Abstract
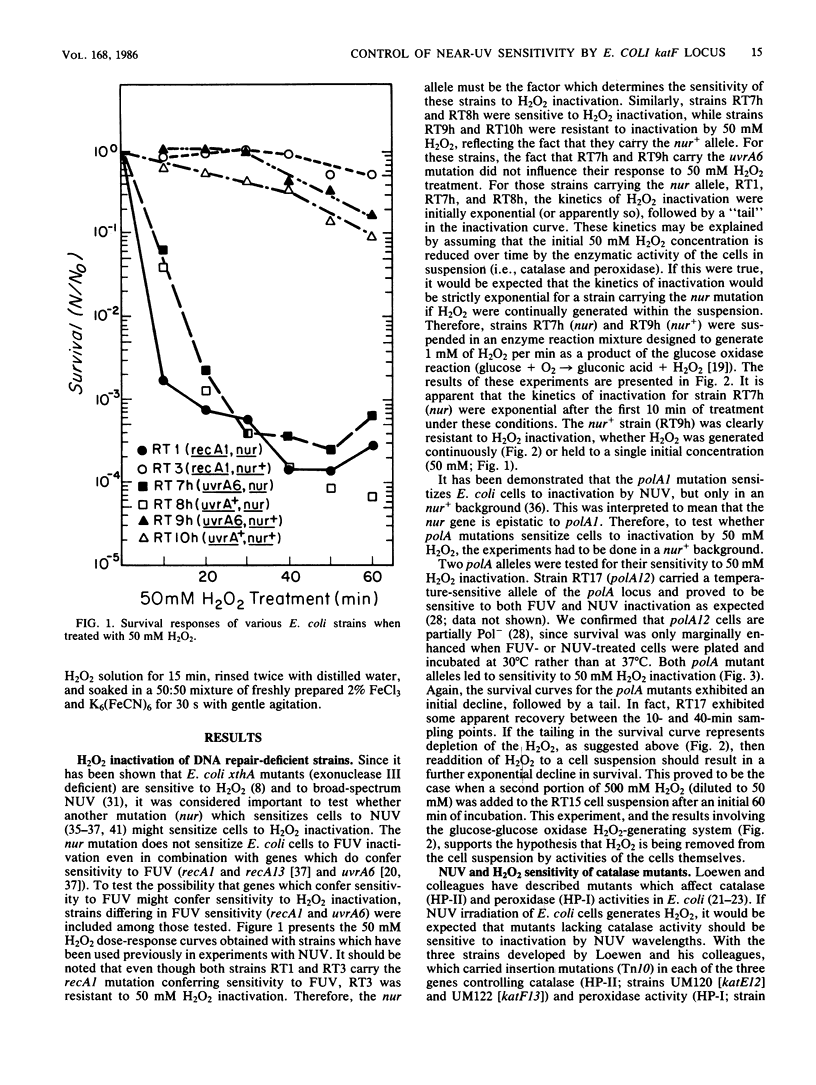
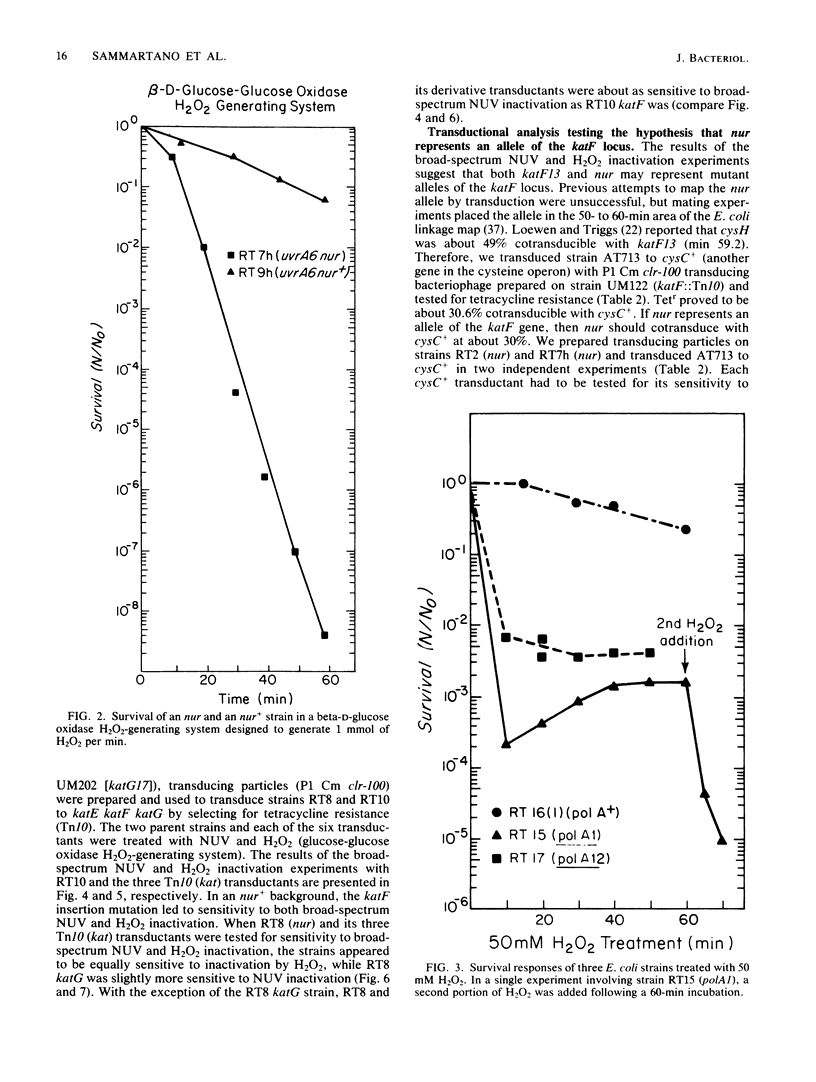
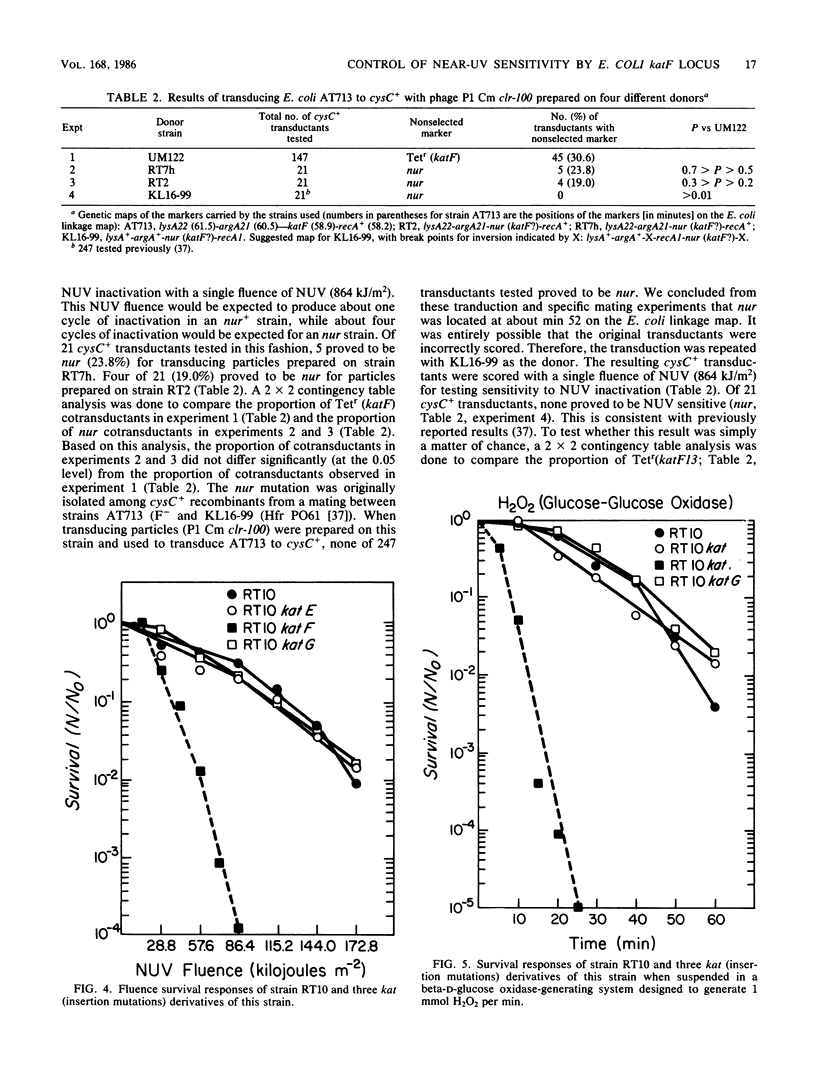
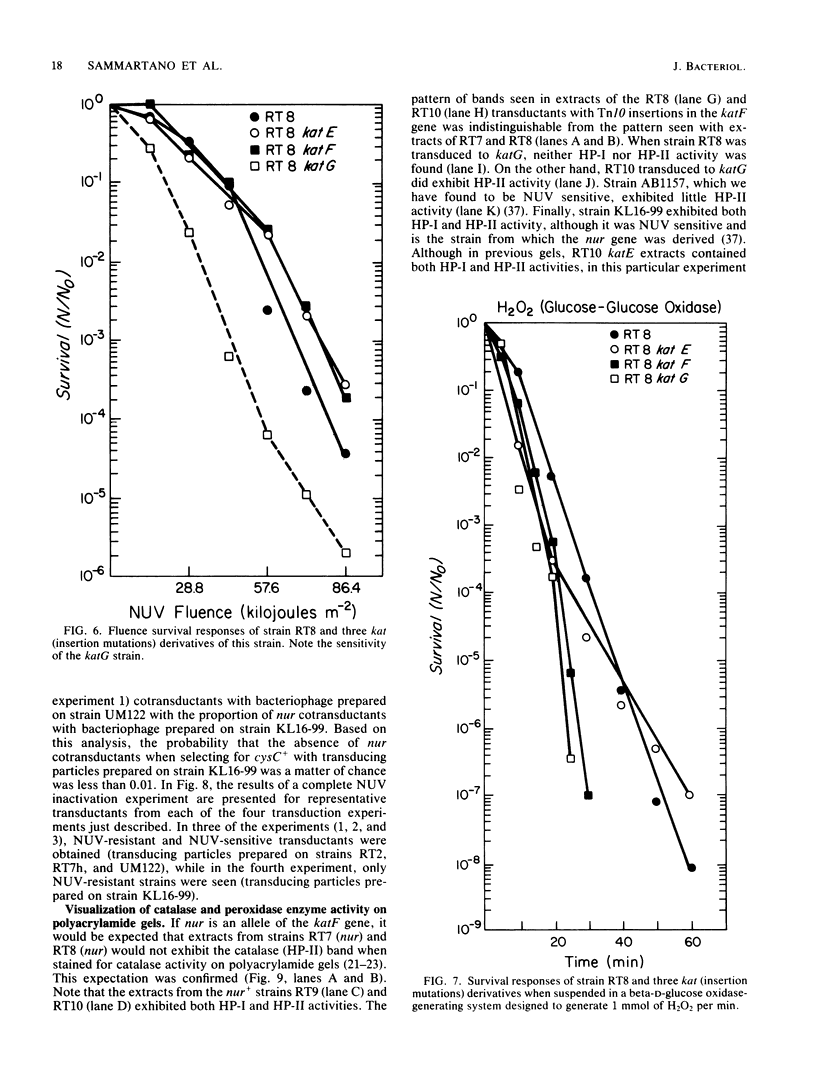
Mutations in the Escherichia coli katF gene (hydroperoxidase II) result in sensitivity to inactivation by H2O2 and broad-spectrum near-UV (NUV; 300 to 400 nm) radiation. Another mutation, nur, originally described as conferring sensitivity to inactivation by broad-spectrum and monochromatic NUV, also confers sensitivity to inactivation by H2O2. Genetic analysis via transduction suggests that the nur mutation allele of the katF locus. As previously reported for broad-spectrum and monochromatic NUV wavelengths, the sensitivity of a particular strain to H2O2 inactivation is also independent of the recA and uvrA alleles. Extracts of nur and katF strains lack catalase (hydroperoxidase II) as revealed by polyacrylamide gels stained for such activity, which is consistent with the genetic results.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthaswamy H. N., Eisenstark A. Near-UV-induced breaks in phage DNA: sensitization by hydrogen peroxide (a tryptophan photoproduct). Photochem Photobiol. 1976 Nov;24(5):439–442. doi: 10.1111/j.1751-1097.1976.tb06851.x. [DOI] [PubMed] [Google Scholar]
- Ananthaswamy H. N., Eisenstark A. Repair of hydrogen peroxide-induced single-strand breaks in Escherichia coli deoxyribonucleic acid. J Bacteriol. 1977 Apr;130(1):187–191. doi: 10.1128/jb.130.1.187-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Carpenter V. S. The recA+ gene product is more important than catalase and superoxide dismutase in protecting Escherichia coli against hydrogen peroxide toxicity. J Bacteriol. 1980 Apr;142(1):319–321. doi: 10.1128/jb.142.1.319-321.