Abstract
The SR proteins, a group of abundant arginine/serine (RS)-rich proteins, are essential pre-mRNA splicing factors that are localized in the nucleus. The RS domain of these proteins serves as a nuclear localization signal. We found that RS domain–bearing proteins do not utilize any of the known nuclear import receptors and identified a novel nuclear import receptor specific for SR proteins. The SR protein import receptor, termed transportin-SR (TRN-SR), binds specifically and directly to the RS domains of ASF/SF2 and SC35 as well as several other SR proteins. The nuclear transport regulator RanGTP abolishes this interaction. Recombinant TRN-SR mediates nuclear import of RS domain– bearing proteins in vitro. TRN-SR has amino acid sequence similarity to several members of the importin β/transportin family. These findings strongly suggest that TRN-SR is a nuclear import receptor for the SR protein family.
Keywords: SR proteins, RS domain, nuclear localization signal, nuclear import receptor, RanGTP
In eukaryotic cells, trafficking of proteins and RNAs between the cytoplasm and the nucleus occurs through large structures in the nuclear envelope, called nuclear pore complexes (NPCs)1 (for reviews see Doye and Hurt, 1997; Nakielny and Dreyfuss, 1997; Izaurralde and Adam, 1998; Ohno et al., 1998; Pemberton et al., 1998; Stutz and Rosbash, 1998). Although small molecules of <60 kD can generally diffuse into the nucleus, most nuclear proteins have nuclear localization signals (NLSs) that mediate their active import into the nucleus. The most extensively characterized NLS is the classical NLS which consists of a short segment of basic amino acids (Kalderon et al., 1984; Robbins et al., 1991). The nuclear import of proteins containing this NLS is mediated by a dimeric soluble factor comprised of importins α and β (also referred to as karyopherins α and β; Görlich et al., 1994, 1995; Chi et al., 1995; Imamoto et al., 1995a,b; Moroianu et al., 1995; Radu et al., 1995). Importin α binds the NLS directly and serves as the adapter to importin β, which interacts with the NLS-importin α complex through the importin β–binding domain (IBB) of importin α (Görlich et al., 1996a; Moroianu et al., 1996a; Weis et al., 1996). The other well characterized NLS is the M9 sequence of heterogeneous nuclear RNP (hnRNP) A1 (Siomi and Dreyfuss, 1995; Weighardt et al., 1995). M9-dependent import is mediated by transportin 1 (TRN1, also referred to as karyopherin β2 or MIP) which is related by sequence to importin β. Unlike importin β, which utilizes importin α as an adapter for NLS binding, TRN1 binds to M9 directly (Nakielny et al., 1996; Pollard et al., 1996; Bonifaci et al., 1997; Fridell et al., 1997). By sequence homology searches or biochemical purification, several importin β/TRN1–related proteins have been identified in several species (Görlich et al., 1997). Some of these have been found to be nuclear import or nuclear export receptors for specific proteins and RNAs (reviewed in Weis, 1998; Wozniak et al., 1998). Importin β homologues are all similar in size (95–125 kD) and show considerable amino acid sequence similarity, particularly in their amino-terminal regions which have been shown to contain a RanGTP-binding domain (Görlich et al., 1997). Ran is a small GTPase that can exist in either a GTP-bound state (RanGTP) or a GDP-bound state (RanGDP) (reviewed by Dahlberg and Lund, 1998; Moore, 1998). One role of RanGTP, considered to be the predominant form of Ran in the nucleus, is to promote dissociation of import receptor-cargo complexes and thus cause cargo release in the nucleus (Rexach and Blobel, 1995; Chi et al., 1996; Görlich et al., 1996b; Moroianu et al., 1996b; Izaurralde et al., 1997; Siomi et al., 1997). RanGTP also plays an important role in nuclear export as it is required for efficient binding of export receptors to their cargoes (Fornerod et al., 1997; Kutay et al., 1997b, 1998; Arts et al., 1998).
In addition to the two NLSs described above, the arginine/serine rich (RS) domain of SR proteins has been shown also to function as an NLS (Li and Bingham, 1991; Hedley et al., 1995; Cáceres et al., 1997). SR proteins are essential splicing factors, characterized by the presence of at least one RNA-binding domain and a domain containing several, often numerous, arginine/serine dipeptide repeats (for review see Fu, 1995). Several SR proteins have been described including SRp20, 30 (ASF/SF2, SC35), 40, 55, and 75 (Fu and Maniatis, 1990, 1992; Ge and Manley, 1990; Krainer et al., 1990, 1991; Ge et al., 1991; Zahler et al., 1992, 1993). In addition to their roles as essential splicing factors, SR proteins can modulate splice site selection and thus also have important regulatory roles in alternative splicing (Ge and Manley, 1990; Krainer et al., 1990, 1991; Ge et al., 1991; Fu et al., 1992; Zahler et al., 1993; Cáceres et al., 1994). SR proteins are generally found throughout the nucleoplasm and are often particularly concentrated in nuclear speckles, or interchromatin granules (Fu and Maniatis, 1990; Fu, 1995; Cáceres et al., 1997; Singer and Green, 1997; Lamond and Earnshaw, 1998; Misteli and Spector, 1998). Several of the SR proteins have also been shown to shuttle between the nucleus and the cytoplasm (Cáceres et al., 1998) and to accompany mRNAs as they are exported through the NPC (Alzhanova-Ericsson et al., 1996).
Although the RS domain has been shown to function as an NLS for SR proteins and to participate in their localization to speckles (Li and Bingham, 1991; Hedley et al., 1995; Cáceres et al., 1997), the nuclear import pathway for SR proteins has not been previously characterized. Here we show that the nuclear import of several of the SR proteins, including ASF/SF2 and SC35, is mediated by a specific import receptor, termed transportin-SR (TRN-SR). TRN-SR is a novel member of the importin β/transportin family and we show that it binds specifically and directly to the RS domains of ASF/SF2, SC35, and to several additional SR proteins. These findings indicate that TRN-SR is the nuclear import receptor for many SR proteins.
