Biol Chem. 1990;265:5409–5413. [PubMed] [Google Scholar]
- Chefalo PJ, Yang JM, Ramaiah KVA, Gehrke L, Chen J-J. Inhibition of protein synthesis in insect cells by baculovirus-expressed heme-regulated eIF-2α kinase. J Biol Chem. 1994;269:25788–25794. [PubMed] [Google Scholar]
- Clark JD, Milona N, Knopf JL. Purification of a 110-kilodalton cytosolic phospholipase A2from the human monocytic cell line U937. Proc Natl Acad Sci USA. 1990;87:7708–7712. doi: 10.1073/pnas.87.19.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JD, Lin L-L, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, Milona N, Knopf JL. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell. 1991;65:1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Clark JD, Schievella AR, Nalefski EA, Lin L-L. Cytosolic phospholipase A2 . J Lipid Mediat Cell Signal. 1995;12:83–117. doi: 10.1016/0929-7855(95)00012-f. [DOI] [PubMed] [Google Scholar]
- Corbalán-García S, Rodríguez-Alfaro JA, Gómez-Fernández JC. Determination of the calcium-binding sites of the C2 domain of protein kinase Cα that are critical for its translocation to the plasma membrane. Biochem J. 1999;337:513–521. [PMC free article] [PubMed] [Google Scholar]
- Davletov B, Perisic O, Williams RL. Calcium-dependent membrane penetration is a hallmark of the C2 domain of cytosolic phospholipase A2whereas the C2A domain of synaptotagmin binds membranes electrostatically. J Biol Chem. 1998;273:19093–19096. doi: 10.1074/jbc.273.30.19093. [DOI] [PubMed] [Google Scholar]
- de Carvalho MGS, Garritano J, Leslie CC. Regulation of lysophospholipase activity of the 85-kD phospholipase A2and activation in mouse peritoneal macrophages. J Biol Chem. 1995;270:20439–20446. doi: 10.1074/jbc.270.35.20439. [DOI] [PubMed] [Google Scholar]
- de Carvalho MGS, McCormack AL, Olson E, Ghomashchi F, Gelb MH, Yates JR, III, Leslie CC. Identification of phosphorylation sites of human 85-kD cytosolic phospholipase A2expressed in insect cells and present in human monocytes. J Biol Chem. 1996;271:6987–6997. doi: 10.1074/jbc.271.12.6987. [DOI] [PubMed] [Google Scholar]
- de Carvalho MS, McCormack FX, Leslie CC. The 85-kD, arachidonic acid-specific phospholipase A2is expressed as an activated phosphoprotein in Sf9 cells. Arch Biochem Biophys. 1993;306:534–540. doi: 10.1006/abbi.1993.1549. [DOI] [PubMed] [Google Scholar]
- Edwards AS, Newton AC. Regulation of protein kinase C βII by its C2 domain. Biochemistry. 1997;36:15615–15623. doi: 10.1021/bi9718752. [DOI] [PubMed] [Google Scholar]
- Glover S, de Carvalho MS, Bayburt T, Jonas M, Chi E, Leslie CC, Gelb MH. Translocation of the 85-kD phospholipase A2from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J Biol Chem. 1995;270:15359–15367. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- Hirabayashi T, Kume K, Hirose K, Yokomizo T, Iino M, Itoh H, Shimizu T. Critical duration of intracellular Ca2+ response required for continuous translocation and activation of cytosolic phospholipase A2 . J Biol Chem. 1999;274:5163–5169. doi: 10.1074/jbc.274.8.5163. [DOI] [PubMed] [Google Scholar]
- Hixon MS, Ball A, Gelb MH. Calcium-dependent and -independent interfacial binding and catalysis of cytosolic group IV phospholipase A2 . Biochemistry. 1998;37:8516–8526. doi: 10.1021/bi980416d. [DOI] [PubMed] [Google Scholar]
- Kramer RM, Roberts EF, Um SL, Borsch-Haubold AG, Watson SP, Fisher MJ, Jakubowski JA. p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets. J Biol Chem. 1996;271:27723–27729. doi: 10.1074/jbc.271.44.27723. [DOI] [PubMed] [Google Scholar]
- Kwa MSG, de Maagd RA, Stiekema WJ, Vlak JM, Bosch D. Toxicity and binding properties of the Bacillus thuringiensisdelta-endotoxin Cry1C to cultured insect cells. J Invertebr Pathol. 1998;71:121–127. doi: 10.1006/jipa.1997.4723. [DOI] [PubMed] [Google Scholar]
- Leslie CC. Kinetic properties of a high molecular mass arachidonoyl-hydrolyzing phospholipase A2that exhibits lysophospholipase activity. J Biol Chem. 1991;266:11366–11371. [PubMed] [Google Scholar]
