Abstract
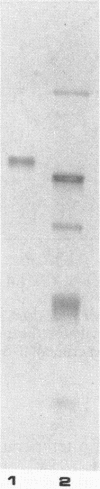
Pyrococcus furiosus is a strictly anaerobic hyperthermophilic archaebacterium with an optimal growth temperature of about 100 degrees C. When this organism was grown in the presence of certain complex carbohydrates, the production of several amylolytic enzymes was noted. These enzymes included an alpha-glucosidase that was located in the cell cytoplasm. This alpha-glucosidase has been purified 310-fold and corresponded to a protein band of 125 kilodaltons as resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The enzyme exhibited optimum activity at pH 5.0 to 6.0 and over a temperature range of 105 to 115 degrees C. Kinetic analysis conducted at 108 degrees C revealed hydrolysis of the substrates p-nitrophenyl-alpha-D-glucopyranoside (PNPG), methyl-alpha-D-glucopyranoside, maltose, and isomaltose. Trace activity was detected towards p-nitrophenyl-beta-D-glucopyranoside, and no activity could be detected towards starch or sucrose. Inhibition studies conducted at 108 degrees C with PNPG as the substrate and maltose as the inhibitor yielded a Ki for maltose of 14.3 mM. Preincubation for 30 min at 98 degrees C in 100 mM dithiothreitol and 1.0 M urea had little effect on enzyme activity, whereas preincubation in 1.0% sodium dodecyl sulfate and 1.0 M guanidine hydrochloride resulted in significant loss of enzyme activity. Purified alpha-glucosidase from P. furiosus exhibited remarkable thermostability; incubation of the enzyme at 98 degrees C resulted in a half life of nearly 48 h.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antranikian G., Herzberg C., Gottschalk G. Production of Thermostable alpha-Amylase, Pullulanase, and alpha-Glucosidase in Continuous Culture by a New Clostridium Isolate. Appl Environ Microbiol. 1987 Jul;53(7):1668–1673. doi: 10.1128/aem.53.7.1668-1673.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono S., Bryant F. O., Adams M. W. A novel and remarkably thermostable ferredoxin from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Bacteriol. 1989 Jun;171(6):3433–3439. doi: 10.1128/jb.171.6.3433-3439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. H., Kelly R. M. Cultivation Techniques for Hyperthermophilic Archaebacteria: Continuous Culture of Pyrococcus furiosus at Temperatures near 100 degrees C. Appl Environ Microbiol. 1989 Aug;55(8):2086–2088. doi: 10.1128/aem.55.8.2086-2088.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant F. O., Adams M. W. Characterization of hydrogenase from the hyperthermophilic archaebacterium, Pyrococcus furiosus. J Biol Chem. 1989 Mar 25;264(9):5070–5079. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Shen G. J., Zeikus J. G. Differential amylosaccharide metabolism of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. J Bacteriol. 1985 Dec;164(3):1153–1161. doi: 10.1128/jb.164.3.1153-1161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madi E., Antranikian G., Ohmiya K., Gottschalk G. Thermostable amylolytic enzymes from a new clostridium isolate. Appl Environ Microbiol. 1987 Jul;53(7):1661–1667. doi: 10.1128/aem.53.7.1661-1667.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihl T. D., Schicho R. N., Kelly R. M., Maier R. J. Characterization of hydrogen-uptake activity in the hyperthermophile Pyrodictium brockii. Proc Natl Acad Sci U S A. 1989 Jan;86(1):138–141. doi: 10.1073/pnas.86.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Yuki T., Kishigami T., Abe S. Purification and properties of extracellular alpha-glucosidase of a thermophile, Bacillus thermoglucosidus KP 1006. Biochim Biophys Acta. 1976 Sep 14;445(2):386–397. doi: 10.1016/0005-2744(76)90092-9. [DOI] [PubMed] [Google Scholar]