Abstract
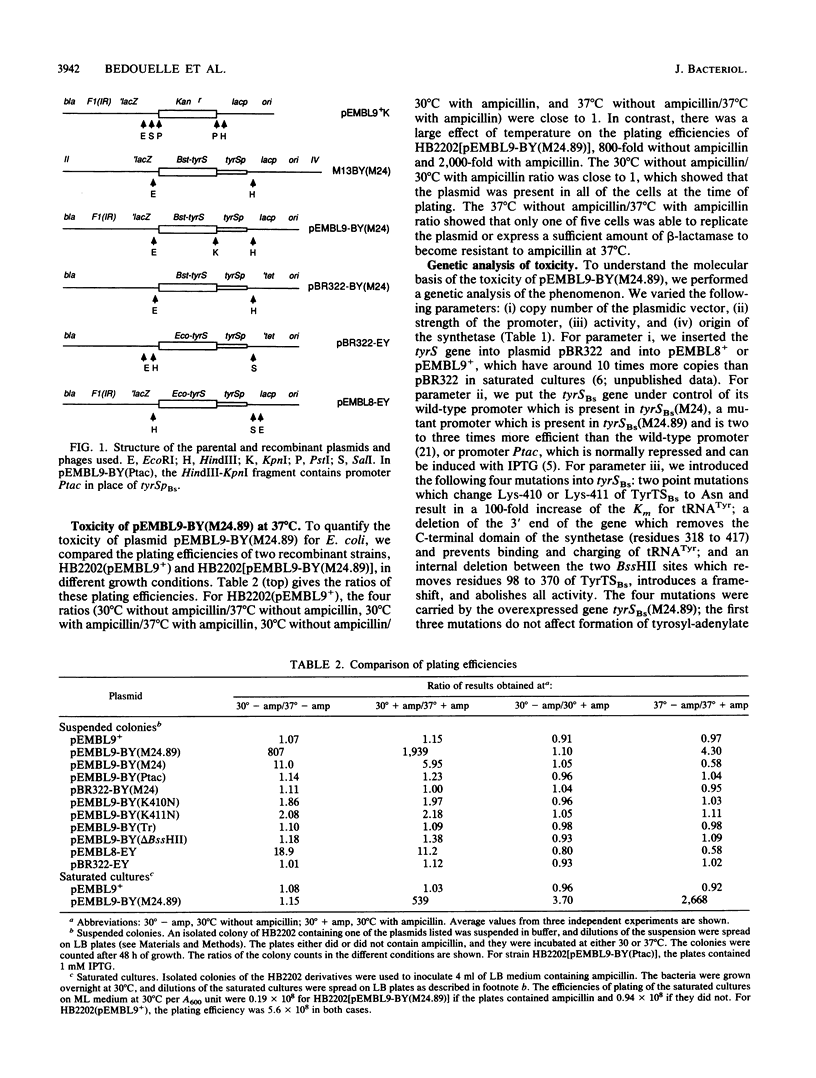
The tyrS genes from Escherichia coli and Bacillus stearothermophilus were toxic to E. coli when they were carried by plasmids with very high copy numbers (pEMBL8 and pEMBL9). We quantified this effect by comparing the efficiencies of plating of E. coli derivatives harboring recombinant plasmids in various experimental conditions. The toxicity was apparent at both 30 and 37 degrees C. It increased with the growth temperature, the strength of the tyrS promoter, and the copy number of the plasmidic vector. Two- to threefold enhancement of tyrS expression raised the toxicity 300-fold. Point mutations in tyrS that prevent interaction between its product, tyrosyl-tRNA synthetase, and tRNA(Tyr) but do not alter the rate of formation of tyrosyl-adenylate abolished the toxicity. Thus, the toxic effect was due to high cellular levels of synthetase activity. At 30 degrees C, the cellular concentration of tyrosyl-tRNA synthetase reached 55% of that of soluble proteins and led to decreased beta-galactosidase stability. We discuss possible causes of this toxic effect and describe its applications to the study of the recognition and interaction between the synthetase and tRNA(Tyr).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker D. G., Bruton C. J., Winter G. The tyrosyl-tRNA synthetase from Escherichia coli. Complete nucleotide sequence of the structural gene. FEBS Lett. 1982 Dec 27;150(2):419–423. doi: 10.1016/0014-5793(82)80781-3. [DOI] [PubMed] [Google Scholar]
- Barker D. G. Cloning and amplified expression of the tyrosyl-tRNA synthetase genes of Bacillus stearothermophilus and Escherichia coli. Eur J Biochem. 1982 Jul;125(2):357–360. doi: 10.1111/j.1432-1033.1982.tb06691.x. [DOI] [PubMed] [Google Scholar]
- Bedouelle H., Duplay P. Production in Escherichia coli and one-step purification of bifunctional hybrid proteins which bind maltose. Export of the Klenow polymerase into the periplasmic space. Eur J Biochem. 1988 Feb 1;171(3):541–549. doi: 10.1111/j.1432-1033.1988.tb13823.x. [DOI] [PubMed] [Google Scholar]
- Bedouelle H., Winter G. A model of synthetase/transfer RNA interaction as deduced by protein engineering. 1986 Mar 27-Apr 2Nature. 320(6060):371–373. doi: 10.1038/320371a0. [DOI] [PubMed] [Google Scholar]
- Dente L., Cortese R. pEMBL: a new family of single-stranded plasmids for sequencing DNA. Methods Enzymol. 1987;155:111–119. doi: 10.1016/0076-6879(87)55011-x. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. Dissection of the structure and activity of the tyrosyl-tRNA synthetase by site-directed mutagenesis. Biochemistry. 1987 Dec 15;26(25):8031–8037. doi: 10.1021/bi00399a001. [DOI] [PubMed] [Google Scholar]
- Fütterer J., Gordon K., Pfeiffer P., Hohn T. The instability of a recombinant plasmid, caused by a prokaryotic-like promoter within the eukaryotic insert, can be alleviated by expression of antisense RNA. Gene. 1988 Jul 15;67(1):141–145. doi: 10.1016/0378-1119(88)90018-2. [DOI] [PubMed] [Google Scholar]
- Hagan C. E., Warren G. J. Viability of palindromic DNA is restored by deletions occurring at low but variable frequency in plasmids of Escherichia coli. Gene. 1983 Oct;24(2-3):317–326. doi: 10.1016/0378-1119(83)90092-6. [DOI] [PubMed] [Google Scholar]