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Claiborne A., Malinowski D. P., Fridovich I. Purification and characterization of hydroperoxidase II of Escherichia coli B. J Biol Chem. 1979 Nov 25;254(22):11664–11668. [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Demple B., Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983 Aug 4;304(5925):466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- Demple B., Halbrook J., Linn S. Escherichia coli xth mutants are hypersensitive to hydrogen peroxide. J Bacteriol. 1983 Feb;153(2):1079–1082. doi: 10.1128/jb.153.2.1079-1082.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S K, Adelberg E A. The Occurrence of a Genetic Transposition in a Strain of Escherichia Coli. Genetics. 1962 May;47(5):577–585. doi: 10.1093/genetics/47.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstark A. Mutagenic and lethal effects of visible and near-ultraviolet light on bacterial cells. Adv Genet. 1971;16:167–198. [PubMed] [Google Scholar]
- Harrison A. P., Jr Survival of bacteria. Harmful effects of light, with some comparisons with other adverse physical agents. Annu Rev Microbiol. 1967;21:143–156. doi: 10.1146/annurev.mi.21.100167.001043. [DOI] [PubMed] [Google Scholar]
- Hartman P. S., Eisenstark A. Killing of Escherichia coli K-12 by near-ultraviolet radiation in the presence of hydrogen peroxide: role of double-strand DNA breaks in absence of recombinational repair. Mutat Res. 1980 Aug;72(1):31–42. doi: 10.1016/0027-5107(80)90217-1. [DOI] [PubMed] [Google Scholar]
- Hartman P. S., Eisenstark A. Synergistic killing of Escherichia coli by near-UV radiation and hydrogen peroxide: distinction between recA-repairable and recA-nonrepairable damage. J Bacteriol. 1978 Feb;133(2):769–774. doi: 10.1128/jb.133.2.769-774.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman P. S. In situ hydrogen peroxide production may account for a portion of NUV (300-400 nm) inactivation of stationary phase Escherichia coli. Photochem Photobiol. 1986 Jan;43(1):87–89. doi: 10.1111/j.1751-1097.1986.tb05595.x. [DOI] [PubMed] [Google Scholar]
