Abstract
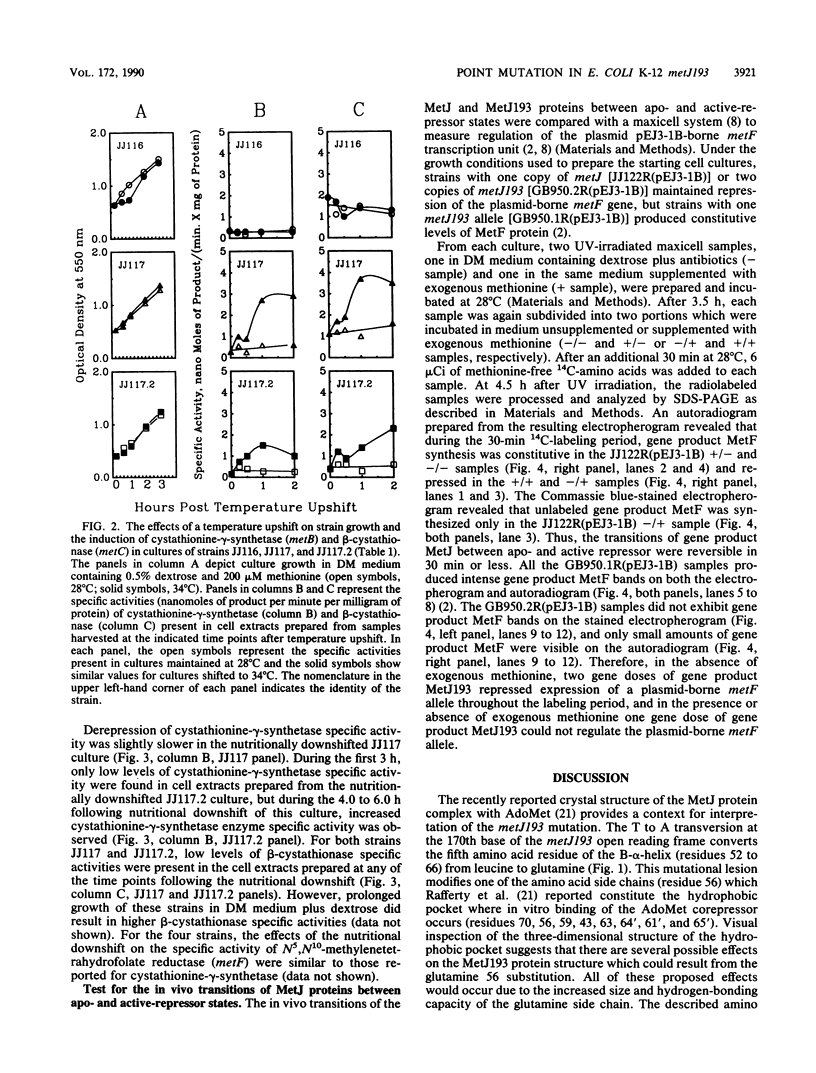
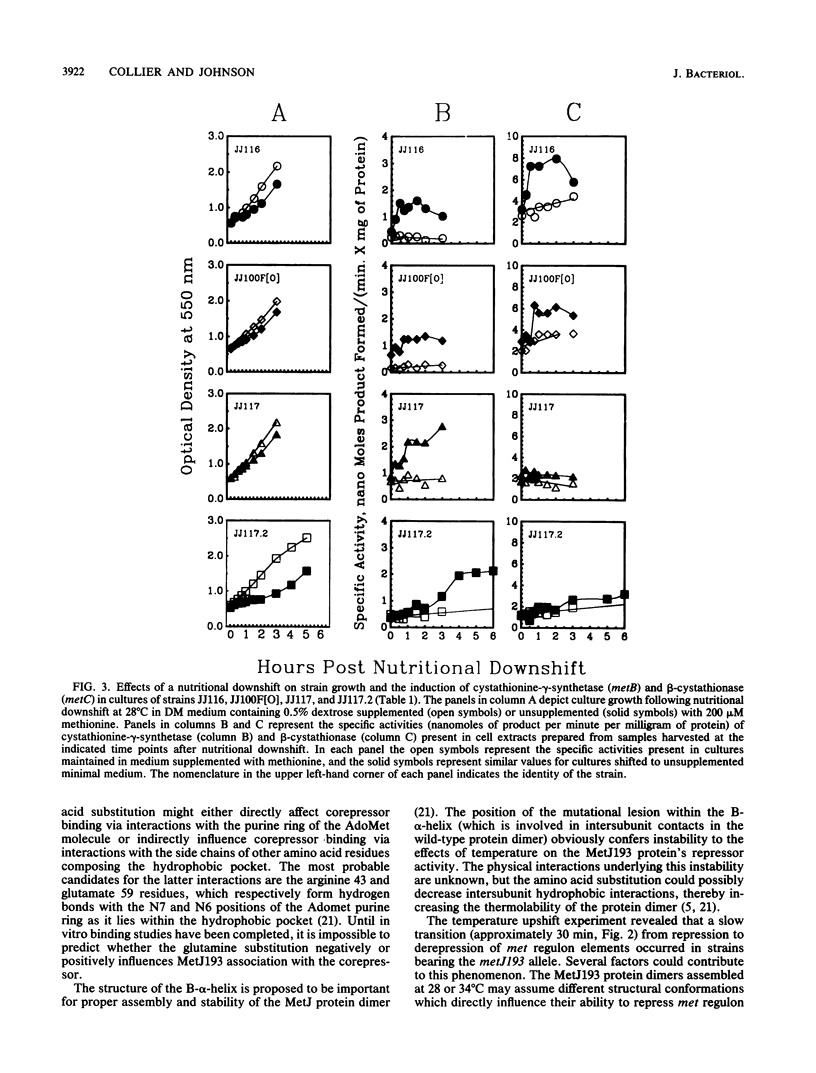
The metJ193 allele encodes one of two identified temperature-sensitive Escherichia coli K-12 met repressors. The nucleotide sequence of the metJ193 allele was determined. The point mutation was a T to A transversion at base 170 of the metJ193 open reading frame and resulted in the substitution of leucine by glutamine at the 56th amino acid residue of the MetJ193 protein. The mutational lesion altered the hydrophobic pocket responsible for in vitro binding of the corepressor S-adenosylmethionine by wild-type MetJ. MetJ193 protein formed at the permissive temperature (28 degrees C) allowed slow derepression of met regulon expression when cultures were shifted to the nonpermissive temperature (34 degrees C). When 28 degrees C cultures of strains bearing two metJ193 alleles were transferred from methionine-containing medium to minimal medium, derepression of met regulon expression did not occur quickly enough to avoid a lag in growth due to the methionine deprivation. The inability of the MetJ193 protein to easily accomplish transition between apo- and active-repressor conformations was also demonstrated by using a maxicell system to study expression of a plasmid-borne copy of the E. coli metF transcription unit. These results confirm the importance of the leucine 56 residue for the structure and function in vivo of the wild-type MetJ protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala G. A., Collier C. D., Emmett M. R., Johnson J. R. Characterization of two mutant metJ proteins with reduced, temperature-dependent capacity to regulate Escherichia coli K-12 met regulon elements. J Bacteriol. 1989 Jul;171(7):4095–4099. doi: 10.1128/jb.171.7.4095-4099.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfaiza J., Guillou Y., Margarita D., Perrin D., Saint Girons I. Operator-constitutive mutations of the Escherichia coli metF gene. J Bacteriol. 1987 Feb;169(2):670–674. doi: 10.1128/jb.169.2.670-674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfaiza J., Parsot C., Martel A., de la Tour C. B., Margarita D., Cohen G. N., Saint-Girons I. Evolution in biosynthetic pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci U S A. 1986 Feb;83(4):867–871. doi: 10.1073/pnas.83.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchange N., Zakin M. M., Ferrara P., Saint-Girons I., Park I., Tran S. V., Py M. C., Cohen G. N. Structure of the metJBLF cluster in Escherichia coli K12. Sequence of the metB structural gene and of the 5'- and 3'-flanking regions of the metBL operon. J Biol Chem. 1983 Dec 25;258(24):14868–14871. [PubMed] [Google Scholar]