Materials and Methods
Construction of Expression Plasmids and Recombinant Protein Preparation
A fragment corresponding to human ASF/SF2 RS domain (amino acids 198–248) was PCR amplified and inserted into BamHI and XhoI sites in either pGEX-5X-1 (Pharmacia Biotech) or pMal-c2 (New England Biolabs). This fragment was also cloned between EcoRI and SalI sites of pLexA (Clontech, Inc.) for the construction of the yeast two-hybrid library screening bait plasmid. The plasmid encoding human SC35 RS domain (amino acids 90–222) was made by ligation of the BamHI-XhoI fragment of SC35 RS domain amplified by PCR into pGEX-5X-1. The full-length of TRN-SR was amplified by PCR with Pfu DNA polymerase (Stratagene) and inserted as an EcoRI-XhoI fragment into pET28A (Novagen). Proteins were overexpressed in the BL21(DE3) Escherichia coli strain and were purified by methods that the manufacturers recommend. Glutathione-S-transferase (GST)-SV-40 T NLS, GST-IBB, and GST-M9 proteins were purified as described (Pollard et al., 1996). His-tagged RanQ69L (GTP form) was purified as described previously (Siomi et al., 1997).
In Vitro Nuclear Import Assays
Nuclear import assays were performed as described (Pollard et al., 1996). Rabbit reticulocyte lysate (Promega) was used as a cytosol source and prepared as described previously (Adam et al., 1990). The transport substrates were added at a concentration of 50 μg/ml. For competition experiments with maltose-binding protein (MBP) fusion proteins, competitors (1 mg/ml) were added to the complete transport mix except transport substrate, incubated on ice for 15 min, and then combined with transport substrate and nuclei. For the import experiments with recombinant receptor protein, recombinant His-tagged TRN-SR and His-tagged RanGDP were added at concentrations of 120 and 40 μg/ml, respectively.
Yeast Two-Hybrid Interaction Screening
The HeLa MATCHMAKER LexA cDNA library, yeast strains, and cloning vectors were obtained from Clontech, Inc. All library screening and yeast manipulations were carried out as recommended by the manufacturer. Saccharomyces cerevisiae strain EGY48 was transformed simultaneously with pLexA-ASF/SF2 RS and the HeLa cell cDNA library. 2 × 106 transformants were plated onto 20 150-mm plates of X-gal–synthetic medium lacking histidine, uracil, tryptophan, and leucine. 32 Leu+ growers that had shown blue color on those plates were isolated. Insert cDNAs were amplified by PCR on these yeast cells using the Advantage-HF™ PCR kit (Clontech, Inc.) and sequenced.
Full-Length TRN-SR Isolation
The PCR fragment from clone 1-1 was used as a hybridization probe to screen the λ phage HeLa cell cDNA library (Clontech, Inc.). Several clones were isolated, and the clone that had the longest insert was sequenced and thus determined as the full-length coding sequence of TRN-SR.
Protein-binding Assays
TRN-SR was produced by in vitro transcription-translation of His-TRN-SR, using a TNT kit (Promega) in rabbit reticulocyte lysate in the presence of [35S]methionine (Amersham) according to the procedure that the manufacturer recommends. Purified recombinant GST and GST fusion proteins (5 μg each) were immobilized on 50 μl of glutathione-Sepharose (Pharmacia) in PBS for at least 1 h at 4°C. The resin was washed with 500 μl of binding buffer (50 mM Tris-HCl, 400 mM NaCl, 5 mM MgOAc, 2 μg/ml of leupeptin, 2 μg/ml of pepstatin, 1% aprotinin, and 0.05% [wt/vol] digitonin; Calbiochem). In vitro translated TRN-SR was added and incubated with these immobilized proteins for 1 h at 4°C. For the experiments to check the effect of exogenous Ran protein, His-tagged RanQ69L (GTP form) was added at a concentration of 2 μM. The resin was washed with 500 μl of binding buffer five times and the bound fraction was eluted by boiling in SDS-PAGE sample buffer. The bound fraction was then analyzed by SDS-PAGE and visualized by fluorography.
The binding experiments with recombinant proteins were done essentially as described above except 20 μg of His- and T7-tagged recombinant TRN-SR was used. Binders were analyzed by 12.5% SDS-PAGE, and detected by an anti-T7 monoclonal antibody (Novagen) and ECL system (Amersham).
Far Western Blotting with SR Proteins
Purified SR proteins were kindly provided by Dr. Akila Mayeda prepared from HeLa cells as described previously (Zahler et al., 1992). 10 μg of proteins was analyzed by SDS-PAGE and transferred to nitrocellulose membrane. Far Western blotting was performed as described previously (Siomi et al., 1997) by using either TRN-SR or TRN1 produced by a TNT kit (Promega) in rabbit reticulocyte lysate in the presence of [35S]methionine (Amersham).
Results
Characterization of SR Protein Nuclear Import
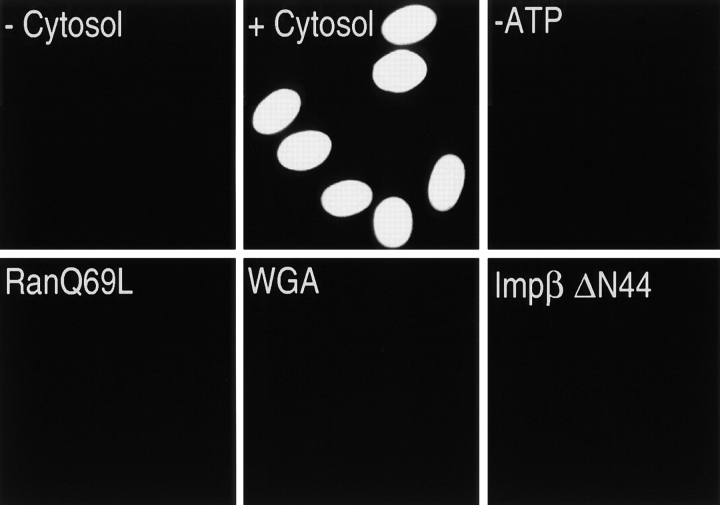
To characterize the import pathway for SR proteins, we carried out in vitro nuclear import assays in digitonin-permeabilized HeLa cells (Adam et al., 1990). As a substrate we used recombinant GST fused to amino acids 198–248 of ASF/SF2 which corresponds to the RS domain (GST-ASF/SF2 RS) of this protein (Ge et al., 1991; Krainer et al., 1991). Efficient nuclear import of GST-ASF/SF2 RS was observed in the presence of cytosol and an ATP-regenerating system (Fig. 1). As no import was detected without addition of cytosol (Fig. 1), this indicates that nuclear import of ASF/SF2 requires additional soluble factor(s). Efficient nuclear import of GST-ASF/SF2 RS was observed in the presence of an ATP-regenerating system and was reduced by incubation with apyrase (Fig. 1), suggesting a role for NTPs in this process. The import of GST-ASF/SF2 RS was strongly inhibited by RanQ69L, a Ran mutant that cannot hydrolyze GTP at a significant rate (Klebe et al., 1995) (Fig. 1), suggesting a role for RanGTP. GST-ASF/ SF2 RS import also has several characteristics of nuclear import that occur through NPCs. Both WGA and an importin β dominant-negative mutant (Impβ ΔN44), reagents which block active nuclear import through NPCs (Forbes, 1992; Görlich et al., 1996b; Kutay et al., 1997a), completely abolished GST-ASF/SF2 RS nuclear import (Fig. 1).
