Abstract
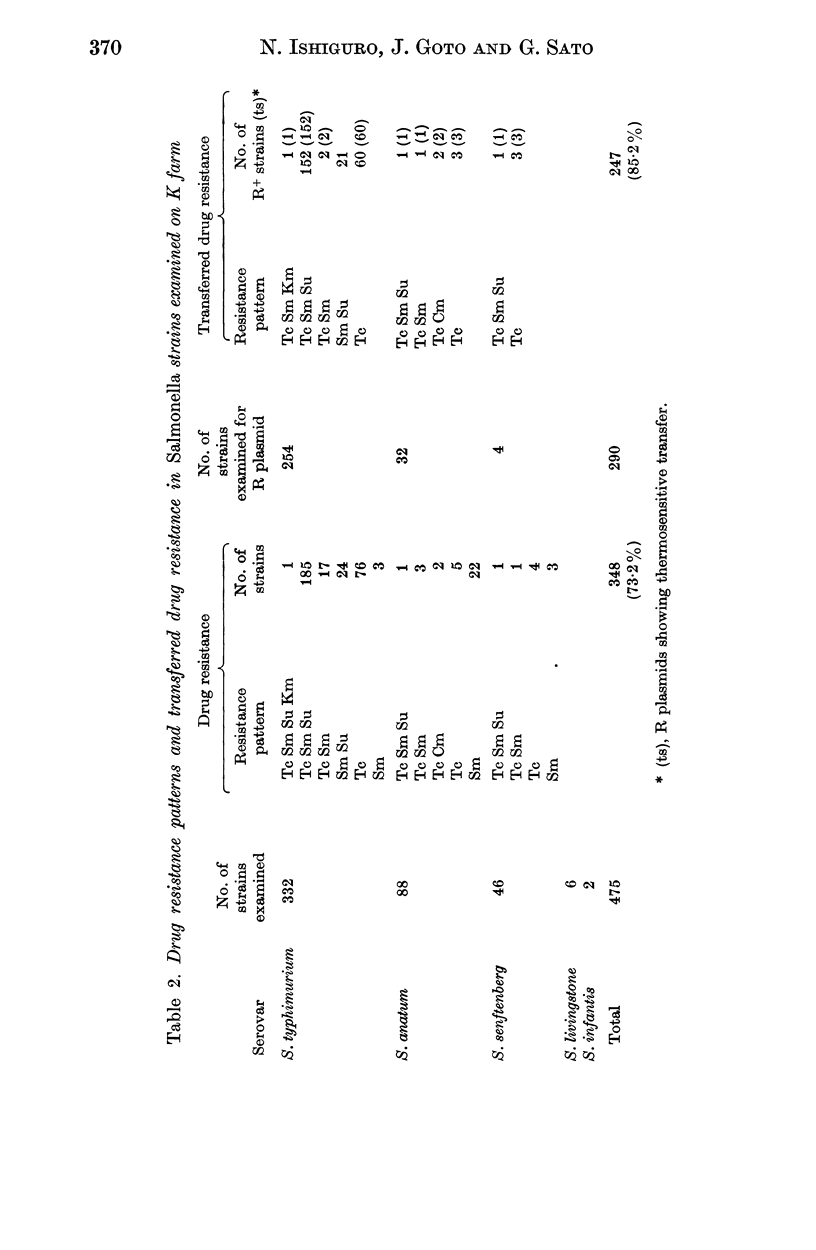
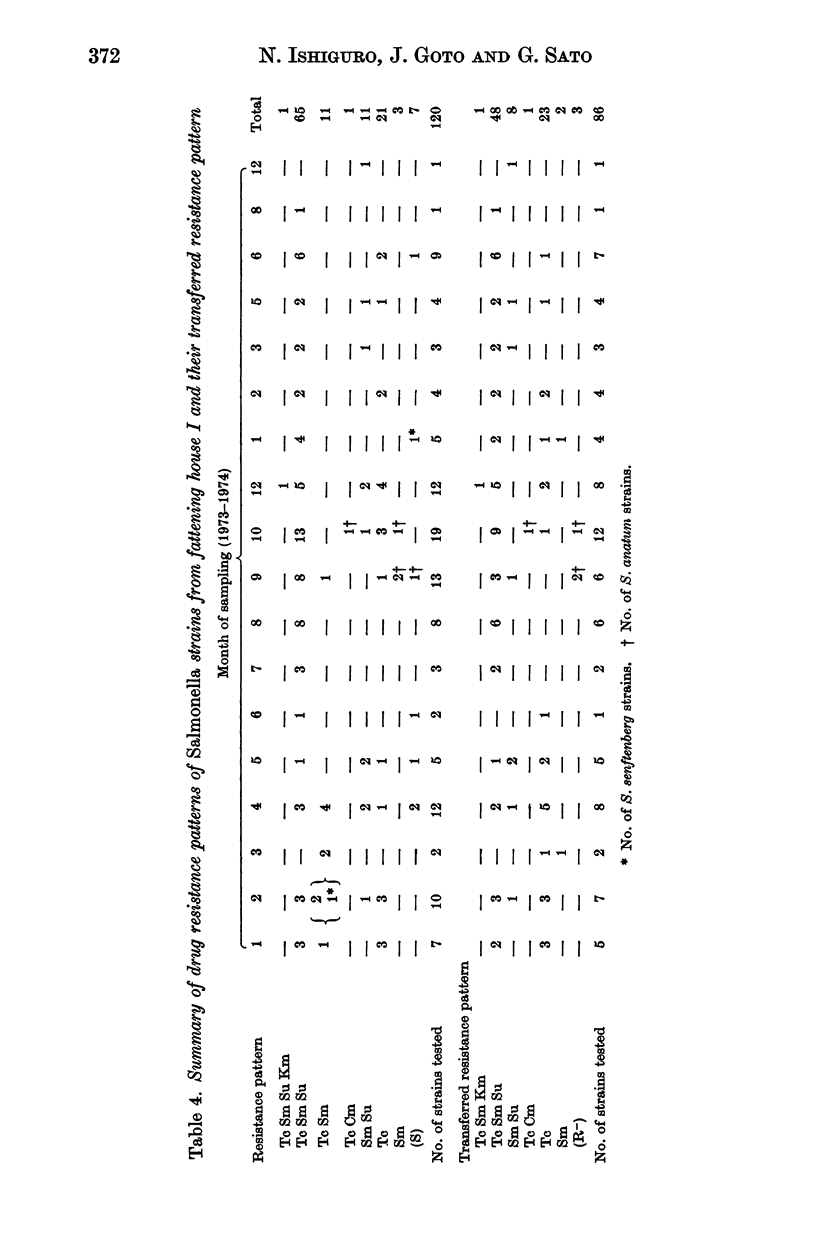
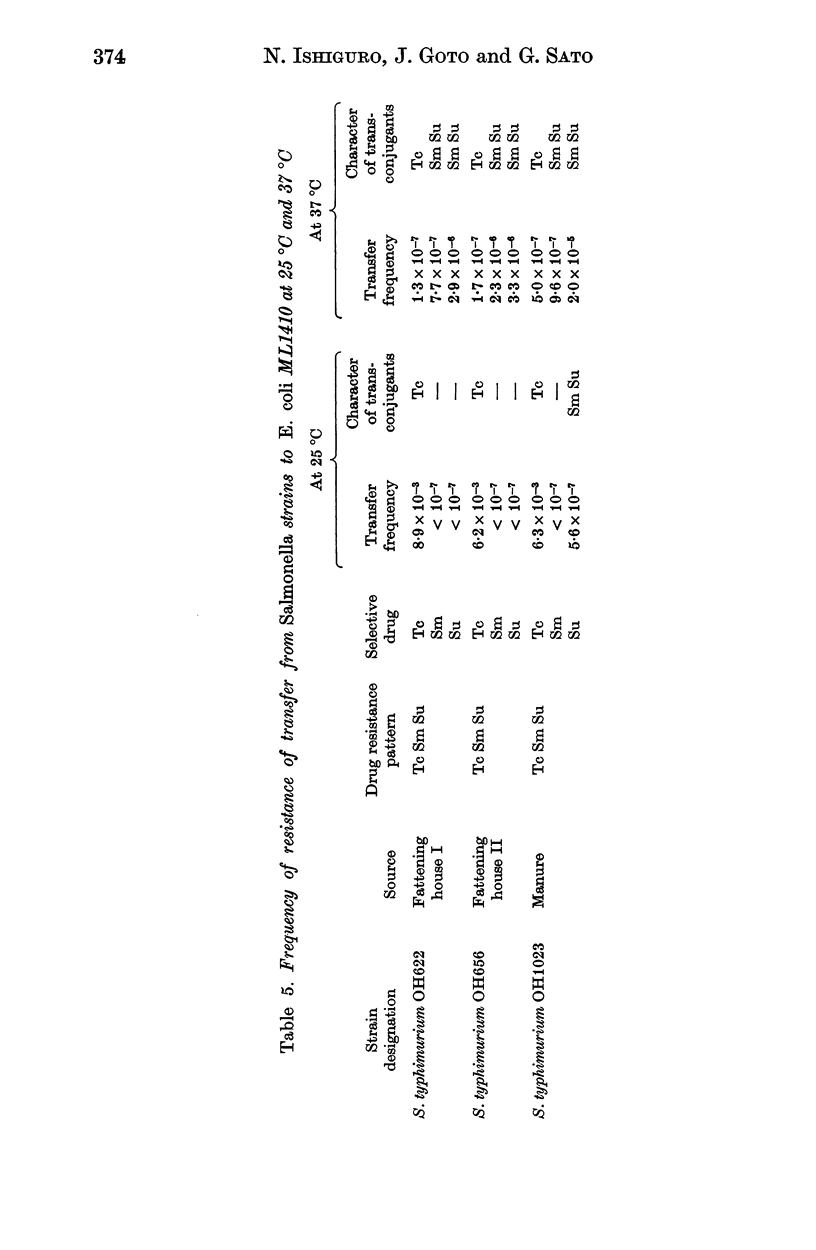
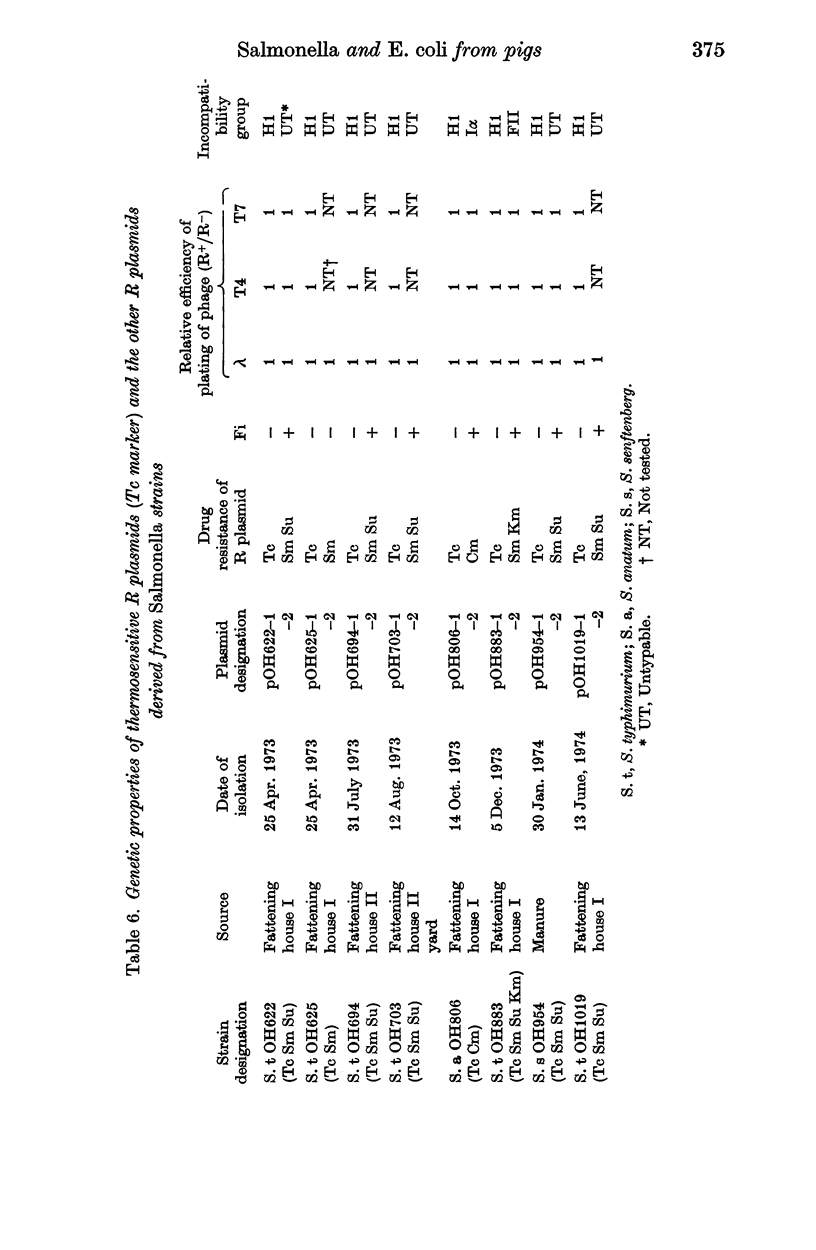
A total of 475 Salmonella strains belonging to 5 serovars, isolated from a pig farm which had been heavily contaminated with Salmonella for the past 2 years were tested for antibiotic susceptibility and detection of R plasmids. Thirty-three Escherichia coli isolates from the same farm were also examined in a similar way. Out of 475 strains 348 (73.2%) were resistant to one or more antibiotics such as tetracycline (Tc), streptomycin (Sm), sulfadimethoxine (Su), chloramphenicol (Cm) and kanamycin (Km), and 247 (85.2%) out of 290 strains belonging to 3 serovars examined harboured conjugative R plasmids. There was no change in the pattern of drug resistance during this survey nor any variation in the pattern of resistance of R plasmids, whatever the serovar. The antibiogram pattern Tc Sm Su, mainly S. typhimurium, was common among Salmonella strains. Among the transferred resistance patterns, the thermosensitive R plasmids conferring the Tc marker detected in this study were Fi-, and belonged to incompatibility group H1, whereas the R plasmids conferring Sm Su resistances which