- Jagger J. Near-UV radiation effects on microorganisms. Photochem Photobiol. 1981 Dec;34(6):761–768. [PubMed] [Google Scholar]
- KLEBANOFF S. J. The relationship of hydrogen peroxide to the inhibition of the glyoxalase activity of intact erythrocytes by x-radiation. J Gen Physiol. 1958 Mar 20;41(4):737–753. doi: 10.1085/jgp.41.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonardo J. M., Reynolds P. R., Tuveson R. W. Mutation induction by 365-nm radiation and far-ultraviolet light in Escherichia coli strains differing in near- and far-ultraviolet light sensitivity. Mutat Res. 1984 Mar;126(1):1–8. doi: 10.1016/0027-5107(84)90163-5. [DOI] [PubMed] [Google Scholar]
- Loewen P. C. Isolation of catalase-deficient Escherichia coli mutants and genetic mapping of katE, a locus that affects catalase activity. J Bacteriol. 1984 Feb;157(2):622–626. doi: 10.1128/jb.157.2.622-626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen P. C., Switala J., Triggs-Raine B. L. Catalases HPI and HPII in Escherichia coli are induced independently. Arch Biochem Biophys. 1985 Nov 15;243(1):144–149. doi: 10.1016/0003-9861(85)90782-9. [DOI] [PubMed] [Google Scholar]
- Loewen P. C., Triggs B. L. Genetic mapping of katF, a locus that with katE affects the synthesis of a second catalase species in Escherichia coli. J Bacteriol. 1984 Nov;160(2):668–675. doi: 10.1128/jb.160.2.668-675.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen P. C., Triggs B. L., George C. S., Hrabarchuk B. E. Genetic mapping of katG, a locus that affects synthesis of the bifunctional catalase-peroxidase hydroperoxidase I in Escherichia coli. J Bacteriol. 1985 May;162(2):661–667. doi: 10.1128/jb.162.2.661-667.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Formation of merodiploids in matings with a class of Rec- recipient strains of Escherichia coli K12. Proc Natl Acad Sci U S A. 1968 May;60(1):160–167. doi: 10.1073/pnas.60.