- Emmett M. R., Johnson J. R. Control of metF gene expression in maxicell preparations of Escherichia coli K-12: reversible action of the metJ protein and effect of vitamin B12. J Bacteriol. 1986 Dec;168(3):1491–1494. doi: 10.1128/jb.168.3.1491-1494.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Hunter J. S., Coch E. H. Properties of metK mutants of Escherichia coli K-12. J Bacteriol. 1973 Jul;115(1):57–67. doi: 10.1128/jb.115.1.57-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Greene R. C., Krueger J. H. Isolation and characterization of specialized lambda transducing bacteriophage carrying the metBJF methionine gene cluster. J Bacteriol. 1977 Sep;131(3):795–800. doi: 10.1128/jb.131.3.795-800.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T. W., Hindenach B. R., Greene R. C. Regulation of in vivo transcription of the Escherichia coli K-12 metJBLF gene cluster. J Bacteriol. 1986 Mar;165(3):671–677. doi: 10.1128/jb.165.3.671-677.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. H., Johnson J. R., Greene R. C., Dresser M. Structural studies of lambda transducing bacteriophage carrying bacterial deoxyribonucleic acid from the metBJLF region of the Escherichia coli chromosome. J Bacteriol. 1981 Aug;147(2):612–621. doi: 10.1128/jb.147.2.612-621.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Liljestrand-Golden C. A., Johnson J. R. Physical organization of the metJB component of the Escherichia coli K-12 metJBLF gene cluster. J Bacteriol. 1984 Feb;157(2):413–419. doi: 10.1128/jb.157.2.413-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfioletti G., Schneider C. A new and fast method for preparing high quality lambda DNA suitable for sequencing. Nucleic Acids Res. 1988 Apr 11;16(7):2873–2884. doi: 10.1093/nar/16.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli S., Mevarech M., Ron E. Z. Regulatory region of the metA gene of Escherichia coli K-12. J Bacteriol. 1984 Dec;160(3):1158–1162. doi: 10.1128/jb.160.3.1158-1162.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. E., Manfield I., Parsons I., Davidson B. E., Rafferty J. B., Somers W. S., Margarita D., Cohen G. N., Saint-Girons I., Stockley P. G. Cooperative tandem binding of met repressor of Escherichia coli. Nature. 1989 Oct 26;341(6244):711–715. doi: 10.1038/341711a0. [DOI] [PubMed] [Google Scholar]