coexisted in the same host were Fi+, and compatible with the reference R plasmids tested. The I alpha plasmid conferring Cm resistance alone was isolated from S. anatum and the FII plasmid conferring Sm Km resistances was also isolated from S. typhimurium. In contrast, the 33 E. coli strains examined were resistant to three or five antibiotics and most of the resistance markers were located on conjugative R plasmids. I alpha plasmids conferring Cm resistance alone or FII plasmids conferring Cm or Km markers were common in the E. coli strains. H1 and H2 plasmids conferring multiple resistance markers were also found in them. The genetic properties of R plasmids derived from Salmonella or E. coli strains are compared, and the potential spread of R plasmids between strains of Salmonella and E. coli is discussed.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S. The problem and implications of chloramphenicol resistance in the typhoid bacillus. J Hyg (Lond) 1975 Apr;74(2):289–299. doi: 10.1017/s0022172400024360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth P. T., Grinter N. J. Comparison of the deoxyribonucleic acid molecular weights and homologies of plasmids conferring linked resistance to streptomycin and sulfonamides. J Bacteriol. 1974 Nov;120(2):618–630. doi: 10.1128/jb.120.2.618-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau P. Y., Shortridge K. F., Huang C. T. Salmonella in pig carcasses for human consumption in Hong Kong: a study on the mode of contamination. J Hyg (Lond) 1977 Apr;78(2):253–260. doi: 10.1017/s002217240005614x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro N., Oka C., Sato G. Isolation of citrate-positive variants of Escherichia coli from domestic pigeons, pigs, cattle, and horses. Appl Environ Microbiol. 1978 Aug;36(2):217–222. doi: 10.1128/aem.36.2.217-222.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro N., Sato G., Takeuchi K., Nakayama A. A longitudinal epizootiological study of Salmonella infection on a piggery: a study on the mode of contamination by biotyping of Salmonella typhimurium and by the antibiogram. Nihon Juigaku Zasshi. 1979 Jun;41(3):261–272. doi: 10.1292/jvms1939.41.261. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Sato G., Asagi M., Oka C., Ishiguro N., Terakado N. Transmissible citrate-utilizing ability in Escherichia coli isolated from pigeons, pigs and cattle. Microbiol Immunol. 1978;22(6):357–360. doi: 10.1111/j.1348-0421.1978.tb00380.x. [DOI] [PubMed] [Google Scholar]
