Abstract
An antibody capture radioimmunoassay was established for the detection of coxsackievirus B4 and B5-specific IgM. A significant feature of the assay was the use of an unrefined coxsackievirus B (CBV) antigen. The antigen was prepared by freeze thawing, ultrasonication and low speed centrifugation of infected Vero cells with no purification or concentration of the antigen being performed. Results of sera tested were expressed as a serum ratio (SR) by comparison with a low positive control serum. To establish an SR indicating positivity in the assays, 100 antenatal sera collected in late February were tested. The mean SR was calculated and the mean plus three standard deviations was taken as the minimum SR indicating positivity. Although resulting in a relatively insensitive assay, such a value was required to exclude sera giving a low level of reactivity which may be due to residual enterovirus-specific IgM resulting from a remote infection.
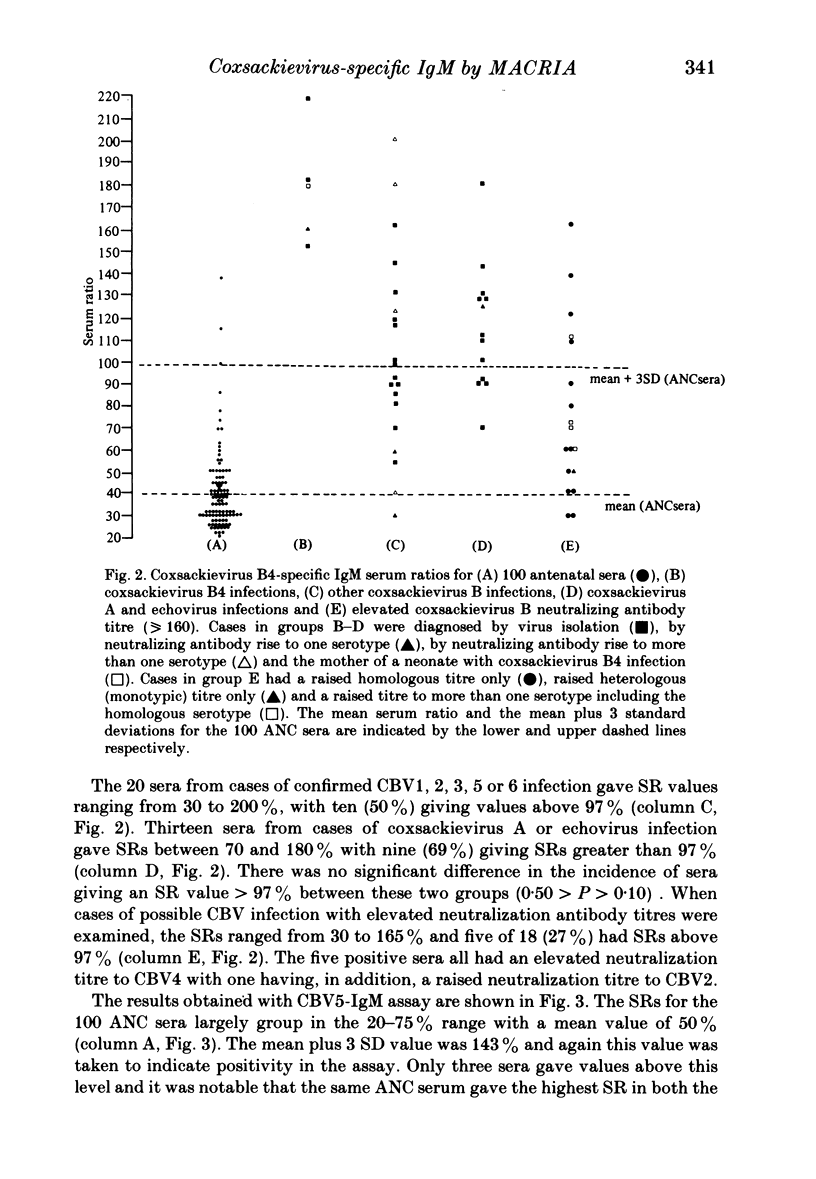
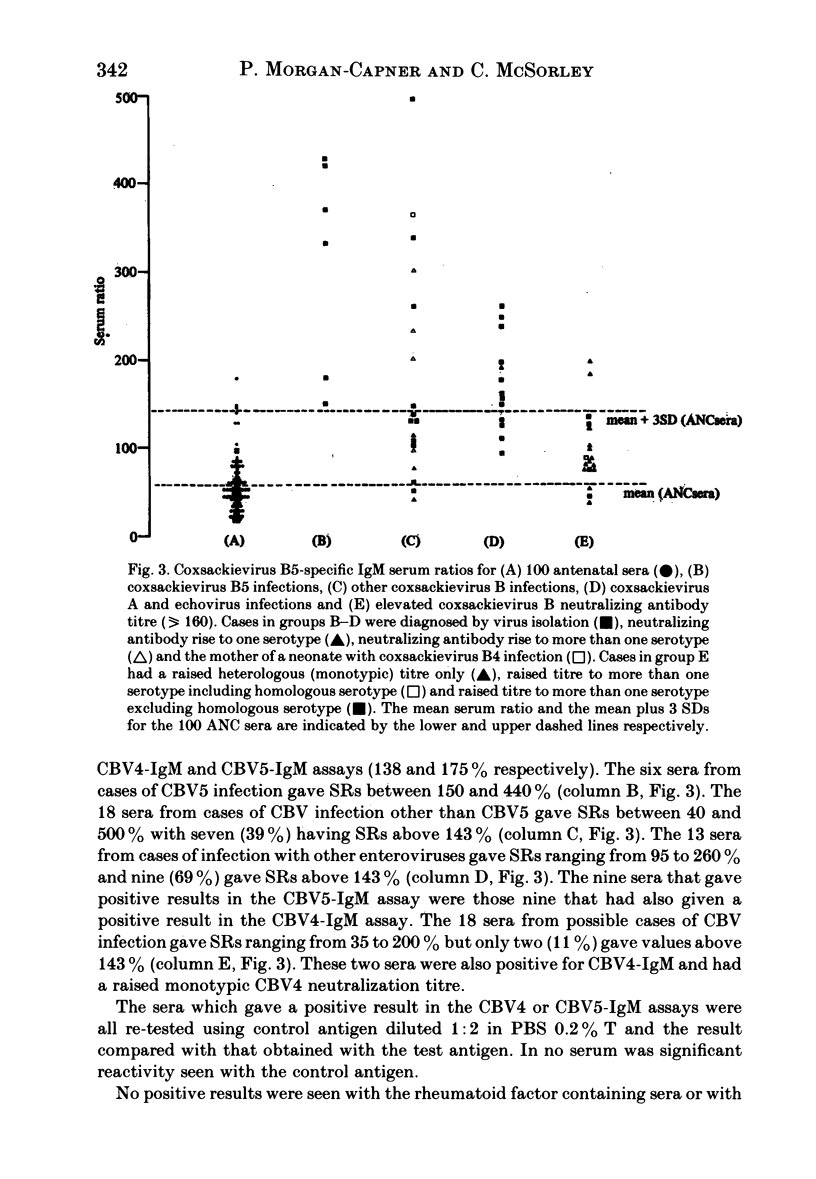
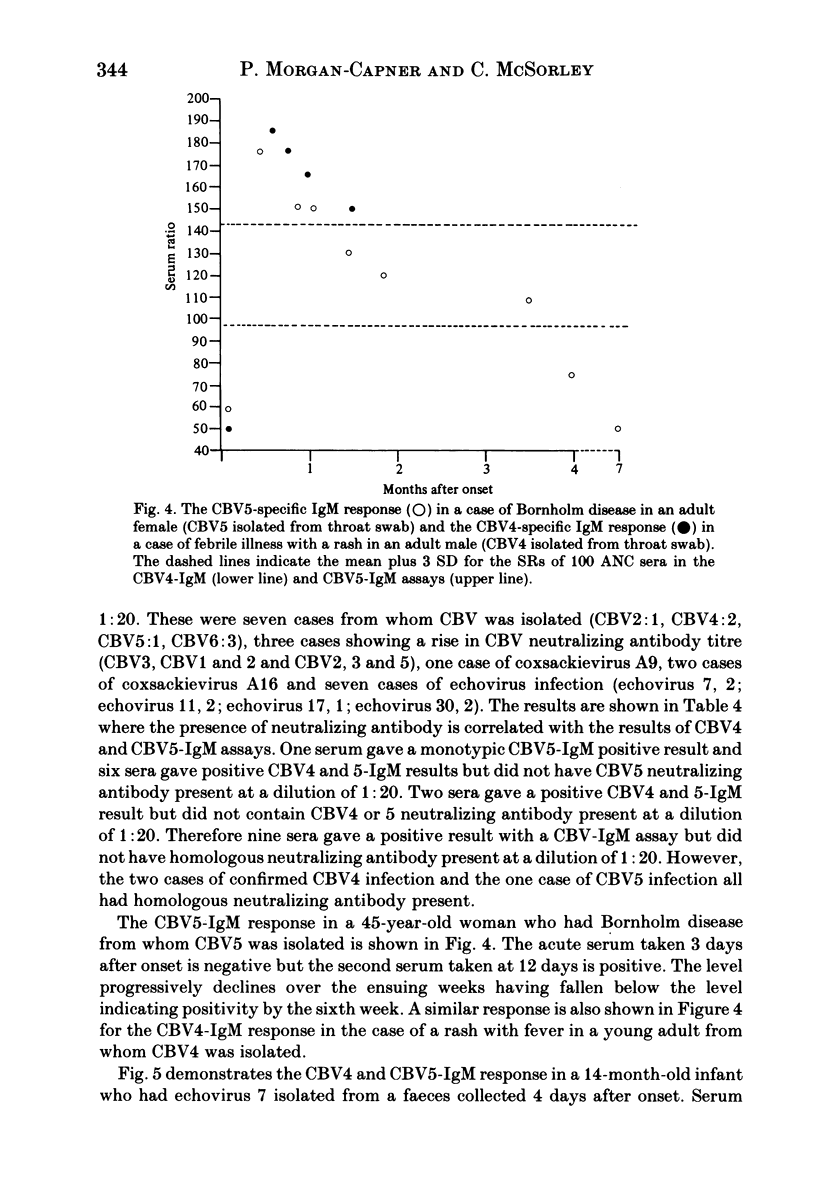
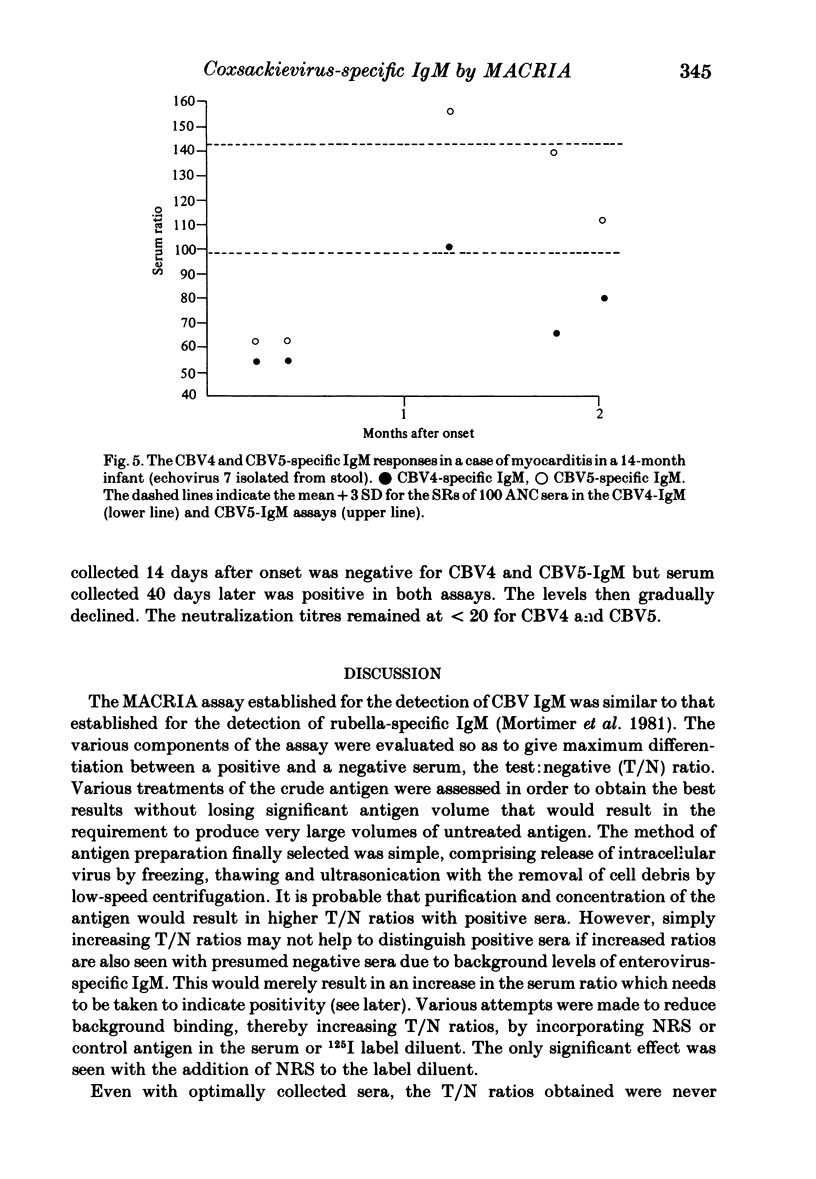
The homologous CBV-IgM assay was positive in four cases of CBV4 infection and six cases of CBV5 infection. For the CBV4 IgM assay, ten of 20 (50%) sera from infections with CBV other than CBV4 were positive and nine of the 13 (69%) sera from infections with echoviruses or coxsackieviruses A were positive. Five of 18 (27%) sera with an elevated CBV neutralization