Abstract
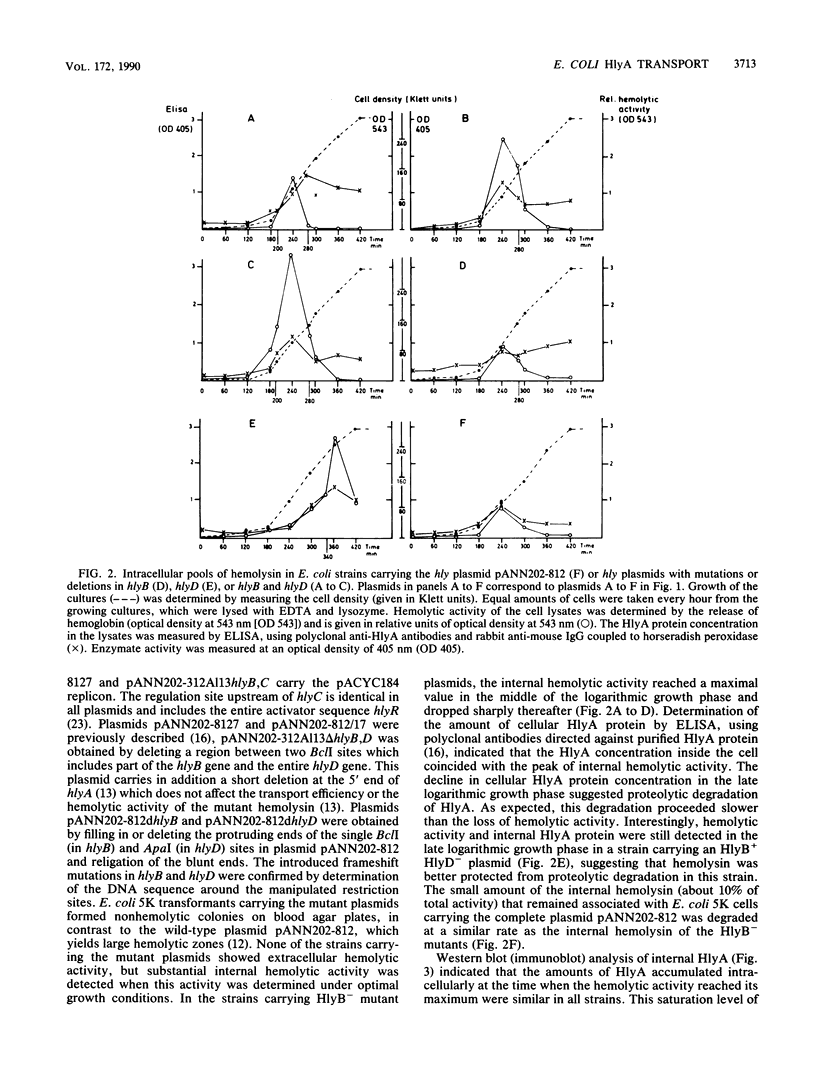
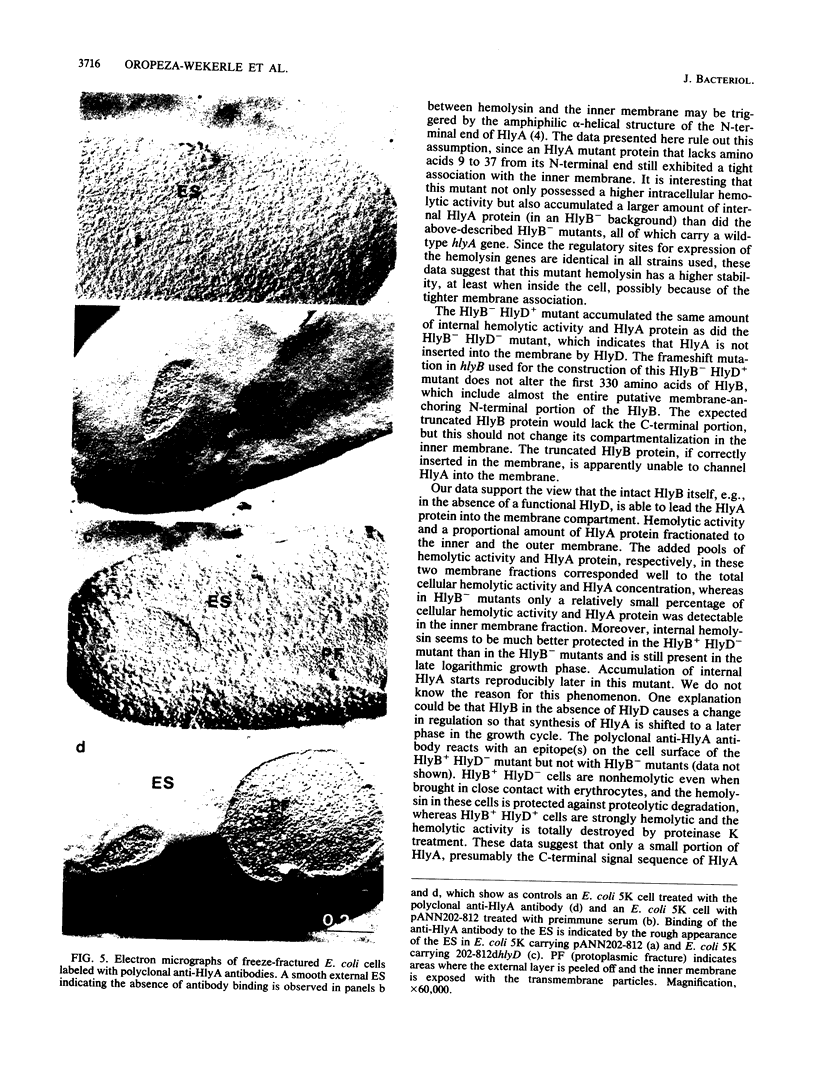
Hemolysin plasmids were constructed with mutations in hlyB, hlyD, or both transport genes. The localization of hemolysin activity and HlyA protein in these mutants was analyzed by biochemical and immunological methods. It was found that mutants defective in hlyB accumulated internal hemolysin, part of which was associated with the inner membrane and was degraded in the late logarithmic growth phase. In an HlyB+ HlyD- mutant, hemolysin was predominantly localized in the membrane compartment. Labeling of these Escherichia coli cells with anti-HlyA antibody indicated that part of HlyA, presumably the C-terminal end but not the pore-forming domains, was already transported to the cellular surface. This finding suggests that HlyB is able to recognize the C-terminal signal of the HlyA protein and to initiate its translocation across the membranes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baty D., Lloubès R., Geli V., Lazdunski C., Howard S. P. Extracellular release of colicin A is non-specific. EMBO J. 1987 Aug;6(8):2463–2468. doi: 10.1002/j.1460-2075.1987.tb02526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb K., Vogel M., Wagner W., Goebel W. Alkaline phosphatase which lacks its own signal sequence becomes enzymatically active when fused to N-terminal sequences of Escherichia coli haemolysin (HlyA). Mol Gen Genet. 1987 Jun;208(1-2):88–93. doi: 10.1007/BF00330427. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J. H., Endicott J. A., Juranka P. F., Henderson G., Sarangi F., Deuchars K. L., Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986 Dec 4;324(6096):485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- Härtlein M., Schiessl S., Wagner W., Rdest U., Kreft J., Goebel W. Transport of hemolysin by Escherichia coli. J Cell Biochem. 1983;22(2):87–97. doi: 10.1002/jcb.240220203. [DOI] [PubMed] [Google Scholar]
- Koronakis V., Koronakis E., Hughes C. Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO J. 1989 Feb;8(2):595–605. doi: 10.1002/j.1460-2075.1989.tb03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D., Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Jarchau T., Benz R., Goebel W. The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet. 1988 Nov;214(3):553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Vogel M., Goebel W. Mutations affecting activity and transport of haemolysin in Escherichia coli. Mol Gen Genet. 1987 Feb;206(2):238–245. doi: 10.1007/BF00333579. [DOI] [PubMed] [Google Scholar]
