Abstract
The filamentous elongation typical of growth-phase cells of the predatory bacterium Bdellovibrio bacteriovorus is mediated by regulatory signals that are derived from the prey cell itself. These signals regulate the differentiation of growth-phase cells into the attack phase and appear to be required for continued filamentous growth by prey-dependent wild-type bdellovibrios and their prey-independent mutant derivatives alike. Using a prey-independent bdellovibrio strain, we have developed an assay for the detection and quantification of the growth-extending signal activity present in extracts of prey cells. This prey-derived regulatory activity was shown to be independent of its nutritional contribution to the bdellovibrios and was found to occur in heat-stable, proteinlike compounds of a variety of native molecular weights within the soluble fraction of extracts from both gram-negative and gram-positive bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers S. F., Fackrell H. B., Huang J. C., Robinson J. Relationship between Bdellovibrio bacteriovorus 6-5-S and autoclaved host bacteria. Can J Microbiol. 1972 Dec;18(12):1941–1948. doi: 10.1139/m72-300. [DOI] [PubMed] [Google Scholar]
- Eksztejn M., Varon M. Elongation and cell division in Bdellovibrio bacteriovorus. Arch Microbiol. 1977 Aug 26;114(2):175–181. doi: 10.1007/BF00410781. [DOI] [PubMed] [Google Scholar]
- Friedberg D. Growth of host dependent Bdellovibrio in host cell free system. Arch Microbiol. 1978 Feb;116(2):185–190. doi: 10.1007/BF00406035. [DOI] [PubMed] [Google Scholar]
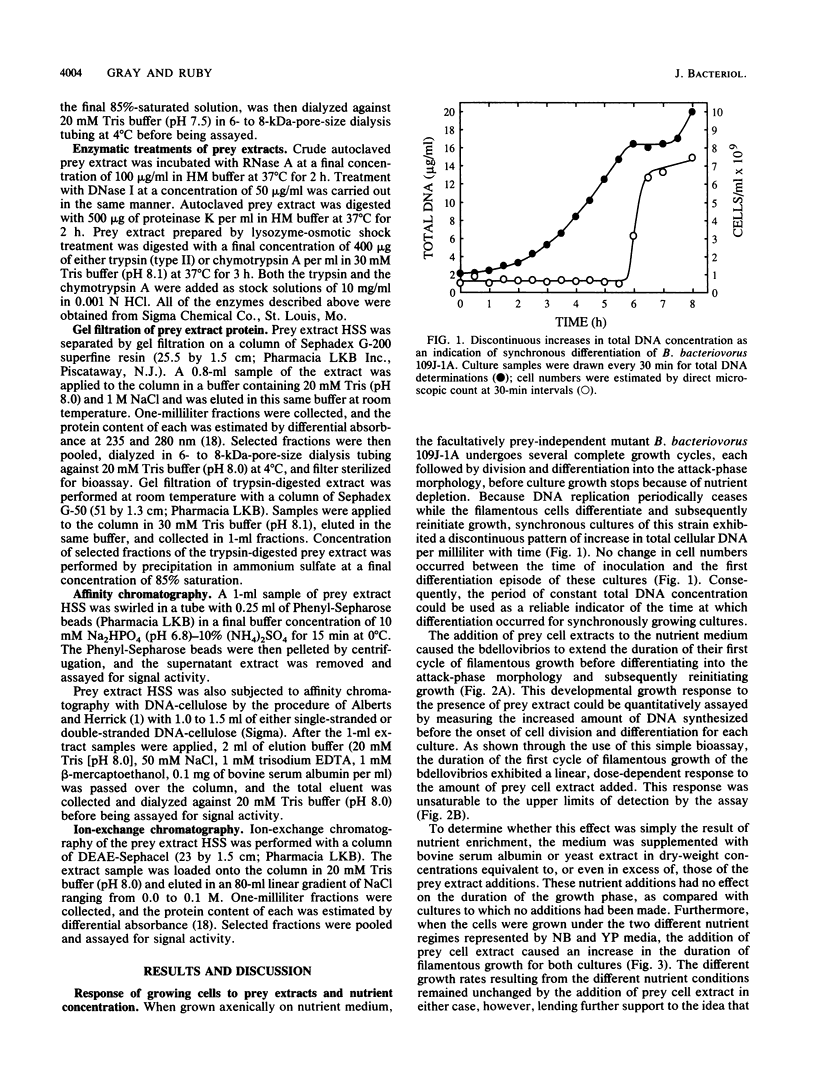
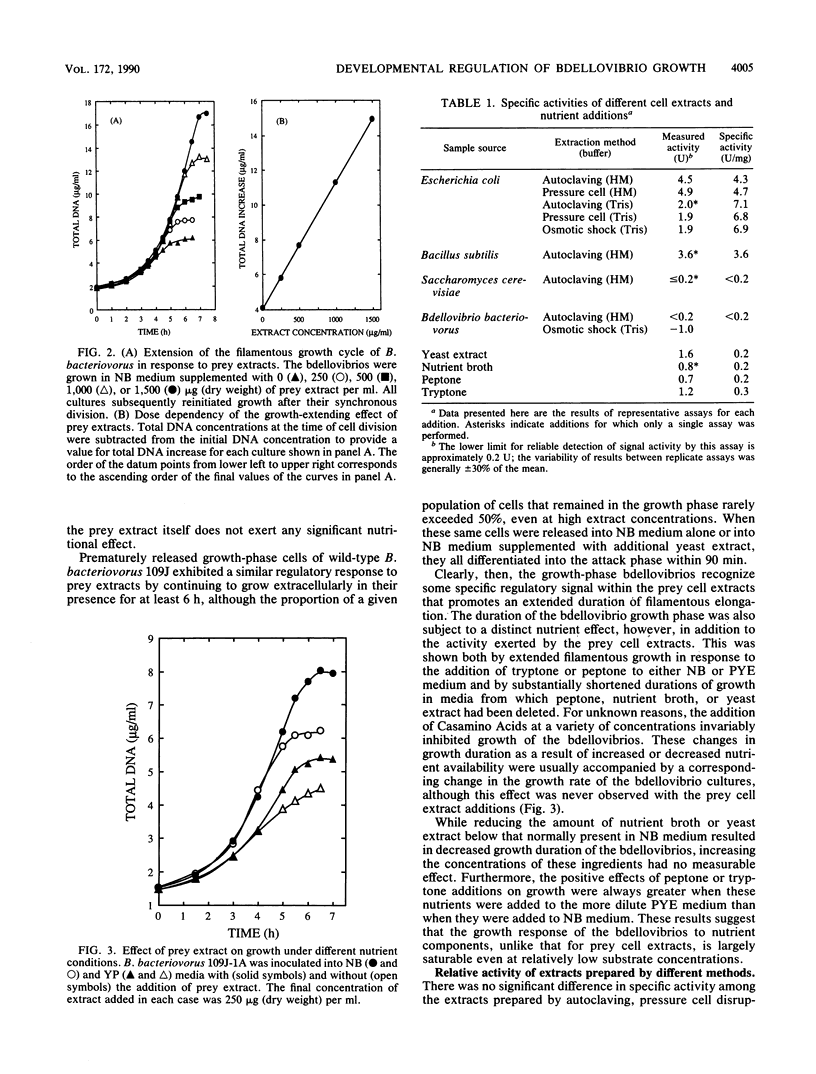
- Gray K. M., Ruby E. G. Unbalanced growth as a normal feature of development of Bdellovibrio bacteriovorus. Arch Microbiol. 1989;152(5):420–424. doi: 10.1007/BF00446922. [DOI] [PubMed] [Google Scholar]
