Abstract
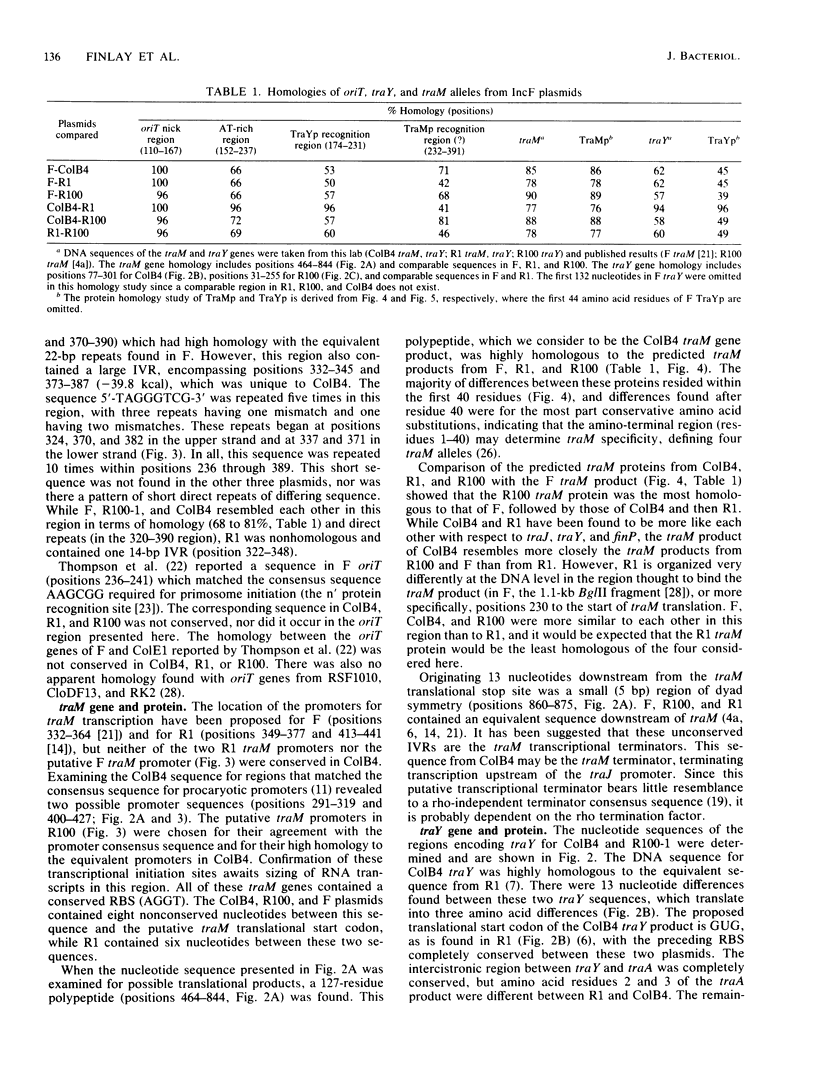
The complete nucleotide sequences of the ColB4-K98 (ColB4) plasmid transfer genes oriT, traM, and traY as well as the traY gene of R100-1 are presented and compared with the corresponding regions from the conjugative plasmids F, R1, and R100. The sequence encoding the oriT nick sites and surrounding inverted repeats identified in F was conserved in ColB4. The adenine-thymine-rich sequence following these nick sites was conserved in R1 and ColB4 but differed in F and R100, indicating that this region may serve as the recognition site for the traY protein. A series of direct repeats unique to the ColB4 plasmid was found in the region of dyad symmetry following this AT-rich region. This area also encodes 21-base-pair direct repeats which are homologous to those in F and R100. The traM gene product may bind in this region. Overlapping and following these repeats is the promoter(s) for the traM protein. The traM protein from ColB4 is similar to the equivalent products from F, R1, and R100. The traY protein from ColB4 is highly homologous to the R1 traY gene product, while the predicted R100-1 traY product differs at several positions. These differences presumably define the different alleles of traM and traY previously identified for IncF plasmids by genetic criteria. The translational start codons of the ColB4 and R100-1 traY genes are GUG and UUG, respectively, two examples of rare initiator codon usage.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Monem M., Taucher-Scholz G., Klinkert M. Q. Identification of Escherichia coli DNA helicase I as the traI gene product of the F sex factor. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4659–4663. doi: 10.1073/pnas.80.15.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram D., Ray A., O'Gorman L., Skurray R. Transcriptional analysis of the leading region in F plasmid DNA transfer. Plasmid. 1984 May;11(3):221–233. doi: 10.1016/0147-619x(84)90028-3. [DOI] [PubMed] [Google Scholar]
- Everett R., Willetts N. Characterisation of an in vivo system for nicking at the origin of conjugal DNA transfer of the sex factor F. J Mol Biol. 1980 Jan 15;136(2):129–150. doi: 10.1016/0022-2836(80)90309-5. [DOI] [PubMed] [Google Scholar]
- Fee B. E., Dempsey W. B. Cloning, mapping, and sequencing of plasmid R100 traM and finP genes. J Bacteriol. 1986 Jul;167(1):336–345. doi: 10.1128/jb.167.1.336-345.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W. Localization, cloning, and sequence determination of the conjugative plasmid ColB2 pilin gene. J Bacteriol. 1984 Oct;160(1):402–407. doi: 10.1128/jb.160.1.402-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W. Nucleotide sequences of the R1-19 plasmid transfer genes traM, finP, traJ, and traY and the traYZ promoter. J Bacteriol. 1986 May;166(2):368–374. doi: 10.1128/jb.166.2.368-374.