Figure 1.
RS domain–mediated nuclear import in vitro. Digitonin-permeabilized HeLa cells were incubated with the import substrate GST-ASF/SF2 RS along with either buffer (−Cytosol) or rabbit reticulocyte lysate (all other panels). An ATP-regenerating system was included in all assays except the −ATP experiment. In addition to omitting the ATP-regenerating system, the −ATP import assay included apyrase at 2.5 U/μl. Where indicated, RanQ69L (2 μM), WGA (0.2 mg/ml), or an importin β fragment containing amino acids 45–462 (Impβ ΔN44, 1 μM) was added to the import mixture. Import of GST-ASF/SF2 RS was detected by immunofluorescence microscopy using an anti-GST antibody.
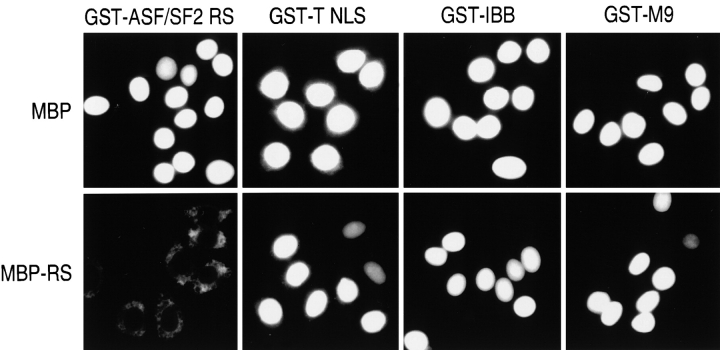
To determine whether a specific and saturable factor(s) participates in RS domain–mediated nuclear import, we tested the effect of excess RS domain on the import of classical NLS, M9, and RS domain–bearing proteins. For these experiments we prepared an MBP fusion of the ASF/SF2 RS domain, termed MBP-RS, as a competitor. Nuclear import assays were carried out in the presence of a 20-fold molar excess of either MBP or MBP-RS. MBP itself had no effect on nuclear import; however, MBP-RS strongly inhibited GST-ASF/SF2 RS import, whereas import of other substrates, GST-SV-40 T NLS, GST-IBB, and GST-M9, was unaffected or only slightly reduced (Fig. 2). These results suggest that a specific nuclear import receptor, distinct from importin β and TRN1, mediates RS domain nuclear import.
Figure 2.
A saturable and specific factor mediates nuclear import of RS domain– containing proteins. Import mixtures were incubated with 1 mg/ml of MBP-RS for 15 min on ice. 50 μg/ml of the import substrates was added to the mixtures, and nuclei were incubated as described in Materials and Methods and detected by immunostaining as described in Fig. 1.
Identification of RS Domain–interacting Proteins
To identify candidate mediator(s) of SR protein nuclear import, we carried out a yeast two-hybrid screening on a HeLa cell cDNA library using the COOH-terminal 51– amino acid region of ASF/SF2 as bait. This fragment contains the RS domain and is sufficient for complete nuclear localization of myc-tagged pyruvate kinase in HeLa cells (data not shown). Several positive interacting clones were isolated and characterized. One of these, clone 1-1, was isolated four times out of 16 clones, and its deduced amino acid sequence showed significant similarity to that of a putative importin β/transportin-related nuclear transport receptors.
The 1-1 DNA insert was subcloned and used for hybridization screening of a λ phage HeLa cDNA library. A 3-kb clone that appears to contain the entire coding region was obtained. The predicted amino acid sequence of this protein, which we termed TRN-SR, because it turned out, like TRN1, to be a transport receptor of pre-mRNA/mRNA-binding proteins, is shown in Fig. 3. TRN-SR is a 975– amino acid protein with a calculated molecular mass of 109,838 D and an estimated pI of 5.29. The amino-terminal domain of TRN-SR shows significant sequence similarity to other importin β/transportin family members, including a region required for RanGTP binding (Görlich et al., 1997). The sequence of the original 1-1 clone isolated from the yeast two-hybrid screening starts at amino acid 590 of the TRN-SR sequence and contains the entire COOH-terminal domain. A BLAST homology search with full-length TRN-SR revealed three proteins that bear significant homology to TRN-SR in other species (Fig. 3). The most similar of these, AF025464 of Caenorhabditis elegans, is 26% identical and 45% similar to TRN-SR. Another apparent homologue is AL022304 of Schizosaccharomyces pombe that is 25% identical and 46% similar, although this clone does not appear to contain the full-length protein sequence. These two sequences are the two closest orthologues of TRN-SR present in available databases. Of previously characterized proteins, the most significant similarity is found with the S. cerevisiae protein Mtr10p (Kadowaki et al., 1994) which has been shown recently to be a nuclear import receptor for Npl3p (Pemberton et al., 1997; Senger et al., 1998). Npl3p is an hnRNP protein in yeast (Bossie et al., 1992; Russell and Tollervey, 1992; Wilson et al., 1994). The amino acid sequences of TRN-SR and Mtr10p are 21% identical and 42% similar.
Figure 3.

Amino acid sequence of TRN-SR and alignment with putative homologues in divergent species. TRN-SR, S. cerevisiae Mtr10p (Mtr10), S. pombe protein AL022304, and the C. elegans protein AF025464 were aligned using the ClustalW program. Identical residues are indicated by dark shading and similar residues are indicated by light shading. The sequence data for TRN-SR are available from GenBank under accession number AF145029.