titre were positive in the CBV4-IgM assay. For the CB5-IgM assay seven of 18 (39%) sera from infections with CBV other than CBV5 were positive and nine of 13 (69%) sera from infections with echoviruses or coxsackieviruses A were positive. The nine sera that were positive from this group in the CBV5-IgM assay were the same nine as were positive in the CBV4-IgM assay. Two of the 18 (11%) sera with an elevated CBV neutralization titre were positive in the CBV5-IgM assay. These two sera were also positive in the CBV4-IgM assay and had an elevated monotypic CBV4 neutralization titre. None of the sera giving positive results gave significant reactivity when tested with control antigen. Twelve rheumatoid factor containing sera and 46 sera from other infections were negative in both assays. Of 24 sera from confirmed CBV infection, seven gave a positive monotypic CBV4 or 5-IgM response, ten were positive in both assays and seven were negative in both assays. The positive results seen with sera from cases of heterologous enterovirus infection may result from an anamnestic IgM response or, more likely, IgM directed against enterovirus cross-reacting antigens. The absence of homologous neutralizing antibody at a dilution of 1:20 in nine of 20 sera that gave a positive CBV-IgM result and the presence of positive results for CBV4 and 5-IgM in a 14 month old infant who had echovirus 7 infection indicates that the IgM need not be directed against neutralizing antigens.
