Abstract
The p21 (Cdc42/Rac) activated kinase Pak1 regulates cell morphology and polarity in most, if not all, eukaryotic cells. We and others have established that Pak's effects on these parameters are mediated by changes in the organization of cortical actin. Because cell motility requires polarized rearrangements of the actin/myosin cytoskeleton, we examined the role of Pak1 in regulating cell movement. We established clonal tetracycline-regulated NIH-3T3 cell lines that inducibly express either wild-type Pak1, a kinase-dead, or constitutively-active forms of this enzyme, and examined the morphology, F-actin organization, and motility of these cells. Expression of any of these forms of Pak1 induced dramatic changes in actin organization which were not inhibited by coexpression of a dominant-negative form of Rac1. Cells inducibly expressing wild-type or constitutively-active Pak1 had large, polarized lamellipodia at the leading edge, were more motile than their normal counterparts when plated on a fibronectin-coated surface, and displayed enhanced directional movement in response to an immobilized collagen gradient. In contrast, cells expressing a kinase-dead form of Pak1 projected multiple lamellipodia emerging from different parts of the cell simultaneously. These cells, though highly motile, displayed reduced persistence of movement when plated on a fibronectin-coated surface and had defects in directed motility toward immobilized collagen. Expression of constitutively activated Pak1 was accompanied by increased myosin light chain (MLC) phosphorylation, whereas expression of kinase-dead Pak1 had no effect on MLC. These results suggest that Pak1 affects the phosphorylation state of MLC, thus linking this kinase to a molecule that directly affects cell movement.
Keywords: p21-activated kinase, motility, GTPases, fibroblasts, phosphorylation
Many cell types are induced to move in response to external factors. Cell migration is essential during embryonic development, in immune response, and wound healing, and aberrant motility is a hallmark of metastatic cells. The migration of fibroblasts in vitro requires acquisition of spacial asymmetry with the extension of lamellipodia, attachment of the cytoskeleton to the extracellular matrix, and generation of force that propels the cell forward while pulling the attachments rearward (Sheetz, 1994). Actin polymerization is thought to drive the formation and extension of the lamellipodial leading edge, while the activation of myosin, particularly myosin II, is thought to be at least partially responsible for creating the traction force needed for cell movement (Condeelis, 1993). In addition, the strength and dynamics of focal adhesion formation play a major role in cell locomotion. The molecular basis of the signaling steps that regulate motile responses are poorly understood; however, Ras and related GTPases of the Rho family clearly play important roles in this process. For example, the scattering response of MDCK cells in response to HGF can be abolished by dominant negative Ras, Rac1, as well as constitutively active RhoA (Ridley et al., 1995), and activated forms of Rac1 and Cdc42 can increase cell motility and the invasive potential of epithelial cells (Keely et al., 1997; Sander et al., 1998). In addition, Rac1 modulates growth factor–driven chemotaxis in fibroblasts and macrophages (Anand-Apte et al., 1997; Allen et al., 1998).
Reorganization of the actin cytoskeleton is a prerequisite for cell motility. The Rho GTPases, Cdc42, Rac1, and RhoA are well known for their ability to induce rearrangements of filamentous actin. In the case of Cdc42 and Rac, several candidate effectors have been identified that might link these GTPases to the actin cytoskeleton. In mammalian cells, these candidate effectors include (a) protein kinases, such as p21-activated kinases (Paks)1, myotonic-dystrophy–related kinases, MEKKs, and mixed-lineage kinases, (b) lipid kinases, such as PI3 kinase and PI5 kinase, (c) formins, such as the mouse homologue of Diaphanous (p140mDia), (d) actin binding proteins, such as IQGAPs, and (e) other proteins that affect actin structure, such as Wiskott-Aldrich protein and POR1 (reviewed in Van Aelst and D'Souza-Schorey, 1997). A major point of controversy in the literature is whether Paks have a role in mediating F-actin assembly by these GTPases in mammalian cells. On the one hand, mutants of Rac1 and Cdc42 that do not efficiently bind the three known mammalian Paks are nonetheless able to induce characteristic actin reorganization in fibroblasts (Joneson et al., 1996; Lamarche et al., 1996; Westwick et al., 1997). On the other hand, expression of activated forms of Pak1 induces changes in actin and focal adhesion structure similar to those seen with activated GTPases (Manser et al., 1997; Sells et al., 1997). A possible resolution to these two seemingly conflicting results has been provided recently by Manser et al., who showed that Pak1 can be activated even in the absence of direct binding to Cdc42 through interaction with the guanine-nucleotide exchange factor PIX (Manser et al., 1998).
In lower eukaryotes, Paks clearly play a role in regulating actin-based morphogenic events. Deletion of the Pak homologue STE20 in budding yeast results in sterile cells, unable to form mating projections or activate the transcription program required for mating (Leberer et al., 1992). Overexpression of STE20 is sufficient to relieve the morphological defects associated with loss of Cdc42p function, consistent with the postulated effector role for Ste20p in regulating cortical actin assembly (Eby et al., 1998). In this system, myosin I has been identified as a potential Pak target (Wu et al., 1996, 1997), providing a possible explanation for Pak's morphogenic effects. In fission yeast, deletion and overexpression studies have shown that both Pak1p and -2p regulate cell morphogenesis (Marcus et al., 1995; Ottilie et al., 1995; Sells et al., 1998; Yang et al., 1998). Interestingly, a temperature-sensitive mutation of pak1 was isolated recently during a screen for rounded (orb) mutants of S. pombe (Verde et al., 1995, 1998), providing independent evidence for a role for this protein in morphogenesis. Genetic analysis has placed this gene (orb2) upstream of another orb gene (orb6), which encodes a homologue of Rok, a Rho-activated kinase (Verde et al., 1998). In vertebrates (Kimura et al., 1996) and probably in C. elegans (Wissmann et al., 1997), Rok inactivates myosin light chain (MLC) phosphatase, resulting in increased MLC phosphorylation. Therefore, the observed effects of S. pombe Paks on cell morphology may involve activation of MLC via Orb6p. Whether these findings will extend to the mammalian system remains to be seen.
In a previous analysis, we noted that the majority of Swiss 3T3 cells transiently expressing an activated form of Pak1 developed large, polarized lamellipodia containing numerous small focal complexes (Sells et al., 1997). Two regions of Pak1 were found to be important for these cytoskeletal effects; proline-rich sequences in the NH2 terminus, which bind to src-homology 3 (SH3) proteins such as the adaptor Nck (Galisteo et al., 1996) and the guanine-nucleotide exchange factor PIX (Manser et al., 1998), and the COOH-terminal protein kinase domain (Manser et al., 1997; Sells et al., 1997). The NH2-terminal proline-rich sequences appear to be important for Pak localization and are required for the development of polarized lamellipodia. The function of the kinase domain is less certain. Inactivating mutations in the kinase domain do not abolish the ability of Pak1 to reorganize actin, but cells expressing such mutants have multiple protrusions and no apparent leading edge (Sells et al., 1997). Therefore, the kinase domain of Pak1 may be important for the disposition of lamellipodia.
The polarized phenotype induced by activated Pak1 is reminiscent of that seen in motile fibroblasts. In this study, we have analyzed the effects of Pak1 on cell motility, using cell lines that inducibly express various forms of Pak1. Here, we show that Pak1 induces the formation of leading edge lamellipodia. These structures are not affected by coexpression of a dominant-negative form of Rac1. Consistent with this morphology, Pak1 overexpression stimulates cell movement. Pak1 overexpression increases random cell movement on fibronectin-coated surfaces, irrespective of its protein kinase activity. In contrast, Pak1 also affects directional cell movement toward an immobilized collagen gradient, but this effect requires its kinase activity. Activated forms of Pak1 induce MLC phosphorylation, whereas a kinase-deficient mutant is without effect. These results suggest that Pak1 plays a role in regulating directional cell motility through its effects on MLC.
Materials and Methods
Materials
Monoclonal anti-hemagglutinin (HA) antibody (12CA5) was obtained from BabCo, polyclonal anti-Myc was obtained from Santa Cruz Biotechnology, Inc., and monoclonal anti-Myc antibody (9E10) was a gift from Phil Tsichlis (Fox Chase Cancer Center, Philadelphia, PA). Antibodies against activated Erk and Jnk were obtained from Promega Corp., anti-vinculin was from Sigma Chemical Co., and anti–phospho-MLC (Matsumura et al., 1998) was a kind gift from Fumio Matsumura (Rutgers University, New Brunswick, NJ). Fluorochrome-conjugated goat anti–mouse and anti–rabbit antibodies were from Jackson ImmunoResearch Laboratories, Inc.