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. P., Fischer J. R., Pachlatko J. P., Eisenstark A. Characterization of a cell-lethal product from the photooxidation of tryptophan: hydrogen peroxide. Science. 1976 Feb 6;191(4226):468–469. doi: 10.1126/science.1108203. [DOI] [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Sammartano L. J., Tuveson R. W. Escherichia coli xthA mutants are sensitive to inactivation by broad-spectrum near-UV (300- to 400-nm) radiation. J Bacteriol. 1983 Nov;156(2):904–906. doi: 10.1128/jb.156.2.904-906.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammartano L. J., Tuveson R. W. Hydrogen peroxide induced resistance to broad-spectrum near-ultraviolet light (300-400 nm) inactivation in Escherichia coli. Photochem Photobiol. 1985 Mar;41(3):367–370. doi: 10.1111/j.1751-1097.1985.tb03499.x. [DOI] [PubMed] [Google Scholar]
- Sammartano L. J., Tuveson R. W. The effects of exogenous catalase on broad-spectrum near-UV (300-400 nm) treated Escherichia coli cells. Photochem Photobiol. 1984 Nov;40(5):607–612. doi: 10.1111/j.1751-1097.1984.tb05348.x. [DOI] [PubMed] [Google Scholar]
- Turner M. A., Eisenstark A. Near-ultraviolet radiation blocks SOS responses to DNA damage in Escherichia coli. Mol Gen Genet. 1984;193(1):33–37. doi: 10.1007/BF00327410. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W. Genetic control of near-UV sensitivity independent of excision deficiency (uvrA6) in Escherichia coli K12. Photochem Photobiol. 1980 Nov;32(5):703–705. doi: 10.1111/j.1751-1097.1980.tb04044.x. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W., Jonas R. B. Genetic control of near-UV (300-400 NM) sensitivity independent of the recA gene in strains of Escherichia coli K12. Photochem Photobiol. 1979 Dec;30(6):667–676. doi: 10.1111/j.1751-1097.1979.tb07197.x. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W., Peak J. G., Peak M. J. Single-strand DNA breaks induced by 365 NM radiation in Escherichia coli strains differing in sensitivity to near and far UV. Photochem Photobiol. 1983 Jan;37(1):109–112. doi: 10.1111/j.1751-1097.1983.tb04442.x. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W., Sammartano L. J. Sensitivity of hemA mutant Escherichia coli cells to inactivation by near-UV light depends on the level of supplementation with delta-aminolevulinic acid. Photochem Photobiol. 1986 Jun;43(6):621–626. doi: 10.1111/j.1751-1097.1986.tb05637.x. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W. The interaction of a gene (nur) controlling near-UV sensitivity and the polA1 gene in strains of E. coli K12. Photochem Photobiol. 1981 Jun;33(6):919–923. doi: 10.1111/j.1751-1097.1981.tb05513.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M. A common pathway for protection of bacteria against damage by solar UVA (334 nm, 365 nm) and an oxidising agent (H2O2). Mutat Res. 1985 May;145(3):129–136. doi: 10.1016/0167-8817(85)90019-7. [DOI] [PubMed] [Google Scholar]
- Webb R. B., Tuveson R. W. Differential sensitivity to inactivation of nur and nur+ strains of Escherichia coli at six selected wavelengths in the UVA, UVB and UVC ranges. Photochem Photobiol. 1982 Nov;36(5):525–530. doi: 10.1111/j.1751-1097.1982.tb04411.x. [DOI] [PubMed] [Google Scholar]
- Yoakum G. H. Tryptophan photoproduct(s): sensitized induction of strand breaks (or alkali-labile bonds) in bacterial deoxyribonucleic acid during near-ultraviolet irradiation. J Bacteriol. 1975 Apr;122(1):199–205. doi: 10.1128/jb.122.1.199-205.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoakum G., Eisenstark A. Toxicity of L-Tryptophan photoproduct on recombinationless (rec) mutants of Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):653–655. doi: 10.1128/jb.112.1.653-655.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