- Rafferty J. B., Somers W. S., Saint-Girons I., Phillips S. E. Three-dimensional crystal structures of Escherichia coli met repressor with and without corepressor. Nature. 1989 Oct 26;341(6244):705–710. doi: 10.1038/341705a0. [DOI] [PubMed] [Google Scholar]
- Saint-Girons I., Belfaiza J., Guillou Y., Perrin D., Guiso N., Bârzu O., Cohen G. N. Interactions of the Escherichia coli methionine repressor with the metF operator and with its corepressor, S-adenosylmethionine. J Biol Chem. 1986 Aug 15;261(23):10936–10940. [PubMed] [Google Scholar]
- Saint-Girons I., Duchange N., Cohen G. N., Zakin M. M. Structure and autoregulation of the metJ regulatory gene in Escherichia coli. J Biol Chem. 1984 Nov 25;259(22):14282–14285. [PubMed] [Google Scholar]
- Saint-Girons I., Duchange N., Zakin M. M., Park I., Margarita D., Ferrara P., Cohen G. N. Nucleotide sequence of metF, the E. coli structural gene for 5-10 methylene tetrahydrofolate reductase and of its control region. Nucleic Acids Res. 1983 Oct 11;11(19):6723–6732. doi: 10.1093/nar/11.19.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Girons I., Parsot C., Zakin M. M., Bârzu O., Cohen G. N. Methionine biosynthesis in Enterobacteriaceae: biochemical, regulatory, and evolutionary aspects. CRC Crit Rev Biochem. 1988;23 (Suppl 1):S1–42. doi: 10.3109/10409238809083374. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Shoeman R., Coleman T., Redfield B., Greene R. C., Smith A. A., Saint-Girons I., Brot N., Weissbach H. Regulation of methionine synthesis in Escherichia coli: effect of metJ gene product and S-adenosylmethionine on the in vitro expression of the metB, metL and metJ genes. Biochem Biophys Res Commun. 1985 Dec 17;133(2):731–739. doi: 10.1016/0006-291x(85)90965-9. [DOI] [PubMed] [Google Scholar]
- Shoeman R., Redfield B., Coleman T., Greene R. C., Smith A. A., Brot N., Weissbach H. Regulation of methionine synthesis in Escherichia coli: Effect of metJ gene product and S-adenosylmethionine on the expression of the metF gene. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3601–3605. doi: 10.1073/pnas.82.11.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. A., Greene R. C., Kirby T. W., Hindenach B. R. Isolation and characterization of the product of the methionine-regulatory gene metJ of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6104–6108. doi: 10.1073/pnas.82.18.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treat M. L., Weaver M. L., Emmett M. R., Johnson J. R. Mutagenesis of the metJBLF gene cluster with transposon Tn5: localization of the metF transcription unit. Mol Gen Genet. 1984;193(2):370–375. doi: 10.1007/BF00330695. [DOI] [PubMed] [Google Scholar]