Expression Plasmids
Wild-type and mutant forms of Pak1 (Sells et al., 1997) were subcloned from pJ3H (Sells and Chernoff, 1995) as SalI/(EcoRI → blunt) fragments into SalI/EcoRV-cut pTet-Splice (Shockett et al., 1995). These plasmids express NH2-terminal HA-tagged Pak1 when cells are grown in the absence of tetracycline. The tetracycline-regulated expression vectors pUM-Rac1 V12 and pUM-Rac1 N17 (both Myc-tagged; Anand-Apte et al., 1997) were kindly donated by Mark Symons (Onyx Corp., Richmond, CA).
Cell Culture
NIH-3T3 cells and derivatives were maintained in DME plus 10% calf serum (CS). The NIH-3T3 variant S2-6 (Shockett et al., 1995), bearing a tetracycline-regulated transactivator, was used to construct cell lines that inducibly express various forms of Pak1 or Rac1. To prepare stable, regulated clonal cell lines, S2-6 cells were cotransfected with either pTet-Splice-Pak1 or pUM-Rac1 plasmids plus a plasmid encoding a puromycin resistance gene using a calcium phosphate precipitation method (Chen and Okayama, 1987). 48 h after transfection, the cells were selected in media containing 2.5 mM histidinol (to retain the tetracycline-VP16 transactivator), 2 μg/ml puromycin (to select for the tetracycline-regulated expression vector), and 1 μg/ml tetracycline (to repress transgene expression during selection). Clonal cell lines were isolated and expanded and at least 24 lines for each form of Pak1 or Rac1 were examined for inducible transgene expression by anti-HA or anti-Myc immunoblot.
Immunoblots
Cells were lysed in NP-40 lysis buffer (50 mM Tris-HCl, pH 8.0, 137 mM NaCl, 10% glycerol, 1% NP-40, 50 mM NaF, 10 mM β-glycerol-phosphate) containing 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, and 10 μg per ml aprotinin. 10 μg extract was fractionated by 10% SDS/PAGE and transferred to polyvinyl difluoride (Immobilon) membranes. Membranes were blocked using fat-free milk, probed with antibodies, and developed using an alkaline-phosphatase–based chemiluminescent system (Dupont/New England Nuclear).
Protein Kinase Assays
HA-tagged Pak1 was immunoprecipitated using mAb 12CA5 (BabCo) from 35-mm wells of S2-6 cells grown in tetracycline-free DME. The immunoprecipitates were washed three times in NP-40 lysis buffer, once in 0.5 M LiCl, and then once in protein kinase buffer (50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 1 mM DTT). The immunoprecipitates were then incubated at 30° C for 20 min in 25 μl protein kinase buffer containing 25 μM ATP plus 5 μCi γ-[32P]ATP. The reactions were stopped by the addition of 5 μl 6× SDS/PAGE sample buffer, boiled for 5 min, then separated by 10% SDS/PAGE. The dried gel was then autoradiographed.
Transient Transfection
Cells were plated at 3.2 × 105 cells/35-mm culture dish in DME plus 10% CS and transfected with expression plasmids using a calcium phosphate precipitation method (Chen and Okayama, 1987).
Immunofluorescence Detection
Cells cultured on coverslips were fixed in 3.5% formaldehyde, permeabilized in 0.1% Tween-20 and blocked with 3% BSA in PBS. After incubation with primary antibodies, the cells were stained with rhodamine X-conjugated goat anti–rabbit antibodies along with AMCA-conjugated and Cy5-conjugated anti–mouse antibodies. For staining of filamentous actin, 0.1 μM Oregon Green or FITC-conjugated phalloidin was included during incubation with the secondary antibodies. Confocal microscopy was performed using a Bio-Rad MRC 600 laser scanning confocal microscope.
Tetracycline Titration
Cells from one inducibly expressing clonal line for each of two constitutive active mutant forms of Pak were plated at 30% confluence into 35-mm wells with and without coverslips. 24 h later the cells were washed one time in PBS and refed with DME plus 10% CS alone or with one of the following: 1, 0.2, 0.1, 0.04, or 0.02 μg/ml tetracycline and incubated for another 8 h. At that time, the cells were again washed and refed with DME plus 0.2% CS, retaining the same tetracycline concentrations as 8 h earlier. 16 h later, HA-tagged Pak was detected in the cells on coverslips by indirect immunofluorescent staining, while the remaining cells were lysed in NP-40 lysis buffer and immunoblotted as described above.
Motility Assays
Pak1 expression was induced by growth in tetracycline-free DME plus 10% CS for 16 h. After protein induction, the cells were treated with trypsin, washed in the presence of soybean trypsin inhibitor and replated in tetracycline-free DME plus 0.1% BSA onto plates coated with 1% BSA for 30 min. Uninduced cells were treated in the same manner. The cells were then transferred to a plate coated with 50 μg/ml fibronectin and allowed to attach for 40 min. Phase contrast images were captured thereafter at 30-s intervals for calculation of cell speed and at 2-min intervals for long term analysis (4 h sampling). The cells were tracked with the aid of Isee™ imaging software and the results were tabulated using Excel™.
For measurements of haptotaxis, Pak1 expression was induced by growth in tetracycline-free DME plus 10% CS for 16 h. Uninduced cells were treated in the same manner in the presence of tetracycline. After this pretreatment, the cells were detached from the plate with trypsin, washed in the presence of soybean trypsin inhibitor, resuspended in DME plus 0.1% BSA in the presence or absence of tetracycline, and then loaded into the top wells of a Boyden chamber at a concentration of 15,000 cells/well. The bottom wells contained DME plus 0.1% BSA, with or without tetracycline. The separating filter was coated on the underside only with 200 μg/ml collagen, type I. The number of cells that successfully migrated through the filter in three to six wells were counted after staining with hematoxylin.
Results
Establishment of Stable Cell Lines That Inducibly Express Pak1 and Rac1 Mutants
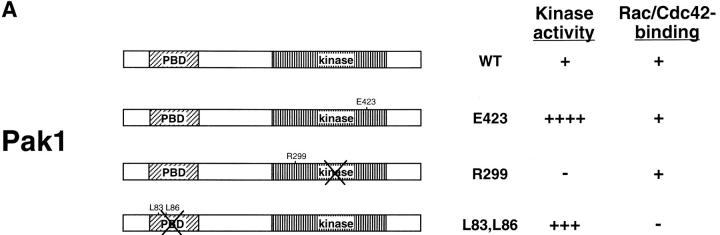
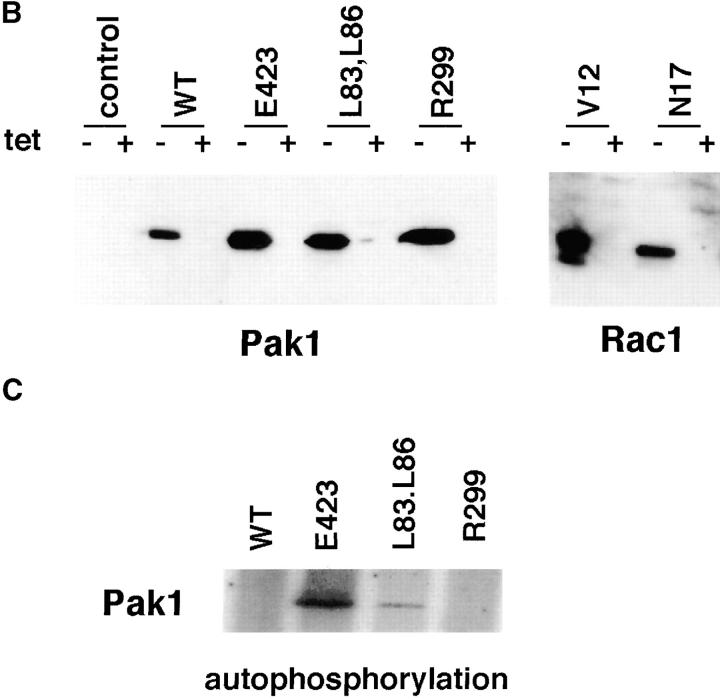
To assess the effects of Pak1 on motility, we established stable cell lines in which Pak1 expression is regulated by tetracycline. These cell lines included HA-tagged wild-type, constitutive-active, and kinase-dead mutants of Pak1, as well as Myc-tagged activated and dominant-negative forms of Rac1 (Fig. 1 a). Two constitutive active forms of Pak1, one that binds Cdc42/Rac normally (Pak1E423), and one that does not (Pak1L83,L86), were included in order to assess the role of p21 binding in any phenotypes induced by Pak1 expression (Sells et al., 1997). Transgene expression was tightly controlled by tetracycline, displaying 5–10-fold induction, as assessed by densitometry of anti-HA immunoblots (Fig. 1 b and data not shown). Importantly, transgene expression was barely detectable in cells grown in tetracycline-containing media, even on prolonged exposure of immunoblots. As expected, immunoprecipitated Pak1E423 displayed the most protein kinase activity, followed by Pak1L83,L86 (Fig 1 c). Neither wild-type nor kinase-dead forms of Pak1 displayed detectable kinase activity, consistent with previous results (Manser et al., 1997; Sells et al., 1997).