- Leslie CC. Properties and regulation of cytosolic phospholipase A2 . J Biol Chem. 1997;272:16709–16712. doi: 10.1074/jbc.272.27.16709. [DOI] [PubMed] [Google Scholar]
- Leslie CC, Channon JY. Anionic phospholipids stimulate an arachidonoyl-hydrolyzing phospholipase A2from macrophages and reduce the calcium requirement for activity. Biochim Biophys Acta. 1990;1045:261–270. doi: 10.1016/0005-2760(90)90129-l. [DOI] [PubMed] [Google Scholar]
- Lichtenbergova L, Yoon ET, Cho W. Membrane penetration of cytosolic phospholipase A2is necessary for its interfacial catalysis and arachidonate specificity. Biochemistry. 1998;37:14128–14136. doi: 10.1021/bi980888s. [DOI] [PubMed] [Google Scholar]
- Lin L-L, Lin AY, Knopf JL. Cytosolic phospholipase A2is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci USA. 1992;89:6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L-L, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Mancini JA, Evans JF. Coupling of recombinant 5-lipoxygenase and leukotriene A4 hydrolase activities and transcellular metabolism of leukotriene A4in Sf9 insect cells. Eur J Biochem. 1993;218:477–484. doi: 10.1111/j.1432-1033.1993.tb18399.x. [DOI] [PubMed] [Google Scholar]
- Medkova M, Cho W. Mutagenesis of the C2 domain of protein kinase C-α. J Biol Chem. 1998;273:17544–17552. doi: 10.1074/jbc.273.28.17544. [DOI] [PubMed] [Google Scholar]
- Monette R, Potvin L, Baines D, Laprade R, Schwartz JL. Interaction between calcium ions and Bacillus thuringiensis toxin activity against Sf9 cells (Spodoptera frugiperda, Lepidoptera) . Appl Environ Microbiol. 1997;63:440–447. doi: 10.1128/aem.63.2.440-447.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosior M, Six DA, Dennis EA. Group IV cytosolic phospholipase A2binds with high affinity and specificity to phosphatidylinositol 4,5-bisphosphate resulting in dramatic increases in activity. J Biol Chem. 1998;273:2184–2191. doi: 10.1074/jbc.273.4.2184. [DOI] [PubMed] [Google Scholar]
- Nagel W, Schilcher P, Zeitlmann L, Kolanus W. The PH domain and the polybasic c domain of cytohesin-1 cooperate specifically in plasma membrane association and cellular function. Mol Biol Cell. 1998;9:1981–1994. doi: 10.1091/mbc.9.8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalefski EA, Falke JJ. The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci. 1996;5:2375–2390. doi: 10.1002/pro.5560051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalefski EA, Falke JJ. Location of the membrane-docking face on the Ca2+-activated C2 domain of cytosolic phospholipase A2 . Biochemistry. 1998;37:17642–17650. doi: 10.1021/bi982372e. [DOI] [PubMed] [Google Scholar]
- Nalefski EA, Sultzman LA, Martin DM, Kriz RW, Towler PS, Knopf JL, Clark JD. Delineation of two functionally distinct domains of cytosolic phospholipase A2, a regulatory Ca2+-dependent lipid-binding domain and a Ca2+-independent catalytic domain. J Biol Chem. 1994;269:18239–18249. [PubMed] [Google Scholar]
- Nalefski EA, Slazas MM, Falke JJ. Ca2+-signaling cycle of a membrane-docking C2 domain. Biochemistry. 1997;36:12011–12018. doi: 10.1021/bi9717340. [DOI] [PubMed] [Google Scholar]
- Nalefski EA, McDonagh T, Somers W, Seehra J, Falke JJ, Clark JD. Independent folding and ligand specificity of the C2 calcium-dependent lipid binding domain of cytosolic phospholipase A2 . J Biol Chem. 1998;273:1365–1372. doi: 10.1074/jbc.273.3.1365. [DOI] [PubMed] [Google Scholar]
- Perisic O, Fong S, Lynch DE, Bycroft M, Williams RL. Crystal structure of a calcium-phospholipid binding domain from cytosolic phospholipase A2 . J Biol Chem. 1998;273:1596–1604. doi: 10.1074/jbc.273.3.1596. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M, Song K, Marshall T, Brock T. Translocation of cytosolic phospholipase A2to the nuclear envelope elicits topographically localized phospholipid hydrolysis. Biochem J. 1996;318:797–803. doi: 10.1042/bj3180797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant PJ, Yeger H, Staub O, Howard P, Rotin D. The C2 domain of the ubiquitin protein ligase Nedd4 mediates Ca2+-dependent plasma membrane localization. J Biol Chem. 1997;272:32329–32336. doi: 10.1074/jbc.272.51.32329. [DOI] [PubMed] [Google Scholar]
