Abstract
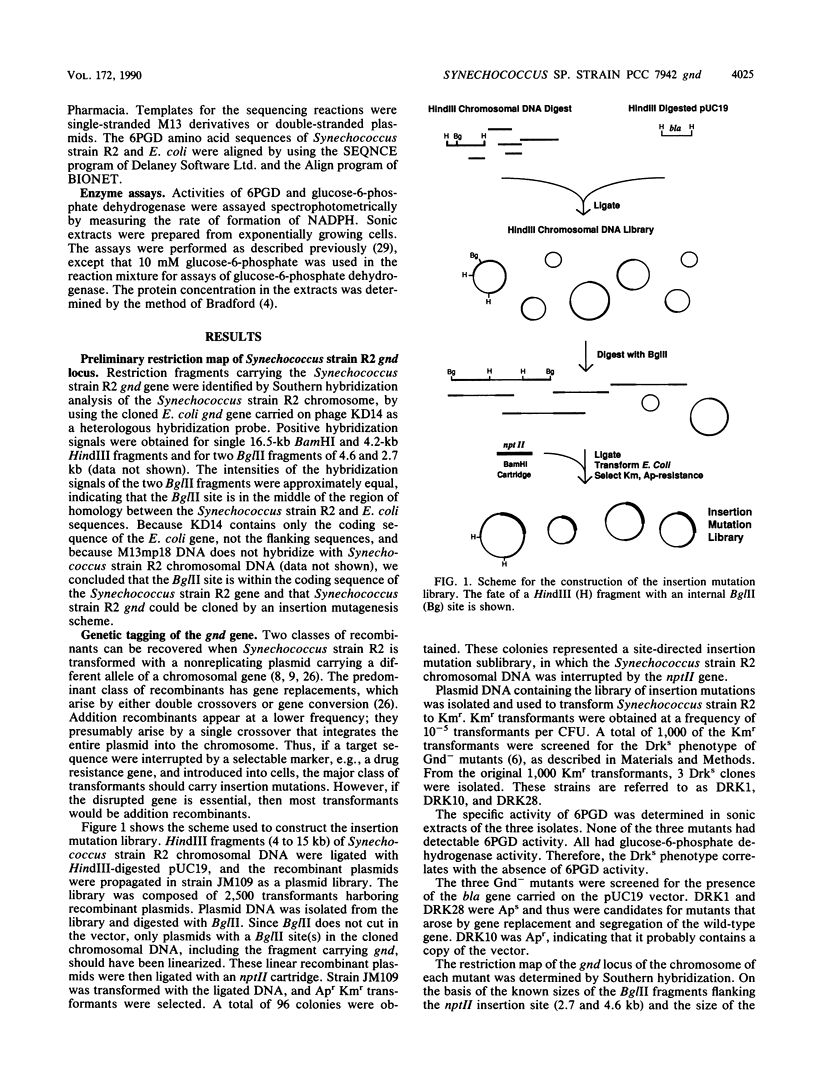
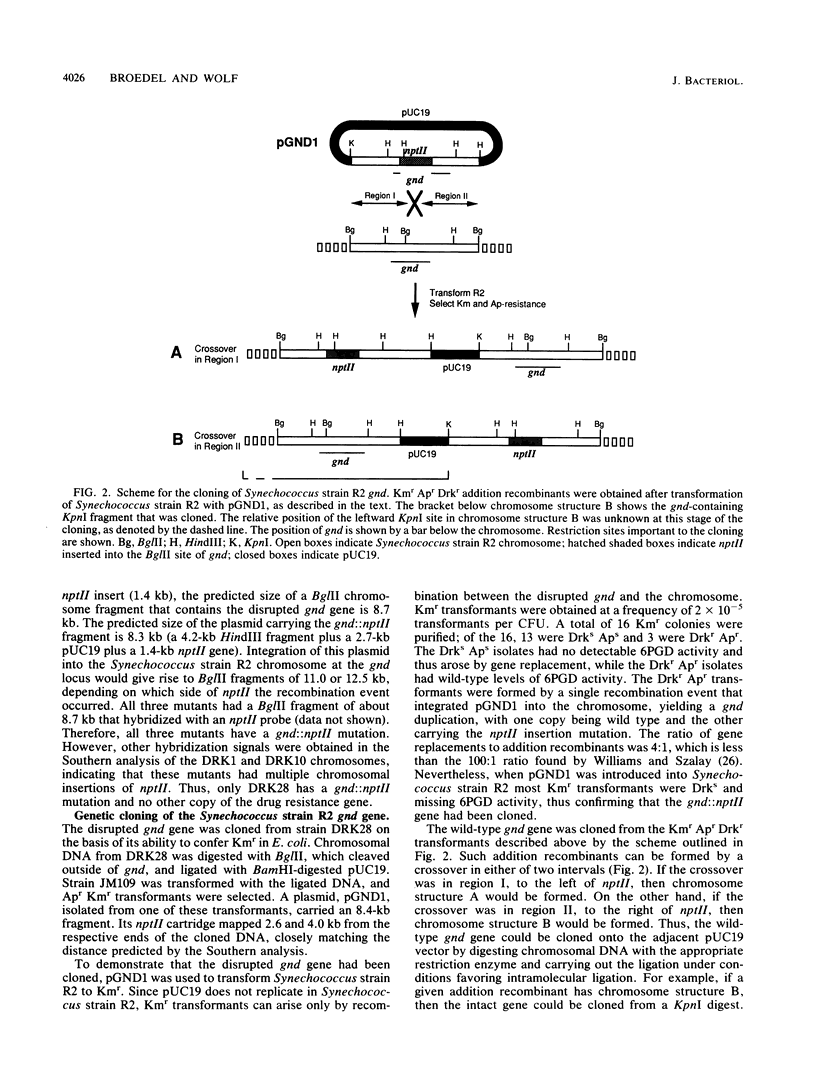
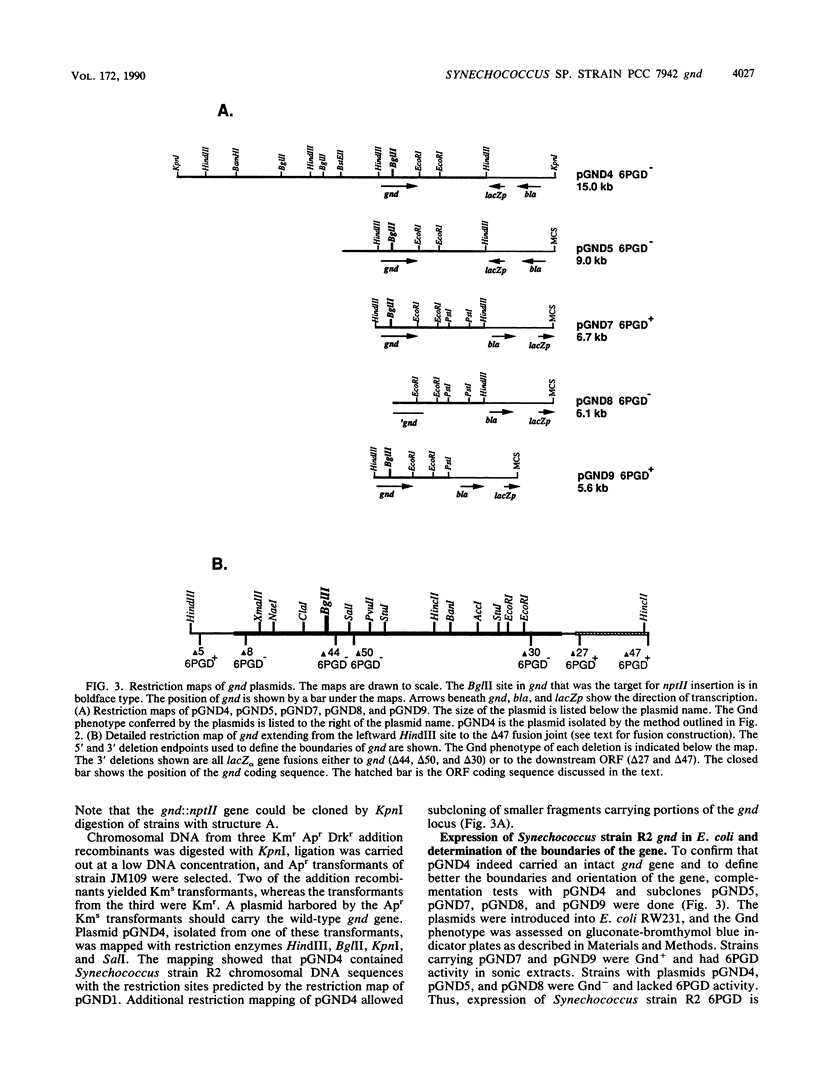
A genetic approach was used for the cloning of the Synechococcus sp. strain PCC 7942 (Synechococcus strain R2) gnd gene which encodes 6-phosphogluconate dehydrogenase (6PGD). A restriction map of the gnd locus was prepared by Southern analysis using the Escherichia coli gene as a heterologous probe. The Synechococcus strain R2 gene was genetically tagged by restriction site-specific insertion of the nptII gene of Tn903 into a pUC19 plasmid library of Synechococcus strain R2 chromosomal DNA. Synechococcus strain R2 was transformed with this insertion mutation library, and isolates carrying the gnd::nptII gene were identified as mutants hypersensitive to incubation in the dark. The interrupted gene was cloned from one of the mutants. A plasmid carrying the gnd::nptII gene was reintroduced into Synechococcus strain R2, and kanamycin-resistant transformants were selected. Transformants arising by gene replacement were dark sensitive and missing 6PGD activity. Transformants arising by plasmid insertion were dark resistant and had 6PGD activity. The wild-type gene was then cloned from a transformant containing a plasmid insertion, making use of the restriction map derived from the interrupted gene. Synechococcus strain R2 6PGD was expressed in E. coli when the cloned gnd gene was transcribed from the lacZ promoter resident on the vector. The boundaries of the gene and the direction of transcription were determined from the phenotypes conferred by plasmids carrying deletions entering gnd from either end. The nucleotide sequence was determined. The deduced amino acid sequence of Synechococcus strain R2 6PGD has 56% homology to that of the E. coli K-12 enzyme.