Figure 1.
Tetracycline regulated expression of various forms of Pak1 and Rac1 in NIH-3T3 clonal cell lines. (a) Structure and activities of Pak1 and Rac1 mutants used in this study. A diagrammatic representation of the wild-type and mutant forms of Pak1 and Rac1 are shown in the top panel with amino acid changes denoted by a single letter amino acid abbreviation of the mutant residue. For Pak1, domains shown include the p21 binding domain (PBD) and the catalytic domain (kinase). (b) Inducible expression of Pak1 and Rac1 proteins. S2-6 NIH-3T3 cells, containing the tTA transactivator, were transfected with tetracycline-regulatable expression plasmids bearing either no insert (control), or various forms of HA epitope–tagged Pak1 or Myc-tagged Rac1. Pak1 constructs: wild-type, WT; constitutively activated, E423; kinase-inactive, R299; constitutively activated and Cdc42/Rac-binding defective, L83,L86. Rac1 constructs: constitutively activated, V12; and dominant-negative, N17. Stable clones were isolated and characterized in both the presence and absence of tetracycline (tet) for Pak1 expression by immunoblot with anti-HA antisera, and for Rac1 expression by immunoblot with anti-Myc antisera. (c) Protein kinase activity of induced cell lines. HA-tagged Pak1 was immunoprecipitated from the indicated cell lines and tested for auto-kinase activity.
Expression of Mutant Forms of Pak1 Modulates Cell Morphology, F-Actin Distribution, and Focal Complex Formation in NIH-3T3 Clonal Cell Lines
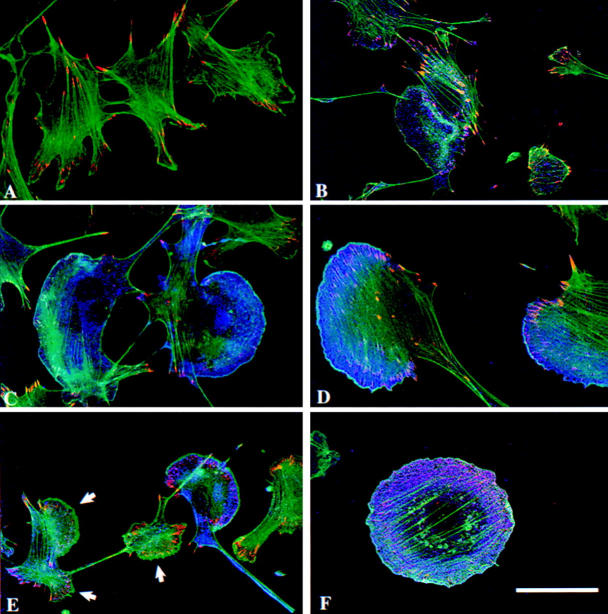
We and others have previously shown that transient expression of Pak1 affects the structure of the actin cytoskeleton in Swiss 3T3 (Sells et al., 1997), HeLa (Manser et al., 1997), and PC12 cells (Daniels et al., 1998). However, the effects of stable Pak1 expression on populations of cells have not been reported. Our tetracycline-regulatable cell lines permit large numbers of cells to be examined, each expressing similar levels of transgenic Pak1. In addition, we can titrate the level of Pak1 expression by varying the concentration of tetracycline. In induced NIH-3T3 cultures, wild-type Pak1 has only modest effects on F-actin distribution as we previously reported in transiently transfected Swiss 3T3 cells (Sells et al., 1997), although dorsal ruffles develop in a few (<20%) cells (Fig. 2 b). Expression of an activated Pak1 mutant, Pak1L83,L86, resulted in formation of not only dorsal ruffles but also of large, asymmetric lamellipodial structures characteristic of the leading edge of the cells (Fig. 2 c). These latter structures resemble those found in motile fibroblasts. Interesting, asymmetric lamellipodia were seen in >50% of cells expressing another activated mutant, Pak1E423 (Fig. 2 d); the remaining cells have a morphology characterized by a marked loss of stress fibers and few lamellipodia similar to those observed upon transient transfection (Manser et al., 1997; Sells et al., 1997). That these differences are due to the different cell types used for these studies is unlikely, given that transient transfection of S2-6 cells with pCMV6M-Pak1E423 (the same vector used in previous studies with Swiss 3T3 cells; Sells et al., 1997), results in a flattened, stress fiber-free cell phenotype (data not shown). A more likely explanation for differences in cell morphology may relate to the very high expression levels achieved in transient transfection versus the more moderate, but stable levels attained in the tetracycline-regulated cells. In support of this latter hypothesis, when a small amount of tetracycline (0.04 μg/ml) was added to the medium, the population of cells as a whole produced lower levels of Pak1E423, with concomitantly higher percentage of the cells having asymmetric lamellipodia as Pak1 production decreased (50.4% lamellipodia in tetracycline-free medium; 76.1% lamellipodia in medium containing 0.04 μg/ml tetracycline; 800 cells counted in total).
Figure 2.
Expression of mutant forms of Pak1 modulates F-actin and vinculin distribution in cells of NIH-3T3 clonal lines. Pak1 expression was induced by growth in tetracycline-free DME plus 10% CS for 8 h, followed by 16 h starvation in tetracycline-free DME plus 0.1% CS. Confocal images are shown. (a) control, (b) wild-type Pak1, (c) Pak1L83,L86, (d) Pak1E423, (e) Pak1R299 (arrows denote the three lamellipodia of a single cell), and (f) Rac1V12. Cells were stained for F-actin (FITC-phalloidin, green), vinculin (anti-vinculin, visualized with rhodamine X-labeled goat anti–mouse, red), and Pak1 or Rac1 (anti-HA or anti-Myc, visualized with Cy5-labeled goat anti–rabbit, blue). Bar, 25 μm.
Expression of a kinase-dead form of Pak1 also induced formation of lamellipodia, but, compared with cells expressing activated Pak1, these cells tended to have increased numbers of such structures dispersed throughout the cell body, giving the cells a multi-lobed appearance (Fig. 2 e). Often, lamellipodia accumulated on multiple sides of the cell, a phenotype that is virtually never observed in cells expressing activated forms of Pak1.
To ensure that the NIH-3T3 derivatives used in these studies respond to Rho-family GTPases like other commonly studied fibroblasts, we examined the cytoskeletal effects of Rac1 in the parental S2-6 NIH-3T3 cells. As in Swiss 3T3 (Ridley et al., 1992) and REF52 cells (Joneson et al., 1996), activated Rac1 induced cell flattening and circumferential lamellipodia, formation of cortical focal complexes, and a loss of central stress fibers (Fig. 2 f), while the dominant-negative version induced compact but elongated cells (data not shown). Thus, Rac1 expression gave rise to phenotypes similar to those described previously in Swiss 3T3 and REF52 cells, indicating that the S2-6 cells behave in a similar manner as these well-described cell systems.
The Morphologic Effects Produced by Overexpression of Pak1 in Fibroblasts Are Not Mediated by Rac1
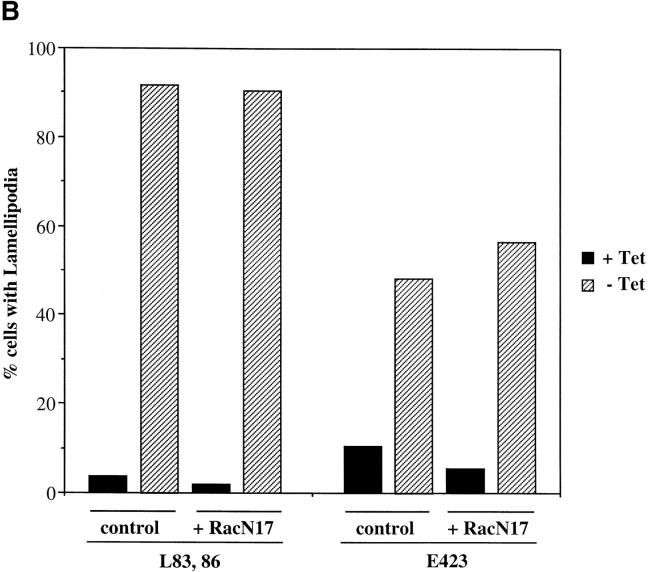
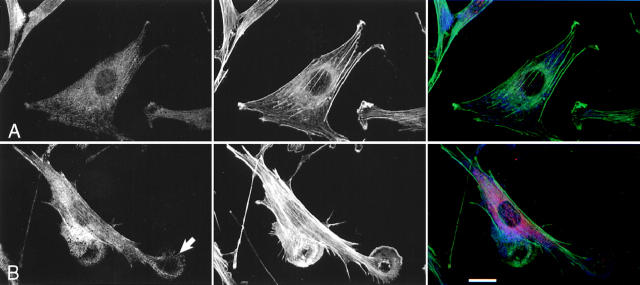
We have previously argued that, in Swiss 3T3 cells, the effects of Pak1L83,L86 on F actin organization are independent of Rac1 (Sells et al., 1997). However, Obermeier et al. (1998) showed that Pak1L83,L86 induces neurite outgrowth in PC12 cells, and that ∼40% of these effects are inhibited by coexpression of a dominant-negative form of Rac1, implying that Pak1 may operate upstream of Rac1. To reexamine this issue in fibroblasts, we transiently transfected Rac1N17 into the tetracycline regulated cell line expressing Pak1L83,L86. Cell expressing Pak1L83,L86 alone have, as expected, large asymmetric lamellipodia (Fig. 3 a, lower cell). Coexpression of Rac1N17 has no apparent effect on this phenotype (Fig. 3 a, upper cell). In cells that lack Pak1L83,L86 expression (i.e., those grown in the presence of tetracycline), Rac1N17 induces cell shrinkage, indicating that this protein is biologically active (data not shown). To quantitate these effects, we scored the phenotypes of 400 cells per group grown in the presence or absence of tetracycline with or without coexpressed Rac1N17. As expected, when grown in the presence of tetracycline, few cells display lamellipodia. Upon induction, ∼91% of cells expressing Pak1L83,L86 alone, and 90% of cells coexpressing Rac1N17 display characteristic lamellipodial structures (Fig. 3 b). A second activated Pak1 mutant, Pak1E423, was less potent in inducing lamellipodia (56% of cells), but, as with Pak1L83,L86, coexpression of Rac1N17 did not inhibit formation of these structures. The results of these studies show that Rac1N17 has very little effect on the phenotype elicited either by Pak1L83,L86 or Pak1E423, and therefore, in this cell type, the effects of these activated Pak1 mutants on F actin organization are independent of Rac1.