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W., Willetts N. S. Nucleotide sequences of five IncF plasmid finP alleles. J Bacteriol. 1986 Aug;167(2):754–757. doi: 10.1128/jb.167.2.754-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler T., Taylor L., Thompson R. The control region of the F plasmid transfer operon: DNA sequence of the traJ and traY genes and characterisation of the traY leads to Z promoter. Gene. 1983 Dec;26(1):79–89. doi: 10.1016/0378-1119(83)90038-0. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Finlay B. B., Opgenorth A., Paranchych W., Lee J. S. Characterization and sequence analysis of pilin from F-like plasmids. J Bacteriol. 1985 Dec;164(3):1238–1247. doi: 10.1128/jb.164.3.1238-1247.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz B., Deonier R. C. Formation of delta tra F' plasmids: specific recombination at oriT. J Mol Biol. 1985 Nov 20;186(2):267–274. doi: 10.1016/0022-2836(85)90103-2. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Kingsman A., Willetts N. The requirements for conjugal DNA synthesis in the donor strain during flac transfer. J Mol Biol. 1978 Jul 5;122(3):287–300. doi: 10.1016/0022-2836(78)90191-2. [DOI] [PubMed] [Google Scholar]
- Koronakis V. E., Bauer E., Högenauer G. The traM gene of the resistance plasmid R1: comparison with the corresponding sequence of the Escherichia coli F factor. Gene. 1985;36(1-2):79–86. doi: 10.1016/0378-1119(85)90071-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann E., Kricek F., Högenauer G. Cloning the origin of transfer region of the resistance plasmid R1. EMBO J. 1984 Aug;3(8):1731–1735. doi: 10.1002/j.1460-2075.1984.tb02039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Thompson R., Taylor L., Kelly K., Everett R., Willetts N. The F plasmid origin of transfer: DNA sequence of wild-type and mutant origins and location of origin-specific nicks. EMBO J. 1984 May;3(5):1175–1180. doi: 10.1002/j.1460-2075.1984.tb01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Taylor L. Promoter mapping and DNA sequencing of the F plasmid transfer genes traM and traJ. Mol Gen Genet. 1982;188(3):513–518. doi: 10.1007/BF00330058. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Suggs S. V., Miyoshi K., Bhatt R., Itakura K. A set of synthetic oligodeoxyribonucleotide primers for DNA sequencing in the plasmid vector pBR322. Gene. 1981 Dec;16(1-3):21–26. doi: 10.1016/0378-1119(81)90057-3. [DOI] [PubMed] [Google Scholar]
- Willetts N., Maule J. Specificities of IncF plasmid conjugation genes. Genet Res. 1986 Feb;47(1):1–11. doi: 10.1017/s0016672300024447. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ende A., Teertstra R., van der Avoort H. G., Weisbeek P. J. Initiation signals for complementary strand DNA synthesis on single-stranded plasmid DNA. Nucleic Acids Res. 1983 Jul 25;11(14):4957–4975. doi: 10.1093/nar/11.14.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]