- Sato G., Terakado N. R factor types found in Salmonella typhimurium and Escherichia coli isolated from calves in a confined environment. Am J Vet Res. 1977 Jun;38(6):743–747. [PubMed] [Google Scholar]
- Smith H. R., Grindley N. D., Humphreys G. O., Anderson E. S. Interactions of group H resistance factors with the F factor. J Bacteriol. 1973 Aug;115(2):623–628. doi: 10.1128/jb.115.2.623-628.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Parsell Z., Green P. Thermosensitive antibiotic resistance plasmids in enterobacteria. J Gen Microbiol. 1978 Nov;109(1):37–47. doi: 10.1099/00221287-109-1-37. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Tucker J. F. The effect of feeding diets containing permitted antibiotics on the faecal excretion of Salmonella typhimurium by experimentally infected chickens. J Hyg (Lond) 1975 Oct;75(2):293–301. doi: 10.1017/s0022172400047318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima M., Kudo Y., Kudow S. Demonstration of intranuclear nucleocapsids in cultured cells infected with Newcastle disease virus. Nihon Juigaku Zasshi. 1977 Oct;39(5):521–530. doi: 10.1292/jvms1939.39.521. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Grant R. B. Incompatibility and bacteriophage inhibition properties of N-1, a plasmid belonging to the H2 incompatibility group. Mol Gen Genet. 1977 May 20;153(1):5–10. doi: 10.1007/BF01035990. [DOI] [PubMed] [Google Scholar]