- Hall B., Gallant J. Defective translation in RC - cells. Nat New Biol. 1972 May 31;237(74):131–135. doi: 10.1038/newbio237131a0. [DOI] [PubMed] [Google Scholar]
- Hermann M., Bedouelle H. A method for monitoring double restriction cuts within a polylinker. Res Microbiol. 1990 Feb;141(2):187–189. doi: 10.1016/0923-2508(90)90028-o. [DOI] [PubMed] [Google Scholar]
- Labouze E., Bedouelle H. Structural and kinetic bases for the recognition of tRNATyr by tyrosyl-tRNA synthetase. J Mol Biol. 1989 Feb 20;205(4):729–735. doi: 10.1016/0022-2836(89)90317-3. [DOI] [PubMed] [Google Scholar]
- Martin B., Alloing G., Boucraut C., Claverys J. P. The difficulty of cloning Streptococcus pneumoniae mal and ami loci in Escherichia coli: toxicity of malX and amiA gene products. Gene. 1989 Aug 15;80(2):227–238. doi: 10.1016/0378-1119(89)90287-4. [DOI] [PubMed] [Google Scholar]
- Miller K. W., Evans R. J., Eisenberg S. P., Thompson R. C. Secretory leukocyte protease inhibitor binding to mRNA and DNA as a possible cause of toxicity to Escherichia coli. J Bacteriol. 1989 Apr;171(4):2166–2172. doi: 10.1128/jb.171.4.2166-2172.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueber D., Bujard H. Transcription from efficient promoters can interfere with plasmid replication and diminish expression of plasmid specified genes. EMBO J. 1982;1(11):1399–1404. doi: 10.1002/j.1460-2075.1982.tb01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R., Hoben P., Sumner-Smith M., Uemura H., Watson L., Söll D. Accuracy of in vivo aminoacylation requires proper balance of tRNA and aminoacyl-tRNA synthetase. Science. 1988 Dec 16;242(4885):1548–1551. doi: 10.1126/science.3144042. [DOI] [PubMed] [Google Scholar]
- Vernet T., Tessier D. C., Laliberté F., Dignard D., Thomas D. Y. The expression in Escherichia coli of a synthetic gene coding for the precursor of papain is prevented by its own putative signal sequence. Gene. 1989 Apr 30;77(2):229–236. doi: 10.1016/0378-1119(89)90071-1. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Waye M. M., Winter G. A transcription terminator in the 5' non-coding region of the tyrosyl tRNA synthetase gene from Bacillus stearothermophilus. Eur J Biochem. 1986 Aug 1;158(3):505–510. doi: 10.1111/j.1432-1033.1986.tb09783.x. [DOI] [PubMed] [Google Scholar]
- Waye M. M., Winter G., Wilkinson A. J., Fersht A. R. Deletion mutagenesis using an 'M13 splint': the N-terminal structural domain of tyrosyl-tRNA synthetase (B. stearothermophilus) catalyses the formation of tyrosyl adenylate. EMBO J. 1983;2(10):1827–1829. doi: 10.1002/j.1460-2075.1983.tb01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson A. J., Fersht A. R., Blow D. M., Winter G. Site-directed mutagenesis as a probe of enzyme structure and catalysis: tyrosyl-tRNA synthetase cysteine-35 to glycine-35 mutation. Biochemistry. 1983 Jul 19;22(15):3581–3586. doi: 10.1021/bi00284a007. [DOI] [PubMed] [Google Scholar]
- Winter G., Koch G. L., Hartley B. S., Barker D. G. The amino acid sequence of the tyrosyl-tRNA synthetase from Bacillus stearothermophilus. Eur J Biochem. 1983 May 2;132(2):383–387. doi: 10.1111/j.1432-1033.1983.tb07374.x. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]