TRN-SR Binds Specifically to the RS Domain of SR Proteins
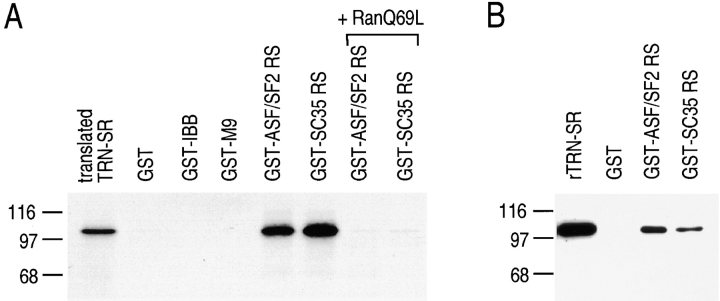
To confirm that TRN-SR binds specifically to SR proteins, we carried out in vitro binding experiments using TRN-SR produced by transcription-translation in rabbit reticulocyte lysate. In the same experiments we also tested another RS domain, that of the SR splicing factor SC35 (amino acids 90–222; Fu and Maniatis, 1992). TRN-SR binds to the RS domains of both ASF/SF2 and SC35, but not to IBB or to hnRNP A1 M9 (Fig. 4 A). RanQ69L abolishes the binding of TRN-SR to RS domains (Fig. 4 A), consistent with the possibility that it is a nuclear import receptor for these proteins. Since rabbit reticulocyte lysate contains many proteins, the binding of TRN-SR detected in Fig. 4 A could be indirect. To examine whether TRN-SR can bind to the RS domains directly, we carried out binding assays using purified recombinant TRN-SR. As shown in Fig. 4 B, bacterially produced TRN-SR binds to both GST-ASF/SF2 RS and GST-SC35 RS directly, but not to GST alone. These results strongly suggest that TRN-SR is a specific import receptor for SR proteins.
Figure 4.
TRN-SR binds to RS domains specifically and directly. (A) Purified GST, GST-M9, GST-IBB, GST-ASF/SF2 RS, and GST-SC35 RS were immobilized on glutathione beads and incubated with in vitro translated 35S-labeled TRN-SR (translated TRN-SR). To the reactions in the lanes marked RanQ69L, 2 μM of His-tagged RanQ69L (GTP form) was added. After binding, beads were washed with buffer containing 400 mM NaCl. Bound proteins were eluted with SDS-containing sample buffer, resolved by SDS-PAGE, and detected by fluorography. An aliquot equivalent to 10% of TRN-SR used for binding was run in the lane marked translation. Molecular mass markers are indicated on the left side of the figure. (B) Bacterially expressed TRN-SR (rTRN-SR) with a T7 tag was incubated with GST alone, GST-ASF/SF2 RS, or GST-SC35 RS. After extensive washing, bound fractions were resolved by SDS-PAGE and detected by Western blotting using an anti-T7 tag antibody. Molecular mass marker positions are shown at the left.
TRN-SR Mediates the Nuclear Import of RS Domain–containing Proteins
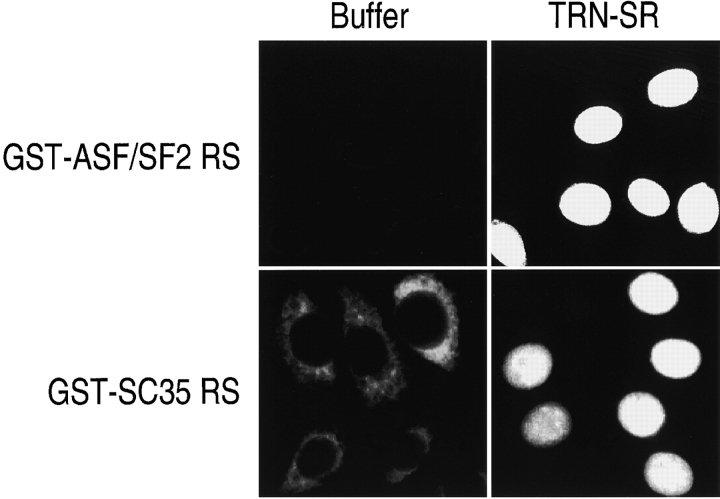
To determine if TRN-SR is the nuclear import receptor of SR proteins, recombinant TRN-SR was used in in vitro nuclear import assays using either GST-ASF/SF2 RS or GST-SC35 RS as a substrate. Neither GST-ASF/SF2 RS nor GST-SC35 RS by itself accumulated in the nucleus (Fig. 5). However, in the presence of ATP, an ATP-regenerating system and RanGDP, TRN-SR efficiently imported GST-ASF/SF2 RS and GST-SC35 RS into the nucleus (Fig. 5). Thus, TRN-SR is a nuclear import receptor for ASF/SF2, SC35, and likely for other RS domain–containing proteins.
Figure 5.
Recombinant TRN-SR can import RS domain–containing proteins in vitro. Import of GST-RS incubated with buffer, TRN-SR, or TRN-SR plus RanGDP was examined on digitonin-permeabilized HeLa cells. Import assays were carried out as detailed in Fig. 1.
TRN-SR Binds to Several SR Proteins
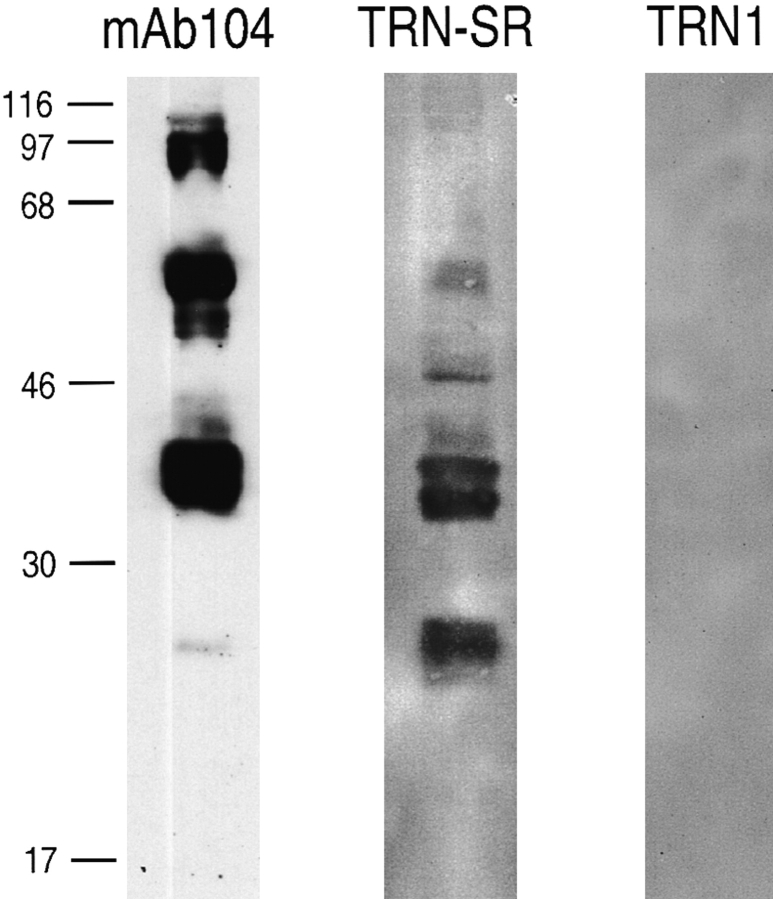
Mammalian cells contain several SR proteins in addition to ASF/SF2 (Zahler et al., 1992; Fu, 1995). Since TRN-SR binds the RS domains of both ASF/SF2 and SC35 (Fig. 4), we examined whether it can also bind other SR proteins. The SR protein fraction was purified from HeLa nuclear extracts (Zahler et al., 1992), resolved by SDS-PAGE, and immobilized on a nitrocellulose membrane. By Western blotting with the anti-RS domain antibody mAb104, these purified SR proteins show the typical pattern reported previously (Fig. 6; Zahler et al., 1992). The capacity of TRN-SR to bind these proteins was determined by far Western blotting using 35S-labeled TRN-SR produced in rabbit reticulocyte lysate (Siomi et al., 1997). TRN-SR bound several of these proteins, whereas TRN1 did not (Fig. 6). In addition to proteins of ∼33 kD, that likely correspond to ASF/SF2 and SC35, proteins of ∼20, 46, and 55 kD also bound specifically to TRN-SR. This observed profile is similar to that detected by Western blotting with mAb104 (Fig. 6), suggesting that TRN-SR is a common nuclear import receptor for many of the SR proteins.