Figure 3.
Pak1-induced cytoskeletal changes are not mediated by activation of Rac1. (a) Cells conditionally expressing activated Pak1 were transfected with an constitutive expression vector for a dominant-negative (N17) form of Rac1. Pak1 expression was induced by growth in tetracycline-free DME plus 10% CS for 8 h, followed by 16 h starvation in tetracycline-free DME plus 0.5% CS. The cells were then fixed and stained for immunofluorescence. HA-tagged Pak1L83,L86 (blue), Myc-tagged Rac1N17 (red), F-actin (green). Bar, 25 μm. (b) At least 400 cells expressing Pak1 or Pak1 plus Rac1 were assessed for presence of lamellipodia. Values represent combined data from three separate experiments.
Expression of Pak1 Regulates Random Cell Locomotion
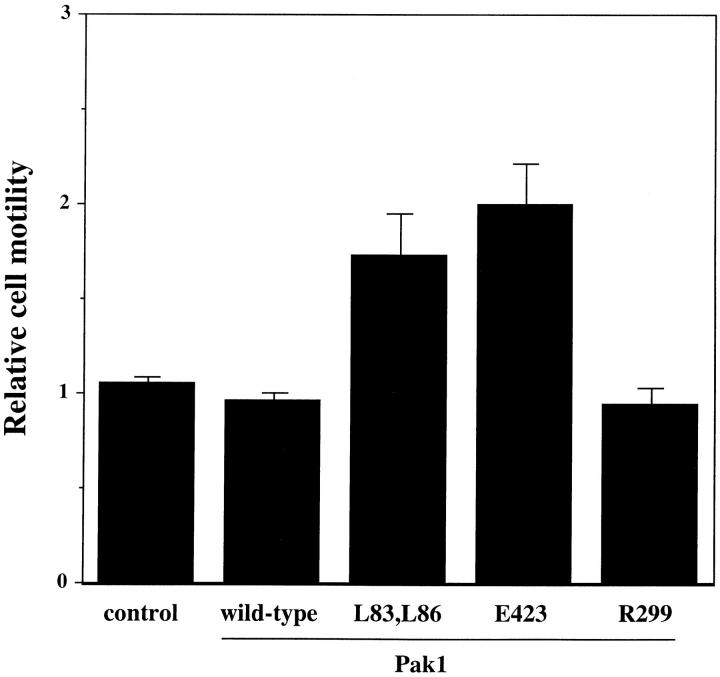
Because the morphology induced by activated Pak1 mimics that seen in motile fibroblasts, we investigated the role of this enzyme in regulating cell movement. Although the control of cell motility at the molecular level is poorly understood, it has been shown in a number of systems that Ras, and related GTPases of the Rho family clearly play important roles in this process (Ridley et al., 1995; Keely et al., 1997; Sander et al., 1998). We assessed the effect of Pak1 expression on cell motility by tracking the movement of individual cells at 30-s intervals over a 1-h time course after their attachment to fibronectin-coated dishes (Fig. 4). In the presence of tetracycline, the cell lines showed minor differences in basal movement rate (16.51 ± 0.85 nm/s for control cells, 12.49 ± 0.66, 12.08 ± 0.56, 14.67 ± 0.75, and 13.86 ± 0.60 nm/s for wild-type Pak1, Pak1L83,L86, Pak1E423, and Pak1R299 expressing cell lines, respectively; Fig. 4). These differences probably reflect intrinsic clonal variation. However, in the absence of tetracycline, the average control cell speed increased by only 0.24 nm/s, never attaining the speed of Pak1-expressing cells, even though these latter cells have a lower basal speed. In fact, the average speed of cells expressing wild-type Pak1, Pak1L83,L86, Pak1E423, and Pak1R299 increased to 17.03 ± 0.91, 17.58 ± 0.83, 21.06 ± 0.88, and 23.25 ± 1.07, respectively, when assayed in the absence of tetracycline. These data show that increased Pak1 levels cause an increase in cell movement, and that this increase is independent of protein kinase activity.
Figure 4.
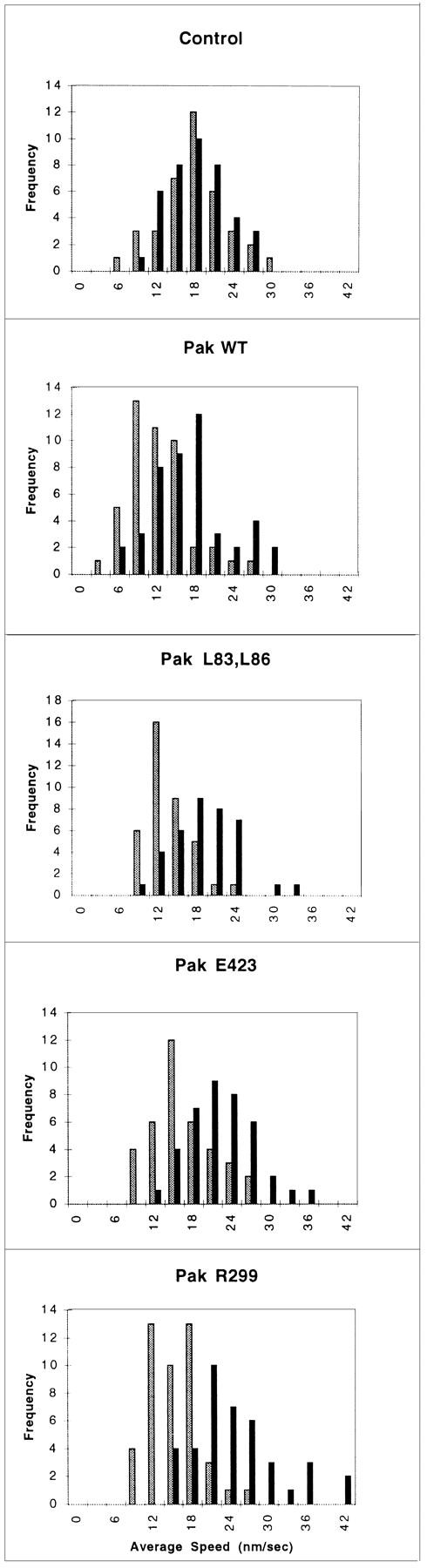
Cell motility is stimulated by Pak1. Pak1 expression was induced by growth in tetracycline-free DME as described in Methods. After protein induction, the cells were treated with trypsin, washed in the presence of soybean-trypsin inhibitor, and then replated in tetracycline-free DME plus 0.1% BSA onto BSA-coated plates for 30 min. The cells were then transferred to a fibronectin-coated plate and allowed to attach for 30 min. A graphic representation of average speed of cells, expressed in nm/s is shown. Cell movement was tracked at 30-s intervals using Inovison Isee™ nano-tracking system. Results shown are representative of four independent experiments. (Gray bars) Plus tetracycline; (black bars) minus tetracycline.