- Mackman N., Baker K., Gray L., Haigh R., Nicaud J. M., Holland I. B. Release of a chimeric protein into the medium from Escherichia coli using the C-terminal secretion signal of haemolysin. EMBO J. 1987 Sep;6(9):2835–2841. doi: 10.1002/j.1460-2075.1987.tb02580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989 Aug 3;340(6232):400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- Oropeza-Wekerle R. L., Müller E., Kern P., Meyermann R., Goebel W. Synthesis, inactivation, and localization of extracellular and intracellular Escherichia coli hemolysins. J Bacteriol. 1989 May;171(5):2783–2788. doi: 10.1128/jb.171.5.2783-2788.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Torrisi M. R., Kachar B. Freeze-fracture cytochemistry: localization of wheat-germ agglutinin and concanavalin A binding sites on freeze-fractured pancreatic cells. J Cell Biol. 1981 Nov;91(2 Pt 1):361–372. doi: 10.1083/jcb.91.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlner J., Halter R., Beyreuther K., Meyer T. F. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. 1987 Jan 29-Feb 4Nature. 325(6103):458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase. EMBO J. 1984 Oct;3(10):2393–2397. doi: 10.1002/j.1460-2075.1984.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Thom J. R. Export of protein: a biochemical view. Annu Rev Microbiol. 1987;41:507–541. doi: 10.1146/annurev.mi.41.100187.002451. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel M., Hess J., Then I., Juarez A., Goebel W. Characterization of a sequence (hlyR) which enhances synthesis and secretion of hemolysin in Escherichia coli. Mol Gen Genet. 1988 Apr;212(1):76–84. doi: 10.1007/BF00322447. [DOI] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert C., Ryter A., Pugsley A. P. Cloning and expression in Escherichia coli of the Klebsiella pneumoniae genes for production, surface localization and secretion of the lipoprotein pullulanase. EMBO J. 1987 Nov;6(11):3531–3538. doi: 10.1002/j.1460-2075.1987.tb02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