Figure 6.
TRN-SR binds to several SR proteins. 10 μg of purified SR proteins was resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was incubated with either in vitro translated 35S-labeled TRN-SR or TRN1, washed, and exposed to an x-ray film for autoradiography.
Discussion
In this report we have identified a novel receptor, TRN-SR, as a nuclear import receptor for SR proteins. Of the known proteins currently present in the sequence databases, we note the considerable amino acid sequence homology of TRN-SR with the S. cerevisiae Mtr10p (Fig. 3). Mtr10p has been shown to be a nuclear import receptor for the yeast pre-mRNA/mRNA-binding protein Npl3 (Pemberton et al., 1997; Senger et al., 1998). Npl3p, which is also referred to as Nop3p and Nab1p, is an hnRNP protein that contains within its carboxyl terminus an RGG-box within which are several serine-arginine (SR) dipeptides (Bossie et al., 1992; Russell and Tollervey, 1992; Wilson et al., 1994; Siebel and Guthrie, 1996; Pemberton et al., 1997). The NLS of Npl3p has not been precisely delineated but is contained in this region of the protein (Senger et al., 1998). The SR dipeptides of Npl3p may be important for Mtr10p recognition, although this has not been determined. Two additional proteins, one in C. elegans and one in S. pombe, show similarity to TRN-SR (Fig. 3). Several candidate SR proteins are found in the C. elegans database, and one SR protein has been recently cloned from S. pombe (Gross et al., 1998). Therefore, these TRN-SR homologues may be the import receptors of SR proteins in these organisms.
TRN-SR binds to the RS domain of ASF/SF2 and of SC35, and these interactions are disrupted by RanQ69L (Fig. 4 A). Furthermore, TRN-SR also binds other proteins enriched in an SR protein fraction (Fig. 6). These results strongly suggest that TRN-SR is a general nuclear import receptor for SR proteins. However, we note that no binding of TRN-SR to SRp75 was detected by far Western blotting, although this protein is abundant in the fraction we tested (Fig. 6). The reason for this is unknown, but it is possible that SRp75 may have a different receptor. There are additional SR proteins, including pre-mRNA splicing factors such as 9G8, U170K, U2AF35, and 65 (Fu, 1995), as well as two large SR proteins (Blencowe et al., 1998), and it remains to be determined whether TRN-SR also mediates the nuclear import of these proteins.
Several abundant hnRNP proteins, including hnRNP A1, A2, and F, are imported by TRN1 (Pollard et al., 1996; Siomi et al., 1997). Thus, in mammalian cells there are at least two nuclear import pathways for pre-mRNA/ mRNA-binding proteins, one mediated by TRN1 and one by TRN-SR. The relative amounts of hnRNP proteins and SR proteins are important for alternative pre-mRNA splicing. For example, the ratio between hnRNP A1 and ASF/SF2 affects 5′ splice site selection (Mayeda and Krainer, 1992; Zahler et al., 1993; Cáceres et al., 1994; Yang et al., 1994). As both of these proteins shuttle between the nucleus and the cytoplasm (Piñol-Roma and Dreyfuss, 1992; Alzhanova-Ericsson et al., 1996; Cáceres et al., 1998), it is conceivable that their relative amounts in the nucleus may be controlled by regulating their rates of nuclear import. Thus, by modifying either the transportins themselves or the respective NLSs, M9 and RS, splice site selection could be modulated. Indeed, several protein kinases have been reported to phosphorylate serine residues in the RS domains of SR proteins (Gui et al., 1994a,b; Colwill et al., 1996; Rossi et al., 1996; Kuroyanagi et al., 1998; Okamoto et al., 1998; Wang et al., 1998). While overexpression of some of these SR protein kinases causes disruption of nuclear speckles (Gui et al., 1994a; Kuroyanagi et al., 1998; Wang et al., 1998), they do not disrupt the nuclear localization of SR proteins. However, overexpression of one SR protein kinase, Clk/Sty kinase, does cause cytoplasmic accumulation of ASF/SF2 in HeLa cells (Cáceres et al., 1998). More recently it was reported that overexpression of kinase-inactive mutant of SR protein kinase-2 causes cytoplasmic accumulation of ASF/SF2 (Koizumi et al., 1999). It will be interesting to determine the effect of RS domain phosphorylation on the SR proteins–TRN-SR interaction.
The physiological function of the shuttling of SR proteins is not known. Both hnRNP A1/A2 proteins and SR proteins are associated with the same mRNAs as they are exported to the cytoplasm (Alzhanova-Ericsson, 1996; Visa et al., 1996) and it is thus possible that they both play a role in mRNA export. Nuclear export signals in the shuttling SR proteins have not been identified yet. The identification of nuclear export signals in shuttling SR proteins, if such exist, and of export receptors for them are issues of considerable interest that remain to be clarified.
Acknowledgments
We thank Dr. James Manley for the ASF/SF2 cDNA; Dr. Xian-dong Fu for SC35 cDNA; Dr. Dirk Görlich for importin β ΔN44 protein and RanQ69L expression plasmids; and Dr. Akila Mayeda for SR proteins. We also thank Lili Wan for help on yeast two-hybrid library screening, and other members of our laboratory, especially Drs. Sara Nakielny, Haruhiko Siomi, Lili Wan, and Robert Perkinson for critical reading and comments on the manuscript.