- Qiu Z-H, de Carvalho MS, Leslie CC. Regulation of phospholipase A2activation by phosphorylation in mouse peritoneal macrophages. J Biol Chem. 1993;268:24506–24513. [PubMed] [Google Scholar]
- Qiu Z-H, Gijón MA, de Carvalho MS, Spencer DM, Leslie CC. The role of calcium and phosphorylation of cytosolic phospholipase A2in regulating arachidonic acid release in macrophages. J Biol Chem. 1998;273:8203–8211. doi: 10.1074/jbc.273.14.8203. [DOI] [PubMed] [Google Scholar]
- Rizo J, Südhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J Biol Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- Schievella AR, Regier MK, Smith WL, Lin L-L. Calcium-mediated translocation of cytosolic phospholipase A2to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30749–30754. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- Schwartz J-L, Garneau L, Masson L, Brousseau R. Early response of cultured lepidopteran cells to exposure to δ-endotoxin from Bacillus thuringiensis: involvement of calcium and anionic channels. Biochim Biophys Acta. 1991;1065:250–260. doi: 10.1016/0005-2736(91)90237-3. [DOI] [PubMed] [Google Scholar]
- Sierra-Honigmann MR, Bradley JR, Pober JS. “Cytosolic” phospholipase A2is in the nucleus of subconfluent endothelial cells but confined to the cytoplasm of confluent endothelial cells and redistributes to the nuclear envelope and cell junctions upon histamine stimulation. Lab Invest. 1996;74:684–695. [PubMed] [Google Scholar]
- Stam JC, Sander EE, Michiels F, van Leeuwen FN, Kain HET, van der Kammen RA, Collard JG. Targeting of Tiam1 to the plasma membrane requires the cooperative function of the n-terminal pleckstrin homology domain and an adjacent protein interaction domain. J Biol Chem. 1997;272:28447–28454. doi: 10.1074/jbc.272.45.28447. [DOI] [PubMed] [Google Scholar]
- Stancato LF, Chow Y-H, Hutchison KA, Perdew GH, Jove R, Pratt WB. Raf exists in a native heterocomplex with hsp90 and p50 that can be reconstituted in a cell-free system. J Biol Chem. 1993;268:21711–21716. [PubMed] [Google Scholar]
- Stancato LF, Silverstein AM, Owens-Grillo JK, Chow Y-H, Jove R, Pratt WB. The hsp90-binding antibiotic geldanamycin decreases Raf levels and epidermal growth factor signaling without disrupting formation of signaling complexes or reducing the specific enzymatic activity of Raf kinase. J Biol Chem. 1997;272:4013–4020. doi: 10.1074/jbc.272.7.4013. [DOI] [PubMed] [Google Scholar]
- Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashior F, Komagata Y, Maki K, Ikuta K, Ouchi Y, Miyazaki J-I, Shimizu T. Role of cytosolic phospholipase A2in allergic response and parturition. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- Williams RL, Katan M. Structural views of phosphoinositide-specific phospholipase C: signalling the way ahead. Curr Biol. 1996;4:1387–1394. doi: 10.1016/s0969-2126(96)00146-3. [DOI] [PubMed] [Google Scholar]
- Xu G-Y, McDonagh T, Hsiang-Ai Y, Nalefski EA, Clark JD, Cumming DA. Solution structure and membrane interactions of the C2 domain of cytosolic phospholipase A2 . J Mol Biol. 1998;280:485–500. doi: 10.1006/jmbi.1998.1874. [DOI] [PubMed] [Google Scholar]
- Yu W, Bozza PT, Tzizik DM, Gray JP, Cassara J, Dvorak AM, Weller PF. Co-compartmentalization of MAP kinases and cytosolic phospholipase A2at cytoplasmic arachidonate-rich lipid bodies. Am J Pathol. 1998;152:759–769. [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Rizo J, Südhof TC. Mechanism of phospholipid binding by the C2A-domain of synaptotagmin I. Biochemistry. 1998;37:12395–12403. doi: 10.1021/bi9807512. [DOI] [PubMed] [Google Scholar]