Thus the CBV4 and 5-IgM assays developed appeared to be specific for an enterovirus infection but because of the cross-reactivity were not type-specific or group-specific.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dörries R., ter Meulen V. Detection of enterovirus specific IgG and IgM antibodies in humans by an indirect solid phase radioimmunoassay. Med Microbiol Immunol. 1980;168(3):159–171. doi: 10.1007/BF02122850. [DOI] [PubMed] [Google Scholar]
- El-Hagrassy M. M., Banatvala J. E., Coltart D. J. Coxsackie-B-virus-specific IgM responses in patients with cardiac and other diseases. Lancet. 1980 Nov 29;2(8205):1160–1162. doi: 10.1016/s0140-6736(80)92595-7. [DOI] [PubMed] [Google Scholar]
- Flehmig B., Ranke M., Berthold H., Gerth H. J. A solid-phase radioimmunoassay for detection of IgM antibodies to hepatitis A virus. J Infect Dis. 1979 Aug;140(2):169–175. doi: 10.1093/infdis/140.2.169. [DOI] [PubMed] [Google Scholar]
- Grist N. R., Bell E. J. A six-year study of coxsackievirus B infections in heart disease. J Hyg (Lond) 1974 Oct;73(2):165–172. doi: 10.1017/s0022172400023998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grist N. R., Bell E. J., Assaad F. Enteroviruses in human disease. Prog Med Virol. 1978;24:114–157. [PubMed] [Google Scholar]
- Katze M. G., Crowell R. L. Immunological studies of the group B coxsackieviruses by the sandwich enzyme-linked immunosorbent assay (ELISA) and immunoprecipitation. J Gen Virol. 1980 Oct;50(2):357–367. doi: 10.1099/0022-1317-50-2-357. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Crowell R. L. Indirect enzyme-linked immunosorbent assay (ELISA) for the detection of Coxsackievirus group B antibodies. J Gen Virol. 1980 May;48(1):225–229. doi: 10.1099/0022-1317-48-1-225. [DOI] [PubMed] [Google Scholar]
- Luqman W. A., Matej L. A., Smith M. L. Comparison of prolactin levels in human semen and seminal plasma. J Endocrinol. 1979 Apr;81(1):131–133. doi: 10.1677/joe.0.0810131. [DOI] [PubMed] [Google Scholar]
- MacWilliam K. M., Cooper M. A. Antibody levels in human sera measured by the fluorescent-antibody technique against the coxsackie B viruses types 1-5 grown in HEp2 cells compared with results obtained by neutralization. J Clin Pathol. 1974 Oct;27(10):825–827. doi: 10.1136/jcp.27.10.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L. Taxonomy and nomenclature of viruses, 1982. Prog Med Virol. 1982;28:208–221. [PubMed] [Google Scholar]
- Minor T. E., Helstrom P. B., Nelson D. B., D'Alessio D. J. Counterimmunoelectrophoresis test for immunoglobulin M antibodies to group B coxsackievirus. J Clin Microbiol. 1979 Apr;9(4):503–506. doi: 10.1128/jcm.9.4.503-506.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Capner P., Tedder R. S., Mace J. E. Rubella-specific IgM reactivity in sera from cases of infectious mononucleosis. J Hyg (Lond) 1983 Jun;90(3):407–413. doi: 10.1017/s0022172400029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer P. P., Tedder R. S., Hamblig M. H., Shafi M. S., Burkhardt F., Schilt U. Antibody capture radioimmunoassay for anti-rubella IgM. J Hyg (Lond) 1981 Apr;86(2):139–153. doi: 10.1017/s0022172400068856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Dennis J. Characterization of antibodies produced in natural and experimental coxsackievirus infections. J Immunol. 1968 Jan;100(1):99–106. [PubMed] [Google Scholar]
- Schmidt N. J., Magoffin R. L., Lennette E. H. Association of group B coxsackie viruses with cases of pericarditis, myocarditis, or pleurodynia by demonstration of immunoglobulin M antibody. Infect Immun. 1973 Sep;8(3):341–348. doi: 10.1128/iai.8.3.341-348.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton S. A., Hodgson J., Morgan-Capner P. The detection of rubella-specific IgM by an immunosorbent assay with solid-phase attachment of red cells (SPARC). J Hyg (Lond) 1982 Jun;88(3):453–461. doi: 10.1017/s0022172400070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder R. S., Yao J. L., Anderson M. J. The production of monoclonal antibodies to rubella haemagglutinin and their use in antibody-capture assays for rubella-specific IgM. J Hyg (Lond) 1982 Apr;88(2):335–350. doi: 10.1017/s0022172400070182. [DOI] [PMC free article] [PubMed] [Google Scholar]