- Terakado N., Sato G. Demonstration of the so-called Mexican type R plasmids in Escherichia coli isolated from domestic animals and pigeons. Microbiol Immunol. 1978;22(4):227–229. doi: 10.1111/j.1348-0421.1978.tb00366.x. [DOI] [PubMed] [Google Scholar]
- Terakado N., Sato G. Detection of an R plasmid of incompatibility group H in Salmonella typhimurium of bovine origin. Natl Inst Anim Health Q (Tokyo) 1978 Winter;18(3-4):180–181. [PubMed] [Google Scholar]
- Timoney J. F. The epidemiology and genetics of antibiotic resistance of Salmonella typhimurium isolated from diseases animals in New York. J Infect Dis. 1978 Jan;137(1):67–73. doi: 10.1093/infdis/137.1.67. [DOI] [PubMed] [Google Scholar]
- Watson W. A. Samonellosis and meat hygiene: red meat. Vet Rec. 1975 Apr 26;96(17):374–376. doi: 10.1136/vr.96.17.374. [DOI] [PubMed] [Google Scholar]
- Wilcock B., Olander H. Influence of oral antibiotic feeding on the duration and severity of clinical disease, growth performance, and pattern of shedding in swine inoculated with Salmonella typhimurium. J Am Vet Med Assoc. 1978 Feb 15;172(4):472–477. [PubMed] [Google Scholar]