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., Archibald I. G., Bugg C. E., Carne A., Gover S., Helliwell J. R., Pickersgill R. W., White S. W. The three dimensional structure of sheep liver 6-phosphogluconate dehydrogenase at 2.6 A resolution. EMBO J. 1983;2(6):1009–1014. doi: 10.1002/j.1460-2075.1983.tb01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcak G. J., Wolf R. E., Jr A method for unidirectional deletion mutagenesis with application to nucleotide sequencing and preparation of gene fusions. Gene. 1986;49(1):119–128. doi: 10.1016/0378-1119(86)90391-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chauvat F., Rouet P., Bottin H., Boussac A. Mutagenesis by random cloning of an Escherichia coli kanamycin resistance gene into the genome of the cyanobacterium Synechocystis PCC 6803: selection of mutants defective in photosynthesis. Mol Gen Genet. 1989 Mar;216(1):51–59. doi: 10.1007/BF00332230. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F., Singer R. A. Mutational analysis of dark endogenous metabolism in the blue-green bacterium Anacystis nidulans. J Bacteriol. 1974 Sep;119(3):677–683. doi: 10.1128/jb.119.3.677-683.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Golden S. S. Mutagenesis of cyanobacteria by classical and gene-transfer-based methods. Methods Enzymol. 1988;167:714–727. doi: 10.1016/0076-6879(88)67083-2. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Sherman L. A. Optimal conditions for genetic transformation of the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1984 Apr;158(1):36–42. doi: 10.1128/jb.158.1.36-42.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck P. C. Molecular aspects of nitrogen fixation by photosynthetic prokaryotes. Crit Rev Microbiol. 1987;14(1):1–48. doi: 10.3109/10408418709104434. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Huisman O., Raymond W., Froehlich K. U., Errada P., Kleckner N., Botstein D., Hoyt M. A. A Tn10-lacZ-kanR-URA3 gene fusion transposon for insertion mutagenesis and fusion analysis of yeast and bacterial genes. Genetics. 