Abstract
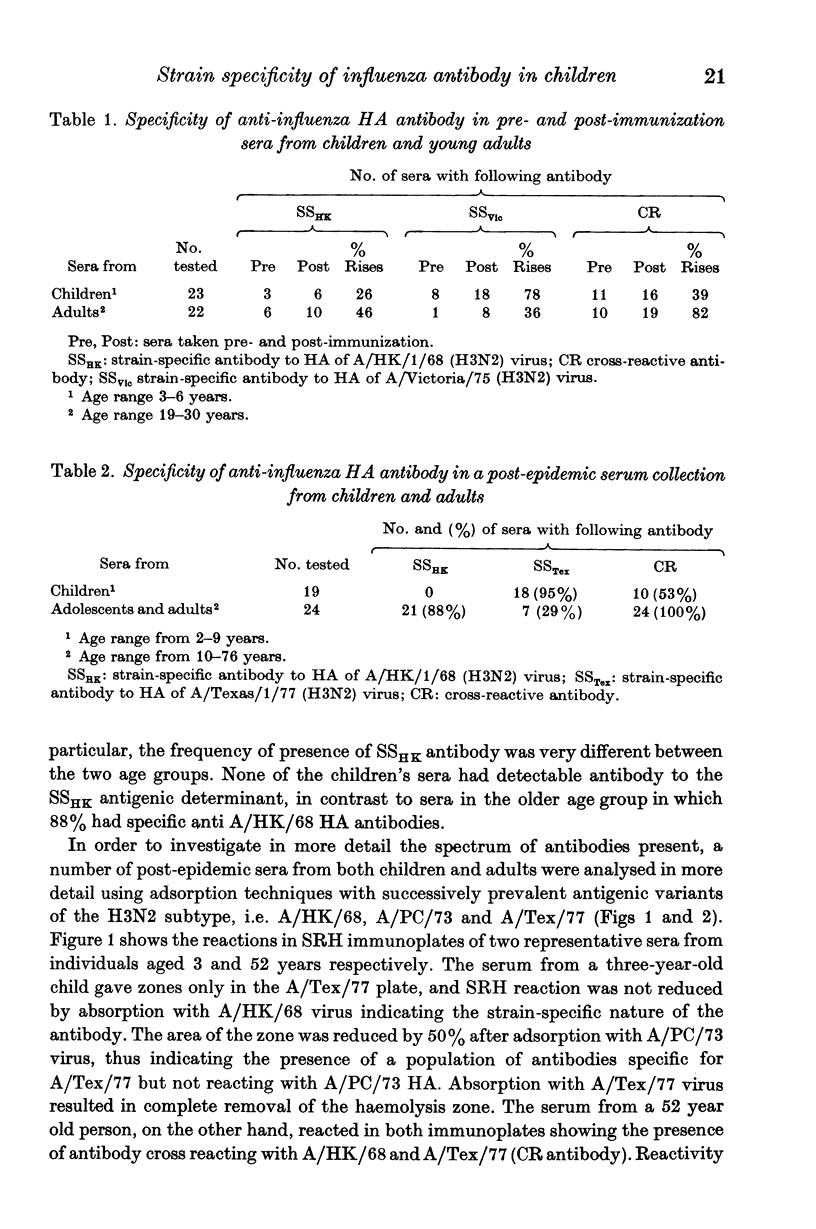
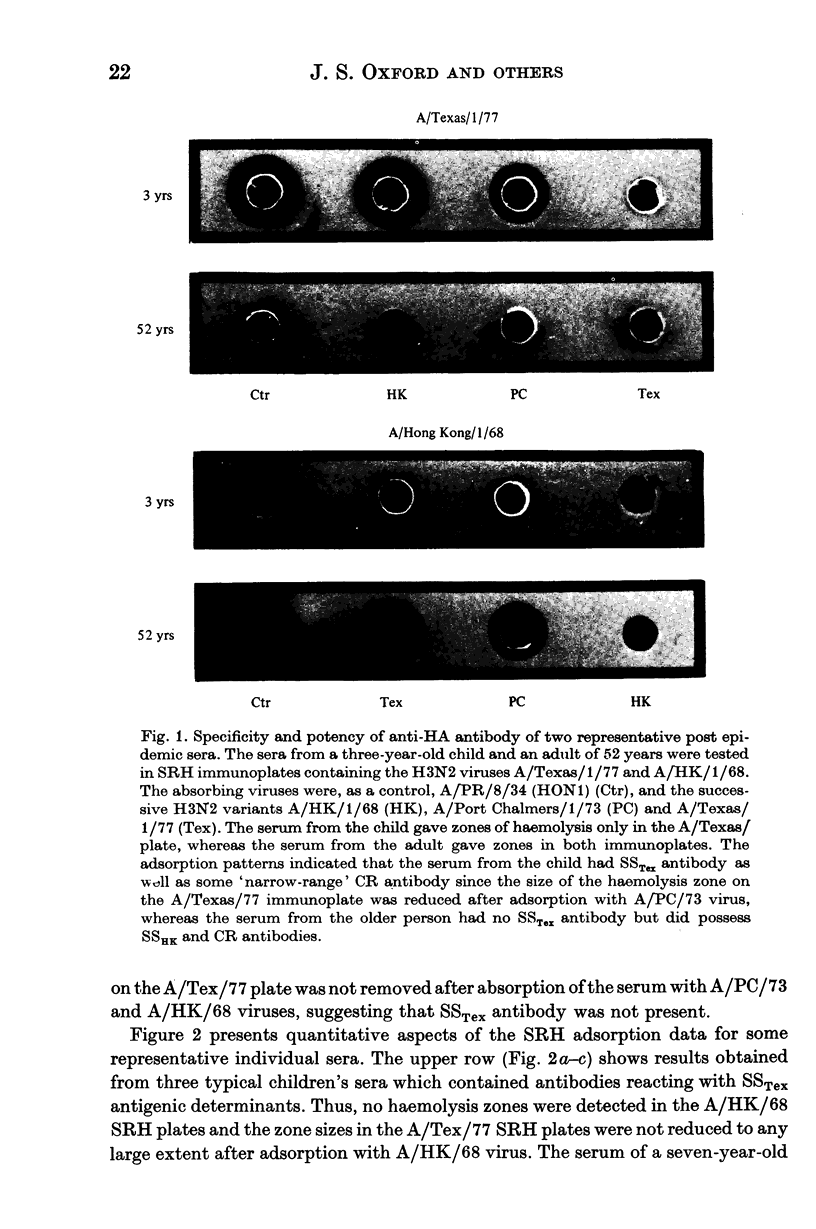
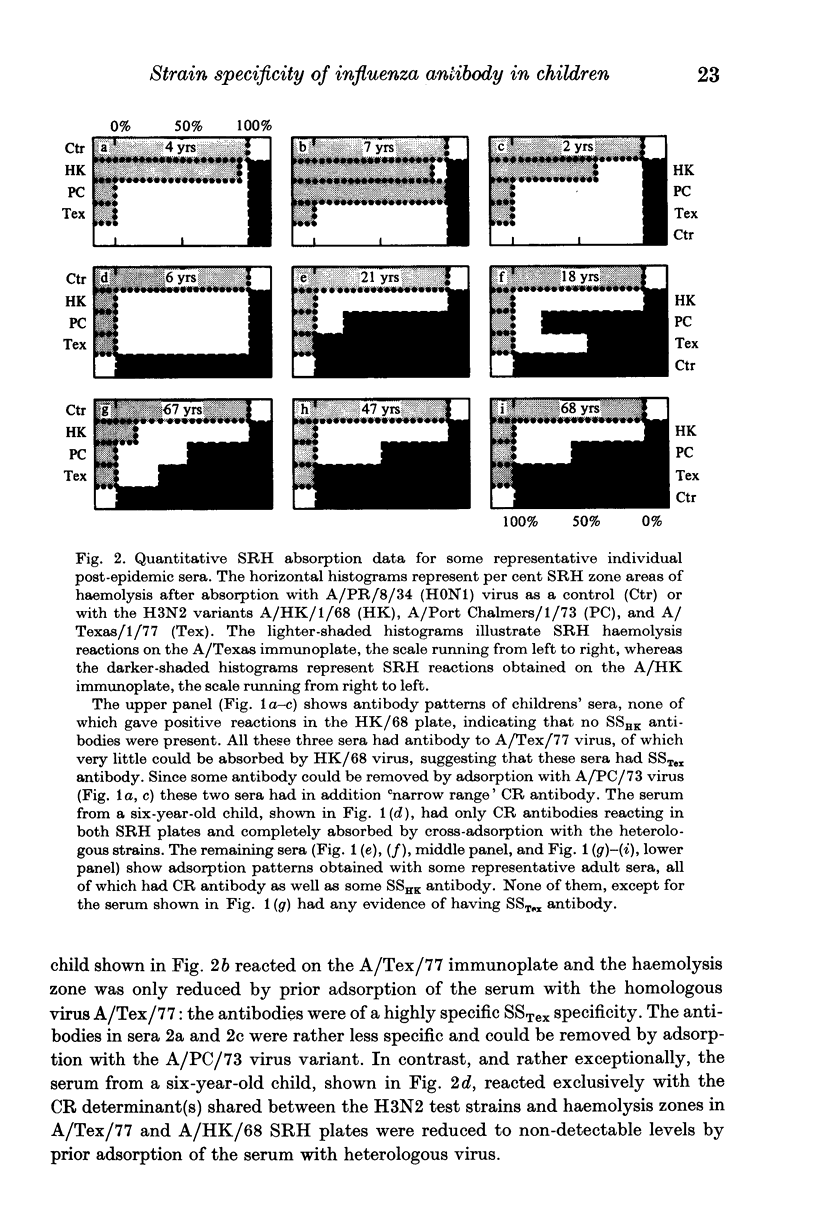
The specificity of serum anti-HA antibody from children immunized or infected with A/Victoria/75 (H3N2 or A/Texas/77 (H3N2) virus was examined using the single radial haemolysis test together with adsorption of antibody with three antigenic variants A/Hong Kong/68 (H3N2), A/Port Chalmers/73 (H3N2) and A/Victoria/75 (H3N2). The majority of young children reacted to vaccination or infection by producing strain-specific (SS) antibody to the homologous virus. A small proportion of children's sera contained cross-reacting (CR) antibodies capable of reacting with the haemagglutinins of all antigenic variants of the sub-type including A/HK/1/68. In contrast, most adults reacted immunologically to either vaccination or infection by producing CR antibody, reacting with all variants of the antigenic subtype including the prototype virus A/HK/1/68 (H3N2).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Couch R. B., Webster R. G., Kasel J. A., Cate T. R. Efficacy of purified influenza subunit vaccines and relation to the major antigenic determinants on the hemagglutinin molecule. J Infect Dis. 1979 Oct;140(4):553–559. doi: 10.1093/infdis/140.4.553. [DOI] [PubMed] [Google Scholar]
- Gerhard W. The analysis of the monoclonal immune response to influenza virus. II. The antigenicity of the viral hemagglutinin. J Exp Med. 1976 Oct 1;144(4):985–995. doi: 10.1084/jem.144.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins T. W., Davies J. R., Smith A. J., Miller C. L., Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet. 1979 Jan 6;1(8106):33–35. doi: 10.1016/s0140-6736(79)90468-9. [DOI] [PubMed] [Google Scholar]
