Abstract
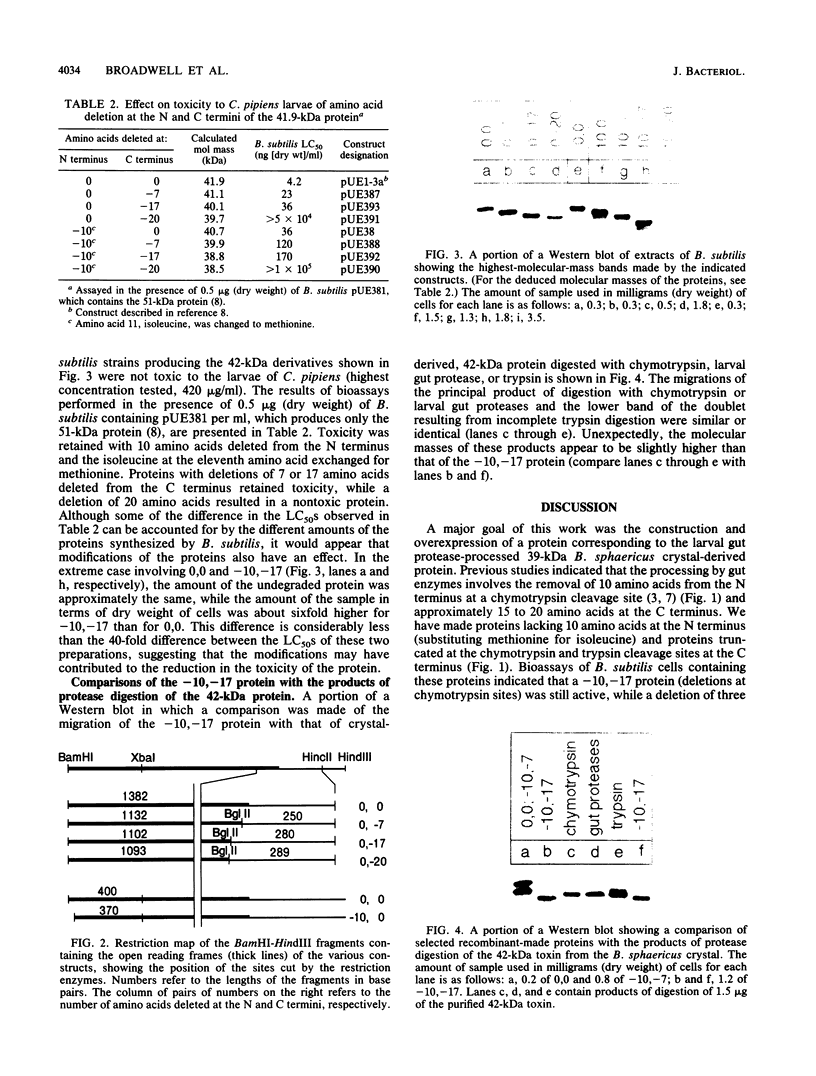
After ingestion of the parasporal crystals of Bacillus sphaericus, mosquito larvae process the 42-kilodalton (kDa) toxin to a protein of 39 kDa, which has an increased toxicity (A. H. Broadwell and P. Baumann, Appl. Environ. Microbiol. 53:1333-1337, 1987). A similar activation is performed by trypsin and chymotrypsin. Using site-directed mutagenesis, we have constructed derivatives of the 42-kDa toxin with a deletion of 10 amino acids at the N terminus and deletions of 7, 17, or 20 amino acids at the C terminus. Toxicity for mosquito larvae was retained upon deletion of 7 or 17 amino acids but was lost upon deletion of 20 amino acids. Evidence is presented indicating that the protein containing deletions of 10 amino acids at the N terminus and 17 amino acids at the C terminus (corresponding to potential chymotrypsin cleavage sites) is similar to the 39-kDa protein produced in mosquito larvae or by digestion with chymotrypsin. Digestion with trypsin appears to generate a protein lacking 16 or 19 amino acids from the N terminus and 7 amino acids from the C terminus. As is the case with the recombinant-made 42-kDa protein, toxicity of its derivatives is dependent on the presence of a 51-kDa protein which is a component of the parasporal crystal of B. sphaericus 2362.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aly C., Mulla M. S., Federici B. A. Ingestion, dissolution, and proteolysis of the Bacillus sphaericus toxin by mosquito larvae. J Invertebr Pathol. 1989 Jan;53(1):12–20. doi: 10.1016/0022-2011(89)90068-2. [DOI] [PubMed] [Google Scholar]
- Arapinis C., de la Torre F., Szulmajster J. Nucleotide and deduced amino acid sequence of the Bacillus sphaericus 1593M gene encoding a 51.4 kD polypeptide which acts synergistically with the 42 kD protein for expression of the larvicidal toxin. Nucleic Acids Res. 1988 Aug 11;16(15):7731–7731. doi: 10.1093/nar/16.15.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann L., Broadwell A. H., Baumann P. Sequence analysis of the mosquitocidal toxin genes encoding 51.4- and 41.9-kilodalton proteins from Bacillus sphaericus 2362 and 2297. J Bacteriol. 1988 May;170(5):2045–2050. doi: 10.1128/jb.170.5.2045-2050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P., Unterman B. M., Baumann L., Broadwell A. H., Abbene S. J., Bowditch R. D. Purification of the larvicidal toxin of Bacillus sphaericus and evidence for high-molecular-weight precursors. J Bacteriol. 1985 Aug;163(2):738–747. doi: 10.1128/jb.163.2.738-747.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowditch R. D., Baumann P., Yousten A. A. Cloning and sequencing of the gene encoding a 125-kilodalton surface-layer protein from Bacillus sphaericus 2362 and of a related cryptic gene. J Bacteriol. 1989 Aug;171(8):4178–4188. doi: 10.1128/jb.171.8.4178-4188.