1987 Jun;116(2):191–199. doi: 10.1093/genetics/116.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarre J., Chauvat F., Thuriaux P. Insertional mutagenesis by random cloning of antibiotic resistance genes into the genome of the cyanobacterium Synechocystis strain PCC 6803. J Bacteriol. 1989 Jun;171(6):3449–3457. doi: 10.1128/jb.171.6.3449-3457.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M., Wöber G. Accumulation, mobilization and turn-over of glycogen in the blue-green bacterium Anacystis nidulans. Arch Microbiol. 1976 Dec 1;111(1-2):93–97. doi: 10.1007/BF00446554. [DOI] [PubMed] [Google Scholar]
- Nasoff M. S., Baker H. V., 2nd, Wolf R. E., Jr DNA sequence of the Escherichia coli gene, gnd, for 6-phosphogluconate dehydrogenase. Gene. 1984 Mar;27(3):253–264. doi: 10.1016/0378-1119(84)90070-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. A., Doolittle W. F. Control of gene expression in blue-green algae. Nature. 1975 Feb 20;253(5493):650–651. doi: 10.1038/253650a0. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Szalay A. A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1983 Sep;24(1):37–51. doi: 10.1016/0378-1119(83)90129-4. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R. E., Jr Integration of specialized transducing bacteriophage lambda cI857 St68 h80 dgnd his by an unusual pathway promotes formation of deletions and generates a new translocatable element. J Bacteriol. 1980 May;142(2):588–602. doi: 10.1128/jb.142.2.588-602.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R. E., Jr, Shea F. M. Combined use of strain construction and affinity chromatography in the rapid, high-yield purification of 6-phosphogluconate dehydrogenase from Escherichia coli. J Bacteriol. 1979 Apr;138(1):171–175. doi: 10.1128/jb.138.1.171-175.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