- Kasel J. A., Couch R. B., Six H. R., Knight V. Antigenicity of licensed whole virion and subvirion influenza vaccines in "high risk" persons. Proc Soc Exp Biol Med. 1976 Apr;151(4):742–747. doi: 10.3181/00379727-151-39298. [DOI] [PubMed] [Google Scholar]
- Kasel J. A., Six H. R., Couch R. B., Greenberg S. B., Cate T. R. Variant-specific antihemagglutinin serum response to type A influenza natural infection and inactivated vaccines in adults. Proc Soc Exp Biol Med. 1979 Sep;161(4):519–521. doi: 10.3181/00379727-161-40587. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Downie J. C., Webster R. G. Studies on antigenic variation in influenza virus. Evidence for multiple antigenic determinants on the hemagglutinin subunits of A-Hong Kong-68 (H3 N2) virus and the A-England-72 strains. Virology. 1974 May;59(1):230–244. doi: 10.1016/0042-6822(74)90218-9. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Kasel J. A., Saglam M., Knight V., Loda F. A. Immunity to influenza to antibody levels. N Engl J Med. 1966 Mar 10;274(10):527–535. doi: 10.1056/NEJM196603102741001. [DOI] [PubMed] [Google Scholar]
- Oxford J. S., Schild G. C., Potter C. W., Jennings R. The specificity of the anti-haemagglutinin antibody response induced in man by inactivated influenza vaccines and by natural infection. J Hyg (Lond) 1979 Feb;82(1):51–61. doi: 10.1017/s0022172400025468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., Jennings R., Nicholson K., Tyrrell D. A., Dickinson K. G. Immunity to attenuated influenza virus WRL 105 infection induced by heterologous, inactivated influenza A virus vaccines. J Hyg (Lond) 1977 Dec;79(3):321–332. doi: 