This work was supported by a grant from the National Institutes of Health (G. Dreyfuss), and by a long-term fellowship from Human Frontier Science Program Organization (N. Kataoka). G. Dreyfuss is an Investigator of the Howard Hughes Medical Institute.
Abbreviations used in this paper
- GST
glutathione-S-transferase
- hnRNP
heterogeneous nuclear RNP
- IBB
importin β–binding domain
- MBP
maltose-binding protein
- NLS
nuclear localization signal
- NPC
nuclear pore complex
- RS domain
arginine/serine rich domain
- TRN1
transportin 1
- TRN-SR
transportin-SR
References
- Adam SA, Marr RS, Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzhanova-Ericsson AT, Sun X, Visa N, Kiseleva E, Wurtz T, Daneholt B. A protein of the SR family of splicing factors binds extensively to exonic Balbiani ring pre-mRNA and accompanies the RNA from the gene to the nuclear pore. Genes Dev. 1996;10:2881–2893. doi: 10.1101/gad.10.22.2881. [DOI] [PubMed] [Google Scholar]
- Arts GJ, Fornerod M, Mattaj IW. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. doi: 10.1016/s0960-9822(98)70130-7. [DOI] [PubMed] [Google Scholar]
- Blencowe BJ, Issner R, Nickerson JA, Sharp PA. A coactivator of pre-mRNA splicing. Genes Dev. 1998;12:996–1009. doi: 10.1101/gad.12.7.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifaci N, Moroianu J, Radu A, Blobel G. Karyopherin beta2 mediates nuclear import of a mRNA binding protein. Proc Natl Acad Sci USA. 1997;94:5055–5060. doi: 10.1073/pnas.94.10.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossie MA, DeHoratius C, Barcelo G, Silver P. A mutant nuclear protein with similarity to RNA binding proteins interferes with nuclear import in yeast. Mol Biol Cell. 1992;3:875–893. doi: 10.1091/mbc.3.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres JF, Stamm S, Helfman DM, Krainer AR. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science. 1994;265:1706–1709. doi: 10.1126/science.8085156. [DOI] [PubMed] [Google Scholar]
- Cáceres JF, Misteli T, Screaton GR, Spector DL, Krainer AR. Role of the modular domains of SR proteins in subnuclear localization and alternative splicing specificity. J Cell Biol. 1997;138:225–238. doi: 10.1083/jcb.138.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres JF, Screaton GR, Krainer AR. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev. 1998;12:55–66. doi: 10.1101/gad.12.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Adam EJ, Adam SA. Sequence and characterization of cytoplasmic nuclear protein import factor p97. J Cell Biol. 1995;130:265–274. doi: 10.1083/jcb.130.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Adam EJ, Visser GD, Adam SA. RanBP1 stabilizes the interaction of Ran with p97 nuclear protein import. J Cell Biol. 1996;135:559–569. doi: 10.1083/jcb.135.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill K, Pawson T, Andrews B, Prasad J, Manley JL, Bell JC, Duncan PI. The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO (Eur Mol Biol Organ) J. 1996;15:265–275. [PMC free article] [PubMed] [Google Scholar]
- Dahlberg JE, Lund E. Functions of the GTPase Ran in RNA export from the nucleus. Curr Opin Cell Biol. 1998;10:400–408. doi: 10.1016/s0955-0674(98)80017-3. [DOI] [PubMed] [Google Scholar]
- Doye V, Hurt E. From nucleoporins to nuclear pore complexes. Curr Opin Cell Biol. 1997;9:401–411. doi: 10.1016/s0955-0674(97)80014-2. [DOI] [PubMed] [Google Scholar]
- Forbes DJ. Structure and function of the nuclear pore complex. Annu Rev Cell Biol. 1992;8:495–527. doi: 10.1146/annurev.cb.08.110192.002431. [DOI] [PubMed] [Google Scholar]
- Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Fridell RA, Truant R, Thorne L, Benson RE, Cullen BR. Nuclear import of hnRNP A1 is mediated by a novel cellular cofactor related to karyopherin-beta. J Cell Sci. 1997;110:1325–1331. doi: 10.1242/jcs.110.11.1325. [DOI] [PubMed] [Google Scholar]
- Fu X-D. The superfamily of arginine/serine-rich splicing factors. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- Fu X-D, Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature. 1990;343:437–441. doi: 10.1038/343437a0. [DOI] [PubMed] [Google Scholar]
- Fu X-D, Maniatis T. Isolation of a complementary DNA that encodes the mammalian splicing factor SC35. Science. 1992;256:535–538. doi: 10.1126/science.1373910. [DOI] [PubMed] [Google Scholar]
- Fu X-D, Mayeda A, Maniatis T, Krainer AR. General splicing factors SF2 and SC35 have equivalent activities in vitro, and both affect alternative 5′ and 3′ splice site selection. Proc Natl Acad Sci USA. 1992;89:11224–11228. doi: 10.1073/pnas.89.23.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H, Manley JL. A protein factor, ASF, controls cell-specific alternative splicing of SV40 early pre-mRNA in vitro. Cell. 1990;62:25–34. doi: 10.1016/0092-8674(90)90236-8. [DOI] [PubMed] [Google Scholar]
- Ge H, Zuo P, Manley JL. Primary structure of the human splicing factor ASF reveals similarities with Drosophilaregulators. Cell. 1991;66:373–382. doi: 10.1016/0092-8674(91)90626-a. [DOI] [PubMed] [Google Scholar]
- Görlich D, Prehn S, Laskey RA, Hartmann E. Isolation of a protein that is essential for the first step of nuclear protein import. Cell. 1994;79:767–778. doi: 10.1016/0092-8674(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Görlich D, Kostka S, Kraft R, Dingwall C, Laskey RA, Hartmann E, Prehn S. Two different subunits of importin cooperate to recognize nuclear localization signals and bind them to the nuclear envelope. Curr Biol. 1995;5:383–392. doi: 10.1016/s0960-9822(95)00079-0. [DOI] [PubMed] [Google Scholar]