Directional Cell Movement Is Affected by Pak1 Kinase Activity
Interestingly, although random cell motility is independent of Pak1's catalytic function, directional movement strongly correlates with kinase activity. 4-h cell tracking experiments indicate that cells expressing kinase-dead Pak1, though highly motile, display poor persistence of movement compared with wild-type or activated forms of Pak1 (Fig. 5). In contrast to cells expressing activated Pak1, cells expressing kinase-dead Pak1 frequently and abruptly change course. These effects are readily apparent in cell path tracings (Fig. 6). Here, plots derived from the paths of 10 randomly selected cells show that the overall motility of cells expressing either activated or kinase-dead Pak1 is increased, but that the complexity of the paths is strongly related to protein kinase activity. Cells expressing activated Pak1 move in relatively straight paths (Fig. 6, b and d), whereas those expressing kinase-dead Pak1 move randomly (Fig 6 f). We speculated that these different types of movement, which are related to Pak1's protein kinase activity, might be reflected in other measurements of directional motility. In agreement with this prediction, cells expressing activated forms of Pak1 display increased haptotactic movement in response to an immobilized collagen gradient, whereas cells expressing kinase-inactive Pak1 show slightly decreased haptotaxis (Fig. 7). As a control, we also assessed haptotaxis in Rac1-expressing cells. Activated Rac1 enhances haptotactic movement, whereas a dominant-negative form inhibits this directional movement (data not shown). Interestingly, neither Pak1 nor Rac1 affect chemotaxis towards soluble fibronectin (data not shown and Anand-Apte et al., 1997), suggesting that this signaling pathway is regulated by a different set of proteins.
Figure 5.
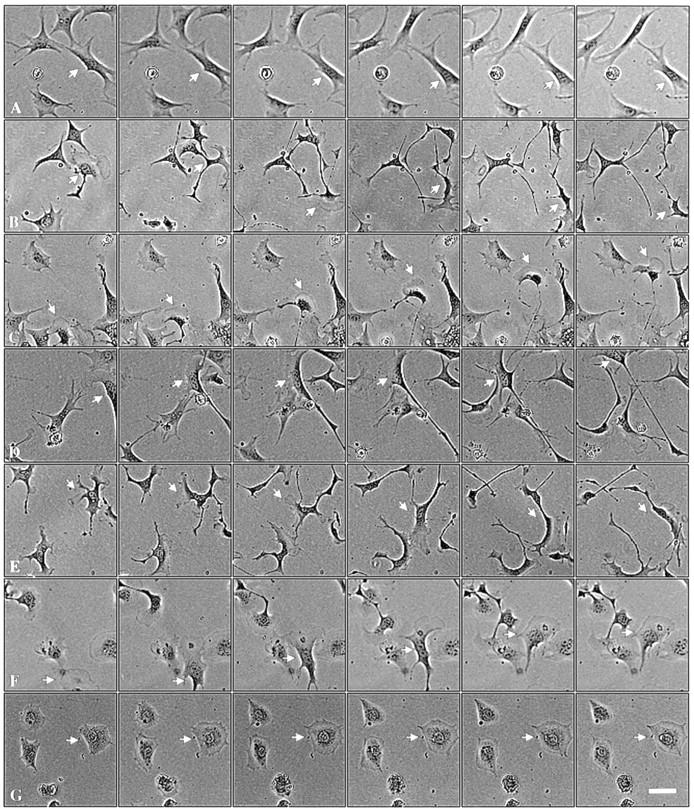
Pak1 kinase activity affects character of movement. Pak1 expression was induced by growth in tetracycline-free DME as in Fig. 4. After attachment to fibronectin-coated plates, individual cells were tracked. Phase contrast micrographs shown were taken at 30-min intervals. (a) control cells; (b) wild-type Pak1, (c) Pak1L83,L86, (d) Pak1E423, (e) Pak1R299 (the cell denoted by an arrow displayed from two to four lamellipodia throughout the course of the experiment), (f) Rac1V12, and (g) Rac1N17. Arrows mark the track of a single motile cell for each sample. Bar, 50 μm.
Figure 6.
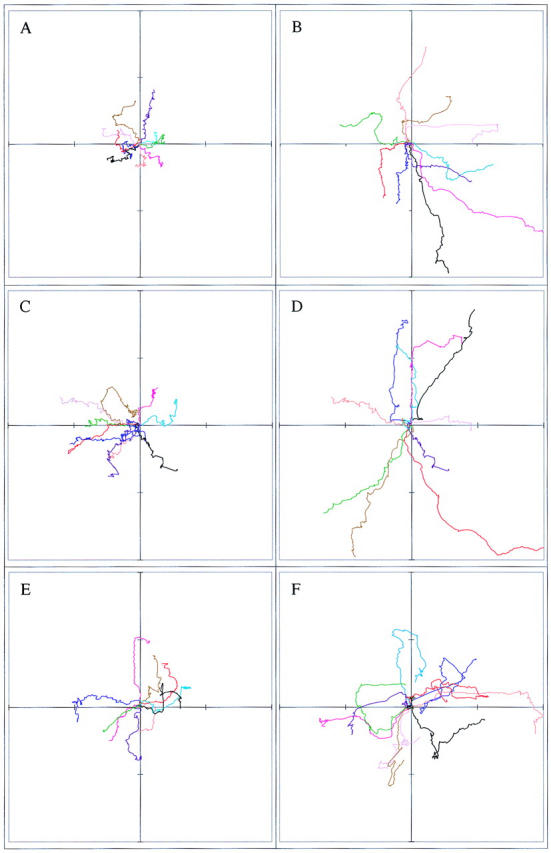
Pak1 kinase activity affects persistence of movement. Pak1 expression was induced by growth in tetracycline-free DME and the cells plated and tracked as in Fig. 5. The paths of ten randomly-chosen cells were plotted for each experimental group. The cells tracked in a, c, and e were grown in the presence of tetracycline; those in b, d, and f were grown in the absence of tetracycline. (a and b) Pak1L83,L86, (c and d) Pak1E423, and (e and f) Pak1R299. Distance traversed from the origin is graphed in μm, with each hashmark representing 30 μm.
Figure 7.
Pak1 kinase activity affects haptotaxis. Cells loaded into the top wells of a modified Boyden chamber and allowed to migrate in response to an immobilized collagen gradient. 18 h post-loading, the number of cells that traversed the membrane was quantified. Results shown are representative of three independent experiments, and are normalized to the results obtained for cells grown in the presence of tetracycline ± SE.
Pak1 Induces Phosphorylation of MLC at Serine 19
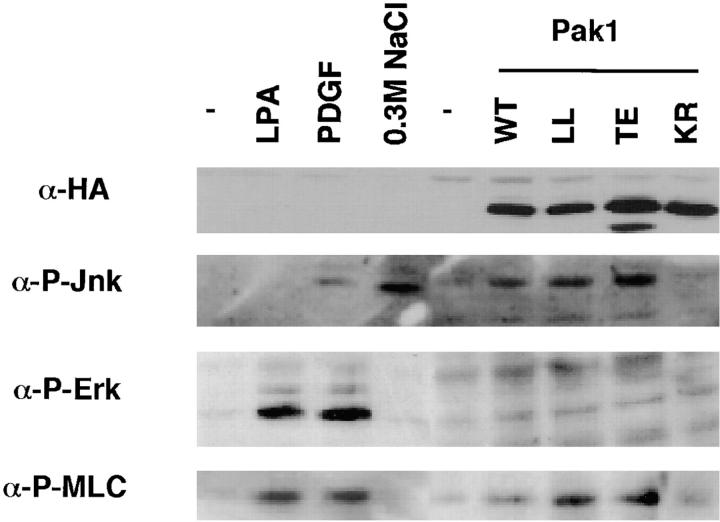
The activation of MLC by phosphorylation has been correlated with cell motility (Wilson et al., 1991, 1992; Matsumura et al., 1998), perhaps due to its role in retracting nondominant pseudopodia (Bailly et al., 1998b). Pak1 has been reported to affect myosin function in a variety of organisms (Brzeska et al., 1996, 1997; Lee et al., 1996; Wu et al., 1996), and mammalian Paks have been shown to phosphorylate MLC at serine 19 in vitro (Ramos et al., 1997; Chew et al., 1998; Van Eyk et al., 1998). We therefore asked whether Pak1 expression in fibroblasts results in increased MLC phosphorylation. For these studies we used an antibody that specifically recognizes the serine-19 phosphorylated form of MLC (Matsumura et al., 1998). Lysates from control cells, or cells expressing WT, activated, or kinase-dead forms of Pak1 were examined by immunoblot for protein expression, MAPK and SAPK activity, and phospho-MLC content. These studies show that activated forms of Pak1, like Rac1, induce activation of a SAPK (Jnk) but not a MAPK (Erk1) pathway (Fig. 8). These results are in agreement with most published studies, which show modest Jnk and p38 activation by activated forms of Pak (reviewed in Sells and Chernoff, 1997). As expected, control cells treated with growth-stimuli such as PDGF or LPA show Erk1 activation, while cells exposed to a hyperosmotic shock show Jnk activation, demonstrating that signaling pathways in the S2-6 cell lines are generally similar to those observed in most other fibroblasts. Phospho-MLC is apparent in growth-factor treated cells, as well as in cells expressing activated forms of Rac1 or Pak1, but not in control cells or cells expressing a kinase-dead form of Pak1. These results indicate that activated Pak1 induces MLC phosphorylation and that this phosphorylation is not mediated via downstream activation of Erk1.