10.1017/s0022172400053158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter C. W., Oxford J. S. Determinants of immunity to influenza infection in man. Br Med Bull. 1979 Jan;35(1):69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- Rovnova Z. I., Kosyakov P. N., Berezina O. N., Isayeva E. I., Zhdanov V. M. Antigenic determinants in influenza virus hemagglutinin. Infect Immun. 1979 Jun;24(3):804–807. doi: 10.1128/iai.24.3.804-807.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Schild G. C. The polypeptide composition of influenza A viruses. Virology. 1971 May;44(2):396–408. doi: 10.1016/0042-6822(71)90270-4. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C., Schild G. C. Antibody responses to antigenic determinants of influenza virus hemagglutinin. II. Original antigenic sin: a bone marrow-derived lymphocyte memory phenomenon modulated by thymus-derived lymphocytes. J Exp Med. 1974 Dec 1;140(6):1571–1578. doi: 10.1084/jem.140.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L. Host defenses against influenza virus: the role of anti-hemagglutinin antibody. J Immunol. 1975 Aug;115(2):434–439. [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Determination of the number of nonoverlapping antigenic areas on Hong Kong (H3N2) influenza virus hemagglutinin with monoclonal antibodies and the selection of variants with potential epidemiological significance. Virology. 1980 Jul 15;104(1):139–148. doi: 10.1016/0042-6822(80)90372-4. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Webster R. G., Gerhard W. U. Antigenic variation in three distinct determinants of an influenza type A haemagglutinin molecule. Nature. 1979 May 17;279(5710):246–248. doi: 10.1038/279246a0. [DOI] [PubMed] [Google Scholar]