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell A. H., Baumann L., Baumann P. The 42- and 51-kilodalton mosquitocidal proteins of Bacillus sphaericus 2362: construction of recombinants with enhanced expression and in vivo studies of processing and toxicity. J Bacteriol. 1990 May;172(5):2217–2223. doi: 10.1128/jb.172.5.2217-2223.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell A. H., Baumann P. Proteolysis in the gut of mosquito larvae results in further activation of the Bacillus sphaericus toxin. Appl Environ Microbiol. 1987 Jun;53(6):1333–1337. doi: 10.1128/aem.53.6.1333-1337.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles J. F. Ultrastructural midgut events in Culicidae larvae fed with Bacillus sphaericus 2297 spore/crystal complex. Ann Inst Pasteur Microbiol. 1987 Jul-Aug;138(4):471–484. doi: 10.1016/0769-2609(87)90064-0. [DOI] [PubMed] [Google Scholar]
- Davidson E. W., Bieber A. L., Meyer M., Shellabarger C. Enzymatic activation of the Bacillus sphaericus mosquito larvicidal toxin. J Invertebr Pathol. 1987 Jul;50(1):40–44. doi: 10.1016/0022-2011(87)90143-1. [DOI] [PubMed] [Google Scholar]
- Hindley J., Berry C. Identification, cloning and sequence analysis of the Bacillus sphaericus 1593 41.9 kD larvicidal toxin gene. Mol Microbiol. 1987 Sep;1(2):187–194. doi: 10.1111/j.1365-2958.1987.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Kawamura F., Doi R. H. Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J Bacteriol. 1984 Oct;160(1):442–444. doi: 10.1128/jb.160.1.442-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Fritz H. J. Oligonucleotide-directed construction of mutations via gapped duplex DNA. Methods Enzymol. 1987;154:350–367. doi: 10.1016/0076-6879(87)54084-8. [DOI] [PubMed] [Google Scholar]
- Park S. S., Wong S. L., Wang L. F., Doi R. H. Bacillus subtilis subtilisin gene (aprE) is expressed from a sigma A (sigma 43) promoter in vitro and in vivo. J Bacteriol. 1989 May;171(5):2657–2665. doi: 10.1128/jb.171.5.2657-2665.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgarrella F., Szulmajster J. Purification and characterization of the larvicidal toxin of Bacillus sphaericus 1593M. Biochem Biophys Res Commun. 1987 Mar 30;143(3):901–907. doi: 10.1016/0006-291x(87)90334-2. [DOI] [PubMed] [Google Scholar]
- Yousten A. A. Bacillus sphaericus: microbiological factors related to its potential as a mosquito larvicide. Adv Biotechnol Processes. 1984;3:315–343. [PubMed] [Google Scholar]
- Zaghloul T. I., Kawamura F., Doi R. H. Translational coupling in Bacillus subtilis of a heterologous Bacillus subtilis-Escherichia coli gene fusion. J Bacteriol. 1985 Nov;164(2):550–555. doi: 10.1128/jb.164.2.550-555.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre F., Bennardo T., Sebo P., Szulmajster J. On the respective roles of the two proteins encoded by the Bacillus sphaericus 1593M toxin genes expressed in Escherichia coli and Bacillus subtilis. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1417–1422. doi: 10.1016/0006-291x(89)91828-7. [DOI] [PubMed] [Google Scholar]