Figure 8.
Activated Pak1 induces MLC phosphorylation in the absence of MAPK activation. Control NIH-3T3 cells or NIH-3T3 cells expressing various forms of Pak1 were starved overnight in media containing 0.5% CS, then treated with the indicated compounds (PDGF, 10 ng/ml or LPA 5 μg/ml) for 10 min. Immunoblots of cell lysates were probed with anti-HA (to detect transgene expression), and antibodies against the activated (phosphorylated) forms of Jnk, Erk, and MLC.
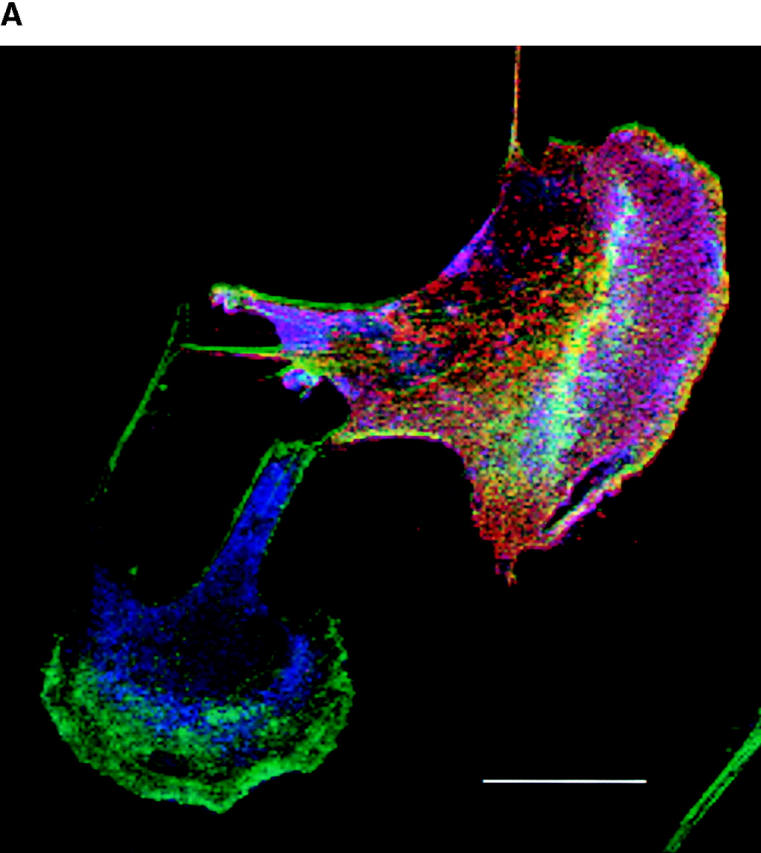
We also used the phospho-MLC antibody to localize activated MLC by immunofluorescence. Cells inducibly expressing an activated form of Pak1 were grown in the presence or absence of tetracycline for 16 h before fixation. These experiments show that cells expressing activated forms of Pak1 induce an accumulation and localization of phospho-MLC to the lamellipodia (Fig. 9). In nonexpressing cells, phospho-MLC levels are low and are found mainly in a perinuclear location (Fig. 9 a, left and right panels). In Pak1-expressing cells, the amount of total phospho-MLC is apparently significantly increased and a substantial fraction of this protein now localizes just beneath the leading edge of lamellipodia (Fig. 9 b, left and right panels). These results are consistent with a previous study which showed that phospho-MLC accumulates in lamellipodia in locomoting cells (Matsumura et al., 1998).
Figure 9.
Pak1-induced phosphorylated MLC localizes to leading edge lamellipodia and to stress fibers. Immunofluorescent staining of a stable clonal line expressing a constitutively active form of Pak1 (Pak1L83,L86) in the presence (+tet; row a) or absence (−tet; row b) of tetracycline was performed after starvation overnight in media containing 0.5% CS. Arrow indicates punctate staining in edge ruffle. MLC phosphorylation (P-MLC) was detected by a polyclonal antibody specific for phospho-MLC (left and right panels, blue stain). Phalloidin stained F-actin is shown in the middle and right panels (green stain) whereas Pak1 expression is shown in the right panel (red stain). Bar, 25 μm.
Discussion
The p21-activated kinases comprise a family of 62–68-kD serine/threonine kinases. These enzymes are catalytically activated by binding specifically to activated forms of Cdc42 and Rac1, two GTPases of the Rho subfamily, thus making Paks good candidate effectors for these signaling molecules (Lim et al., 1996; Sells and Chernoff, 1997). In fact, a number of the Paks have been implicated as the downstream effectors for several Cdc42 and Rac1 regulated signaling pathways including modulation of the actin cytoskeleton and establishment of cell polarity (Cvrckova et al., 1995; Marcus et al., 1995; Ottilie et al., 1995; Manser et al., 1997; Sells et al., 1997), processes that are required for cell motility. Although it has been shown recently that both Cdc42 and Rac1 regulate motility and invasiveness of epithelial cells (Hordijk et al., 1997; Keely et al., 1997), and that Rac1 modulates growth factor–driven chemotaxis in fibroblasts and macrophages (Anand-Apte et al., 1997; Allen et al., 1998) whether any of the Paks play a role in regulating these processes has not been established.
Pak1 has two types of effects on cell morphology, one related to its protein kinase activity and one that is kinase independent (Manser et al., 1997; Sells et al., 1997). We and others have previously shown that at least some of Pak's kinase-independent effects on the actin cytoskeleton are mediated by one or more SH3-containing proteins that associate with proline-rich regions located in the NH2-terminal half of Pak1 (Sells et al.; 1997, Daniels et al., 1998; Obermeier et al., 1998). These SH3-containing proteins include, but are not necessarily limited to, the adaptor Nck and the guanine-nucleotide exchange factor PIX and its relatives (Bokoch et al., 1996; Galisteo et al., 1996; Lu et al., 1997; Bagrodia et al., 1998; Manser et al., 1998). Because PIX is an activator of Rac1, Obermeier et al., have proposed that the kinase-independent activity of Paks on actin polymerization is mediated by Rac1 (Obermeier et al., 1998). In support of this model, they have shown that the kinase-independent effects of Pak3 on neurite extension in PC12 cells are partially inhibited by a dominant-negative from of Rac1. In contrast, we have argued that, in microinjected Swiss 3T3 cells, the kinase-independent effects of Pak1 are not inhibited by dominant-negative Rac1 (Sells et al., 1997). Possibly, cell-type differences, or the isoforms of Paks studied, account for these apparent discrepancies. However, it should be noted that less than half of Pak3's effects in PC12 cells are blocked by dominant-negative Rac1, that a nearly identical degree of inhibition is seen in control cells, and that dominant-negative Cdc42 has an even more inhibitory effect (Obermeier et al., 1998). Therefore, it is not clear if or how Pak3 functionally links Cdc42 to Rac1. Whatever the situation in PC12 cells, our present data show that dominant-negative Rac1 has no measurable effect on Pak1-driven actin reorganization in NIH-3T3 cells. Therefore, at least in cells of mesenchymal origin, the kinase-independent effects of Pak1 do not require Rac1.
As with cell morphology, the effects of Pak1 on cell movement also have a kinase-dependent and independent component. Overexpression of wild-type or mutant forms of Pak1 all increase cell movement, but protein kinase activity affects the persistence of these movements. Cells expressing kinase-dead Pak1 are characterized by multiple, randomly arrayed, lamellipodia, that continuously form and recycle. These cells move more quickly than controls, but do so in a haphazard manner, frequently and abruptly switching direction. In contrast, cells expressing wild-type or activated forms of Pak1 are characterized by a single lamellipodium at the leading edge. These cells move more quickly than controls, and also display increased persistence. As with activated Cdc42 and Rac1 (Keely et al., 1997), such cells also display increased haptotaxis towards a collagen gradient. These differences are likely to reflect the failure of cells expressing kinase-dead Pak1 to polarize F-actin appropriately. It is also possible that the increased motility of these cells is related to weakened adhesion to the substratum, as activated Pak1 induces aberrant formation of focal adhesion complexes (Allen et al., 1997; Manser et al., 1997).