- Horowitz A. T., Kessel M., Shilo M. Growth cycle of predacious Bdellovibrios in a host-free extract system and some properties of the host extract. J Bacteriol. 1974 Jan;117(1):270–282. doi: 10.1128/jb.117.1.270-282.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E. A growth initiation factor for host-independent derivatives of Bdellovibrio bacteriovorus. J Bacteriol. 1973 Jul;115(1):243–252. doi: 10.1128/jb.115.1.243-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M., Shilo M. Relationship of Bdellovibrio elongation and fission to host cell size. J Bacteriol. 1976 Oct;128(1):477–480. doi: 10.1128/jb.128.1.477-480.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D., Schatten G., Sale W. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J Cell Biol. 1975 Jul;66(1):198–200. doi: 10.1083/jcb.66.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., McCabe J. B., Barke J. I. Uptake of intact nucleoside monophosphates by Bdellovibrio bacteriovorus 109J. J Bacteriol. 1985 Sep;163(3):1087–1094. doi: 10.1128/jb.163.3.1087-1094.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Isolation and characterization of host-independent Bdellovibrios. J Bacteriol. 1969 Nov;100(2):769–785. doi: 10.1128/jb.100.2.769-785.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow M. F., Rittenberg S. C. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: solubilization of Escherichia coli peptidoglycan. J Bacteriol. 1978 Sep;135(3):998–1007. doi: 10.1128/jb.135.3.998-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J. R., Granum P. E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980 Nov 15;109(1):156–159. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]