- Görlich D, Henklein P, Laskey RA, Hartmann E. A 41 amino acid motif in importin-alpha confers binding to importin-beta and hence transit into the nucleus. EMBO (Eur Mol Biol Organ) J. 1996a;15:1810–1817. [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Pante N, Kutay U, Aebi U, Bischoff FR. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO (Eur Mol Biol Organ) J. 1996b;15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Dabrowski M, Bischoff FR, Kutay U, Bork P, Hartmann E, Prehn S, Izaurralde E. A novel class of RanGTP binding proteins. J Cell Biol. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross T, Richert K, Mierke C, Lutzelberger M, Kaufer NF. Identification and characterization of srp1, a gene of fission yeast encoding a RNA binding domain and a RS domain typical of SR splicing factors. Nucl Acids Res. 1998;26:505–511. doi: 10.1093/nar/26.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui JF, Lane WS, Fu X-D. A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature. 1994a;369:678–682. doi: 10.1038/369678a0. [DOI] [PubMed] [Google Scholar]
- Gui JF, Tronchere H, Chandler SD, Fu X-D. Purification and characterization of a kinase specific for the serine- and arginine-rich pre-mRNA splicing factors. Proc Natl Acad Sci USA. 1994b;91:10824–10828. doi: 10.1073/pnas.91.23.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley ML, Amrein H, Maniatis T. An amino acid sequence motif sufficient for subnuclear localization of an arginine/serine-rich splicing factor. Proc Natl Acad Sci USA. 1995;92:11524–11528. doi: 10.1073/pnas.92.25.11524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto N, Shimamoto T, Kose S, Takao T, Tachibana T, Matsubae M, Sekimoto T, Shimonishi Y, Yoneda Y. The nuclear pore-targeting complex binds to nuclear pores after association with a karyophile. FEBS Lett. 1995a;368:415–419. doi: 10.1016/0014-5793(95)00699-a. [DOI] [PubMed] [Google Scholar]
- Imamoto N, Shimamoto T, Takao T, Tachibana T, Kose S, Matsubae M, Sekimoto T, Shimonishi Y, Yoneda Y. In vivo evidence for involvement of a 58 kD component of nuclear pore-targeting complex in nuclear protein import. EMBO (Eur Mol Biol Organ) J. 1995b;14:3617–3626. doi: 10.1002/j.1460-2075.1995.tb00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E, Adam S. Transport of macromolecules between the nucleus and the cytoplasm. RNA. 1998;4:351–364. [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E, Kutay U, von Kobbe C, Mattaj IW, Görlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO (Eur Mol Biol Organ) J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Chen S, Hitomi M, Jacobs E, Kumagai C, Liang S, Schneiter R, Singleton D, Wisniewska J, Tartakoff AM. Isolation and characterization of Saccharomyces cerevisiaemRNA transport-defective (mtr) mutants. J Cell Biol. 1994;126:649–659. doi: 10.1083/jcb.126.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D, Roberts BL, Richardson WD, Smith AE. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Klebe C, Bischoff FR, Ponstingl H, Wittinghofer A. Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry. 1995;34:639–647. doi: 10.1021/bi00002a031. [DOI] [PubMed] [Google Scholar]
- Koizumi J, Okamoto Y, Onogi H, Mayeda A, Krainer AR, Hagiwara M. The subcellular localization of SF2/ASF is regulated by direct interaction with SR protein kinases (SRPKs) J Biol Chem. 1999;274:11125–11131. doi: 10.1074/jbc.274.16.11125. [DOI] [PubMed] [Google Scholar]
- Krainer AR, Conway GC, Kozak D. The essential pre-mRNA splicing factor SF2 influences 5′ splice site selection by activating proximal sites. Cell. 1990;62:35–42. doi: 10.1016/0092-8674(90)90237-9. [DOI] [PubMed] [Google Scholar]
- Krainer AR, Mayeda A, Kozak D, Binns G. Functional expression of cloned human splicing factor SF2: homology to RNA-binding proteins, U170K, and Drosophilasplicing regulators. Cell. 1991;66:383–394. doi: 10.1016/0092-8674(91)90627-b. [DOI] [PubMed] [Google Scholar]
- Kuroyanagi N, Onogi H, Wakabayashi T, Hagiwara M. Novel SR-protein-specific kinase, SRPK2, disassembles nuclear speckles. Biochem Biophys Res Commun. 1998;242:357–364. doi: 10.1006/bbrc.1997.7913. [DOI] [PubMed] [Google Scholar]
- Kutay U, Izaurralde E, Bischoff FR, Mattaj IW, Görlich D. Dominant-negative mutants of importin-beta block multiple pathways of import and export through the nuclear pore complex. EMBO (Eur Mol Biol Organ) J. 1997a;16:1153–1163. doi: 10.1093/emboj/16.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Bischoff FR, Kostka S, Kraft R, Görlich D. Export of importin alpha from the nucleus is mediated by a specific nuclear transport factor. Cell. 1997b;90:1061–1071. doi: 10.1016/s0092-8674(00)80372-4. [DOI] [PubMed] [Google Scholar]
- Kutay U, Lipowsky G, Izaurralde E, Bischoff FR, Schwarzmaier P, Hartmann E, Görlich D. Identification of a tRNA-specific nuclear export receptor. Mol Cell. 1998;1:359–369. doi: 10.1016/s1097-2765(00)80036-2. [DOI] [PubMed] [Google Scholar]
- Lamond AI, Earnshaw WC. Structure and function in the nucleus. Science. 1998;280:547–553. doi: 10.1126/science.280.5363.547. [DOI] [PubMed] [Google Scholar]
- Li H, Bingham PM. Arginine/serine-rich domains of the su(wa) and tra RNA processing regulators target proteins to a subnuclear compartment implicated in splicing. Cell. 1991;67:335–342. doi: 10.1016/0092-8674(91)90185-2. [DOI] [PubMed] [Google Scholar]
- Mayeda A, Krainer AR. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell. 1992;68:365–375. doi: 10.1016/0092-8674(92)90477-t. [DOI] [PubMed] [Google Scholar]
- Misteli T, Spector DL. The cellular organization of gene expression. Curr Opin Cell Biol. 1998;10:323–331. doi: 10.1016/s0955-0674(98)80007-0. [DOI] [PubMed] [Google Scholar]
- Moore MS. Ran and nuclear transport. J Biol Chem. 1998;273:22857–22860. doi: 10.1074/jbc.273.36.22857. [DOI] [PubMed] [Google Scholar]
- Moroianu J, Blobel G, Radu A. Previously identified protein of uncertain function is karyopherin alpha and together with karyopherin beta docks import substrate at nuclear pore complexes. Proc Natl Acad Sci USA. 1995;92:2008–2011. doi: 10.1073/pnas.92.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroianu J, Blobel G, Radu A. The binding site of karyopherin alpha for karyopherin beta overlaps with a nuclear localization sequence. Proc Natl Acad Sci USA. 1996a;93:6572–6576. doi: 10.1073/pnas.93.13.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroianu J, Blobel G, Radu A. Nuclear protein import: Ran-GTP dissociates the karyopherin alphabeta heterodimer by displacing alpha from an overlapping binding site on beta. Proc Natl Acad Sci USA. 1996b;93:7059–7062. doi: 10.1073/pnas.93.14.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny S, Dreyfuss G. Nuclear export of proteins and RNAs. Curr Opin Cell Biol. 1997;9:420–429. doi: 10.1016/s0955-0674(97)80016-6. [DOI] [PubMed] [Google Scholar]