The formation of membrane extensions such as pseudopodia is thought to be driven mainly by actin polymerization (Condeelis, 1993; Mitchison and Cramer, 1996). This process is evident in cells expressing either kinase-dead or kinase-active versions of Pak1. As mentioned above, these effects are likely to be mediated by interactions between the NH2-terminal, non-catalytic, domain of Pak1 and other proteins that affect actin polymerization. Although our data argue against the possibility that Rac1 acts downstream of Pak1, it is possible that other GTPases that affect actin organization, such as Arf6, might be activated by Pak1 (Radhakrishna et al., 1999). Activation of this or a similar GTPase might account for the kinase-independent ruffling induced by Pak1. Actin polymerization by itself, however, is insufficient for directed cell movement. In gradient-directed cell movement, one or more pseudopodia become dominant, while the others are suppressed, and these dominant pseudopodia are stabilized as leading edge lamellipodia, whose firm attachment to the substratum are thought to provide an anchorage point for contractile forces. The process of selection, generation, and propagation of a leading edge lamellipodium from a group of random pseudopodia is poorly understood, but may involve stabilization of filaments through the formation of strong focal contacts. In addition, nonadherent extensions may be actively suppressed once a leading edge lamellipodium is established (Bailly et al., 1998a; Wyckoff et al., 1998). In NIH-3T3 cells, expression of activated, but not kinase-dead, Pak1 results in large, polarized lamellipodia and persistent cell movement. Phospho-MLC is increased, both in the trailing edge and at the leading edge of the motile cells. A similar distribution has been noted previously in other motile cells (DeBiasio et al., 1996; Matsumura et al., 1998). Phosphorylation at the tail is thought to be responsible for pushing the cell body forward by tail contraction. The function of phosphorylated MLC at the leading edge is less clear, but may contribute to the membrane extensions at the leading edges, either by translocating actin filaments to this location or by suppressing lamellipodia formation in the absence of the reinforcement provided by stable focal contact (Goeckeleer and Wysolmerski, 1995; Welch et al., 1997; Bailly et al., 1998a; Matsumura et al., 1998; Wyckoff et al., 1998). According to this scenario, active Pak1 may concentrate at the incipient leading edge in resting cells, perhaps directed there by the exchange factor PIX, where it acts upon myosin II, augmenting the polarity of the cell. This might explain the effects of active Pak1 on directional cell movement. Whatever the exact function of myosin II in locomoting cells, our data are consistent with the notion that Pak1 has two types of effects on cell morphology and motility; a kinase-independent activity that affects actin polymerization, and a kinase-dependent function that increases phospho-MLC levels and stabilizes leading edge lamellipodia.
How does Pak1 affect the phosphorylation state of the MLC? The effect of Pak1 on MLC may be direct, as Pak1 has been shown to phosphorylate serine 19 of the MLC in vitro (Ramos et al., 1997; Chew et al., 1998; Van Eyk et al., 1998). Alternatively, Pak1 might induce MLC phosphorylation indirectly, via activation of MAPK or Rho kinase, both of which affect MLC kinase activity (Amano et al., 1996; Klemke et al., 1997). Although Paks are usually considered to be activators of SAPKs such as Jnk and p38, rather than of MAPKs such as Erk1 and -2 (Kyriakis and Avruch, 1996; Sells and Chernoff, 1997), Pak2 has recently been shown to phosphorylate Raf-1 (King et al., 1998). (The Pak isoform that phosphorylates Raf is actually Pak2, not Pak3 as stated in King et al., 1998. This error in nomenclature can be traced to mistakes in the GenBank annotations for γ-Pak, which is also known as Pak2 [see Sells and Chernoff, 1997].) This phosphorylation is required for efficient Raf-1 activation. In addition, Pak1 phosphorylates MEK on serine 298, increasing its affinity for its activator, Raf-1 (Frost et al., 1997). These data suggest that Pak1 activity positively regulates the MAPK pathway. As activation of MAPK is associated with increased MLC phosphorylation and cell movement (Klemke et al., 1997), this pathway might explain Pak1's effects on motility. However, in our inducible cell lines, neither wild-type nor mutant forms of Pak1 appreciably stimulate Erk1 or -2, while activated forms do modestly activate Jnk. Therefore, activation of MLC via MAPKs cannot explain our findings. Alternatively, since Pak1 is associated with PIX, a guanine-nucleotide exchange factor for Rac1 (Manser et al., 1998), Pak1 might activate Rho kinase through a GTPases cascade (Cdc42 to Rac1 to RhoA) (Nobes and Hall, 1995). However, coexpression of a dominant-negative form of Rac1 did not interfere with Pak1-induced cell polarity changes, making it unlikely that its motility effects are mediated through a known GTPase cascade. Therefore, if Pak2 activates Rok, it is not likely via downstream activation of Rac1 and RhoA. In this regard, it is interesting to note that fission yeast Pak1p has recently been shown to operate upstream of the Rok homologue Orb6p (Verde et al., 1998). Whether fission yeast Rho1p is interposed between Pak1p and Orb6p is not known, but these data do reinforce the notion that some of Pak's effects on myosin phosphorylation might be mediated by Rok. Therefore, at present we are uncertain if Pak1 directly phosphorylates MLC or does so indirectly through Rho, Rok, and/or MLCK. These possibilities may be distinguishable through the use of C3 toxin or dominant-negative forms of RhoA or Rok.
Acknowledgments
We thank Fumio Matsumura for his kind gift of anti-phospho-MLC antisera, Sybil Genther and Amanda Pfaff for technical assistance, Joshua Park for assistance with statistical analysis, and Peter Adams and Erica Golemis for review of the manuscript.
This work was supported by grants from the National Institutes of Health (RO1-GM54168) and the American Cancer Society (CB-189). J. Chernoff is a scholar of the Leukemia Society of America.
Abbreviations used in this paper
- CS
calf serum
- HA
hemagglutinin
- LPA
lysophosphatidic acid
- MAPK
mitogen-activated protein kinase
- MLC
myosin light chain
- Pak
p21-activated kinase
- PBS
phosphate-buffered saline
- PDGF
platelet-derived growth factor
- PI3
phosphatidyl inositol 3
- PIX
Pak-interacting exchange factor
- SH3
src-homology 3
- SAPK
stress-activated protein kinase
References
- Allen WE, Jones GE, Pollard JW, Ridley AJ. Rho, Rac, and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110:707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- Allen WE, Zicha D, Ridley AJ, Jones GE. A role for Cdc42 in macrophage chemotaxis. J Cell Biol. 1998;141:1147–1157. doi: 10.1083/jcb.141.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Anand-Apte B, Zetter B, Viswanathan A, Qiu R-g, Chen J, Ruggieri R, Symons M. PDGF and fibronectin-stimulated migration are differentially regulated by the Rac and ERK pathways. J Biol Chem. 1997;272:30688–30692. doi: 10.1074/jbc.272.49.30688. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Jordon KA, Van Aelst L, Cerione RA. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- Bailly M, Condeelis JS, Segall JE. Chemoattractant-induced lamellipod extension. Microscopy Res Tech. 1998a;43:433–443. doi: 10.1002/(SICI)1097-0029(19981201)43:5<433::AID-JEMT9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bailly M, Yan L, Whitesides GM, Condeelis JS, Segall JE. Regulation of protrusion shape and adhesion to the substratum during chemotactic responses of mammalian carcinoma cells. Exp Cell Res. 1998b;241:285–299. doi: 10.1006/excr.1998.4031. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Wang Y, Bohl BP, Sells MA, Quilliam LA, Knaus UG. Interaction of Nck adapter protein with p21-activated kinase (PAK1) J Biol Chem. 1996;271:25746–25749. doi: 10.1074/jbc.271.42.25746. [DOI] [PubMed] [Google Scholar]
- Brzeska H, Martin BM, Korn ED. The catalytic domain of Acanthamoeba myosin I heavy chain kinase. I. Identification and characterization following tryptic cleavage of the native enzyme. J Biol Chem. 1996;271:27049–27055. doi: 10.1074/jbc.271.43.27049. [DOI] [PubMed] [Google Scholar]
- Brzeska H, Knaus UG, Wang Z-Y, Bokoch GM, Korn ED. p21-activated kinase has substrate specificity similar to Acanthamoeba myosin I heavy chain kinase and activates Acanthamoebamyosin I. Proc Natl Acad Sci USA. 1997;94:1092–1095. doi: 10.1073/pnas.94.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cell by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew T-L, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (γ-PAK) Muscle Res Cell Motil. 1998;19:839–854. doi: 10.1023/a:1005417926585. [DOI] [PubMed] [Google Scholar]
- Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- Cramer LP, Mitchison TJ. Myosin is involved in postmitotic cell spreading. J Cell Biol. 1995;131:179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvrckova F, Manser E, Nasmyth K. Ste20-like protein kinases are required for localized cell growth and cytokinesis in budding yeast. Genes Dev. 1995;9:1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- Daniels RH, Hall PS, Bokoch GM. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO (Eur Mol Biol Organ) J. 1998;17:754–764. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBiasio RL, LaRocca GM, Post PL, Taylor DL. Myosin II transport, organization, and phosphorylation: evidence for cortical flow/ solation-contraction coupling during cytokinesis and cell locomotion. Mol Biol Cell. 1996;7:1259–1282. doi: 10.1091/mbc.7.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eby JJ, Holly SP, van Drogen F, Grishin AV, Peter M, Drubin DG, Blumer KJ. Actin cytoskeleton organization regulated by the PAK family of protein kinases. Curr Biol. 1998;8:967–970. doi: 10.1016/s0960-9822(98)00398-4. [DOI] [PubMed] [Google Scholar]
- Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw PE, Cobb MH. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO (Eur Mol Biol Organ) J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galisteo ML, Chernoff J, Su Y-C, Skolnik EY, Schlessinger J. The adaptor protein Nck links tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- Goeckeleer MZ, Wysolmerski RB. Myosin light chain kinase–regulated endothelial cell contraction: The relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol. 1995;130:613–627. doi: 10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordijk P, ten Klooster J, van der Kammen R, Michiels F, Oomen L, Collard J. Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- Joneson T, McDonough M, Bar-Sagi D, Van Aelst L. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 1996;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall MS. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallegher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activate by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenström P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65Pak and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Leberer E, Dignard D, Harcus D, Thomas DY, Whiteway M. The protein kinase homolgue Ste20p is required to link the yeast pheromone response G-protein βγ subunits to downstream signalling components. EMBO (Eur Mol Biol Organ) J. 1992;11:4815–4824. doi: 10.1002/j.1460-2075.1992.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SF, Egelhoff TT, Mahasneh A, Cote GP. Cloning and characterization of a Dictyosteliummyosin I heavy chain kinase activated by Cdc42 and Rac. J Biol Chem. 1996;271:27044–27048. doi: 10.1074/jbc.271.43.27044. [DOI] [PubMed] [Google Scholar]
- Lim L, Manser E, Leung T, Hall C. Regulation of phosphorylation pathways by p21 GTPases: the p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways. Eur J Biochem. 1996;242:171–185. doi: 10.1111/j.1432-1033.1996.0171r.x. [DOI] [PubMed] [Google Scholar]
- Lu W, Katz S, Gupta R, Mayer BJ. Activation of Pak by membrane localization mediated by Nck SH3 domain. Curr Biol. 1997;7:88–94. doi: 10.1016/s0960-9822(06)00052-2. [DOI] [PubMed] [Google Scholar]
- Manser E, Huang H-Y, Loo T-H, Chen XQ, Leung T, Lim L. Expression of constitutively active α-Pak reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Loo T-H, Koh C-G, Zhao Z-S, Tan I, Leung T, Lim L. A family of guanine nucleotide exchange factors (PIXs) is directly coupled to the p21(Cdc42 and Rac)-activated kinase αPAK. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Marcus S, Polverino A, Chang E, Robbins D, Cobb MH, Wigler MH. Shk1, a homolog of the Saccharomyces cerevisiae Ste20 and mammalian p65PAK protein kinases, is a component of a Ras/Cdc42 signaling module in the fission yeast Schizosaccharomyces pombe. . Proc Natl Acad Sci USA. 1995;92:6180–6184. doi: 10.1073/pnas.92.13.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F, Ono S, Yamakita Y, Totsukawa G, Yamashiro S. Specific localization of serine 19 phosphorylated myosin II during cell locomotion and mitosis in cultured cells. J Cell Biol. 1998;140:119–129. doi: 10.1083/jcb.140.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multi-molecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Obermeier A, Ahmed S, Manser E, Yen SC, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO (Eur Mol Biol Organ) J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottilie S, Miller PJ, Johnson DI, Creasy C, Sells MA, Bagrodia S, Forsburg SL, Chernoff J. Fission yeast pak1+ encodes a protein kinase that interacts with Cdc42p and is involved in the control of cell polarity and mating. EMBO (Eur Mol Biol Organ) J. 1995;14:5908–5919. doi: 10.1002/j.1460-2075.1995.tb00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson JG. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- Ramos E, Wysolmerski RB, Masaracchia RA. Myosin phosphorylation by human cdc42-dependent S6/H4 kinase/γPAK from placenta and lymphoid cells. Recept Signal Transduct. 1997;7:99–110. [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factors-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/ hepatocyte growth factor response by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander EE, van Delft S, ten Klooster JP, Reid T, van der Kammen RA, Michiels F, Collard JG. Matrix-dependent Tiam1/Rac signaling in epithelial cells promotes either cell–cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J Cell Biol. 1998;143:1385–1398. doi: 10.1083/jcb.143.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Chernoff J. Epitope tagging vectors for eukaryotic protein production. Gene. 1995;152:187–189. doi: 10.1016/0378-1119(94)00685-l. [DOI] [PubMed] [Google Scholar]
- Sells MA, Chernoff J. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 1997;7:162–167. doi: 10.1016/S0962-8924(97)01003-9. [DOI] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Sells MA, Barratt JT, Caviston J, Ottilie S, Leberer E, Chernoff J. Characterization of Pak2p, a pleckstrin homology domain-containing, p21-activated protein kinase from fission yeast. J Biol Chem. 1998;273:18490–18498. doi: 10.1074/jbc.273.29.18490. [DOI] [PubMed] [Google Scholar]
- Sheetz MP. Cell migration by graded attachment to substrates and contraction. Semin Cell Biol. 1994;5:149–155. doi: 10.1006/scel.1994.1019. [DOI] [PubMed] [Google Scholar]
- Shockett P, DiFilippantonio M, Hellman N, Schatz DG. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci USA. 1995;92:6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Van Eyk JE, Arrell DK, Foster DB, Strauss JD, Heinonen TY, Furmaniak-Kazmierczak E, Cote GP, Mak AS. Different molecular mechanisms for Rho family GTPase-dependent, Ca2+- independent contraction of smooth muscle. J Biol Chem. 1998;273:23433–23439. doi: 10.1074/jbc.273.36.23433. [DOI] [PubMed] [Google Scholar]
- Verde F, Mata J, Nurse P. Fission yeast cell morphogenesis: identification of new genes and analysis of their role during the cell cycle. J Cell Biol. 1995;131:1529–1538. doi: 10.1083/jcb.131.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F, Wiley DJ, Nurse P. Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc Natl Acad Sci USA. 1998;95:7526–7531. doi: 10.1073/pnas.95.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch MD, Mallavarapu A, Rosenblatt J, Mitchison TJ. Actin dynamics in vivo. Curr Opin Cell Biol. 1997;9:54–61. doi: 10.1016/s0955-0674(97)80152-4. [DOI] [PubMed] [Google Scholar]
- Westwick JK, Lambert QT, Clark GJ, Symons M, Van Aelst L, Pestell RG, Der CJ. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AK, Gorgas G, Claypool WD, de Lanerolle P. An increase or a decrease in myosin II phosphorylation inhibits macrophage motility. J Cell Biol. 1991;114:277–283. doi: 10.1083/jcb.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AK, Pollenz RS, Chisholm RL, de Lanerolle P. The role of myosin I and II in cell motility. Cancer Metastasis Rev. 1992;11:79–91. doi: 10.1007/BF00047605. [DOI] [PubMed] [Google Scholar]
- Wissmann A, Ingles J, McGhee JD, Mains PE. Caenorhabditis elegansLET-502 is related to the Rho-binding kinases and human myotonic dystrophy kinase and interacts genetically with a homolog of the regulatory subunit of smooth muscle myosin phosphatase to affect cell shape. Genes Dev. 1997;11:409–422. doi: 10.1101/gad.11.4.409. [DOI] [PubMed] [Google Scholar]
- Wu C, Lee S-F, Furmaniak-Kazmierczak E, Côte GP, Thomas DY, Leberer E. Activation of myosin-I by members of the Ste20p protein kinase family. J Biol Chem. 1996;271:31787–31790. doi: 10.1074/jbc.271.50.31787. [DOI] [PubMed] [Google Scholar]
- Wu C, Lytvyn V, Thomas DY, Leberer E. The phosphorylation site for Ste20p-like protein kinases is essential for the function of myosin-I in yeast. J Biol Chem. 1997;272:30623–30626. doi: 10.1074/jbc.272.49.30623. [DOI] [PubMed] [Google Scholar]
- Wyckoff JB, Insel L, Khazaie K, Lichtner RB, Condeelis JS, Segall JE. Suppression of ruffling by the EGF receptor in chemotactic cells. Exp Cell Res. 1998;242:100–109. doi: 10.1006/excr.1998.4093. [DOI] [PubMed] [Google Scholar]
- Yang P, Sanjay S, Kansra, Pimental RA, Gilbreth M, Marcus S. Cloning and characterization of shk2, a gene encoding a novel p21-activated protein kinase from fission yeast. J Biol Chem. 1998;273:18481–18489. doi: 10.1074/jbc.273.29.18481. [DOI] [PubMed] [Google Scholar]