- Nakielny S, Siomi MC, Siomi H, Michael WM, Pollard V, Dreyfuss G. Transportin: nuclear transport receptor of a novel nuclear protein import pathway. Exp Cell Res. 1996;229:261–266. doi: 10.1006/excr.1996.0369. [DOI] [PubMed] [Google Scholar]
- Ohno M, Fornerod M, Mattaj IW. Nucleocytoplasmic transport: the last 200 nanometers. Cell. 1998;92:327–336. doi: 10.1016/s0092-8674(00)80926-5. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Onogi H, Honda R, Yasuda H, Wakabayashi T, Nimura Y, Hagiwara M. cdc2 kinase-mediated phosphorylation of splicing factor SF2/ASF. Biochem Biophys Res Commun. 1998;249:872–878. doi: 10.1006/bbrc.1998.9247. [DOI] [PubMed] [Google Scholar]
- Pemberton LF, Rosenblum JS, Blobel G. A distinct and parallel pathway for the nuclear import of an mRNA-binding protein. J Cell Biol. 1997;139:1645–1653. doi: 10.1083/jcb.139.7.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton LF, Blobel G, Rosenblum JS. Transport routes through the nuclear pore complex. Curr Opin Cell Biol. 1998;10:392–399. doi: 10.1016/s0955-0674(98)80016-1. [DOI] [PubMed] [Google Scholar]
- Piñol-Roma S, Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature. 1992;355:730–732. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- Pollard VW, Michael WM, Nakielny S, Siomi MC, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Radu A, Blobel G, Moore MS. Identification of a protein complex that is required for nuclear protein import and mediates docking of import substrate to distinct nucleoporins. Proc Natl Acad Sci USA. 1995;92:1769–1773. doi: 10.1073/pnas.92.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- Robbins J, Dilworth SM, Laskey RA, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991;64:615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- Rossi F, Labourier E, Forne T, Divita G, Derancourt J, Riou JF, Antoine E, Cathala G, Brunel C, Tazi J. Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature. 1996;381:80–82. doi: 10.1038/381080a0. [DOI] [PubMed] [Google Scholar]
- Russell ID, Tollervey D. NOP3 is an essential yeast protein which is required for pre-rRNA processing. J Cell Biol. 1992;119:737–747. doi: 10.1083/jcb.119.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger B, Simos G, Bischoff FR, Podtelejnikov A, Mann M, Hurt E. Mtr10p functions as a nuclear import receptor for the mRNA-binding protein Npl3p. EMBO (Eur Mol Biol Organ) J. 1998;17:2196–2207. doi: 10.1093/emboj/17.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebel CW, Guthrie C. The essential yeast RNA binding protein Np13p is methylated. Proc Natl Acad Sci USA. 1996;93:13641–13646. doi: 10.1073/pnas.93.24.13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RH, Green MR. Compartmentalization of eukaryotic gene expression: causes and effects. Cell. 1997;91:291–294. doi: 10.1016/s0092-8674(00)80411-0. [DOI] [PubMed] [Google Scholar]
- Siomi H, Dreyfuss G. A nuclear localization domain in the hnRNP A1 protein. J Cell Biol. 1995;129:551–560. doi: 10.1083/jcb.129.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi MC, Eder PS, Kataoka N, Wan L, Liu Q, Dreyfuss G. Transportin-mediated nuclear import of heterogeneous nuclear RNP proteins. J Cell Biol. 1997;138:1181–1192. doi: 10.1083/jcb.138.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz F, Rosbash M. Nuclear RNA export. Genes Dev. 1998;12:3303–3319. doi: 10.1101/gad.12.21.3303. [DOI] [PubMed] [Google Scholar]
- Visa N, Alzhanova-Ericsson AT, Sun X, Kiseleva E, Bjorkroth B, Wurtz T, Daneholt B. A pre-mRNA-binding protein accompanies the RNA from the gene through the nuclear pores and into polysomes. Cell. 1996;84:253–264. doi: 10.1016/s0092-8674(00)80980-0. [DOI] [PubMed] [Google Scholar]
- Wang HY, Lin W, Dyck JA, Yeakley JM, Songyang Z, Cantley LC, Fu X-D. SRPK2: a differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells. J Cell Biol. 1998;140:737–750. doi: 10.1083/jcb.140.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weighardt F, Biamonti G, Riva S. Nucleo-cytoplasmic distribution of human hnRNP proteins: a search for the targeting domains in hnRNP A1. J Cell Sci. 1995;108:545–555. doi: 10.1242/jcs.108.2.545. [DOI] [PubMed] [Google Scholar]
- Weis K. Importins and exportins: how to get in and out of the nucleus. Trends Biochem Sci. 1998;23:185–189. doi: 10.1016/s0968-0004(98)01204-3. [DOI] [PubMed] [Google Scholar]
- Weis K, Ryder U, Lamond AI. The conserved amino-terminal domain of hSRP1 alpha is essential for nuclear protein import. EMBO (Eur Mol Biol Organ) J. 1996;15:1818–1825. [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Datar KV, Paddy MR, Swedlow JR, Swanson MS. Characterization of nuclear polyadenylated RNA-binding proteins in Saccharomyces cerevisiae. . J Cell Biol. 1994;127:1173–1184. doi: 10.1083/jcb.127.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak RW, Rout MP, Aitchison JD. Karyopherins and kissing cousins. Trends Cell Biol. 1998;8:184–188. doi: 10.1016/s0962-8924(98)01248-3. [DOI] [PubMed] [Google Scholar]
- Yang X, Bani MR, Lu SJ, Rowan S, Ben-David Y, Chabot B. The A1 and A1B proteins of heterogeneous nuclear ribonucleoparticles modulate 5′ splice site selection in vivo. Proc Natl Acad Sci USA. 1994;91:6924–6928. doi: 10.1073/pnas.91.15.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler AM, Lane WS, Stolk JA, Roth MB. SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev. 1992;6:837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- Zahler AM, Neugebauer KM, Lane WS, Roth MB. Distinct functions of SR proteins in alternative pre-mRNA splicing. Science. 1993;260:219–222. doi: 10.1126/science.8385799. [DOI] [PubMed] [Google Scholar]