Abstract
The 97-kD O-linked glycoprotein, Nup98, is a component of the Xenopus laevis nuclear pore complex and the only vertebrate GLFG nucleoporin identified (Powers, M.A., C. Macauley, F. Masiarz, and D.J. Forbes. 1995. J. Cell Biol. 128:721–736). We have investigated possible roles of xNup98 in the nucleocytoplasmic transport of proteins and RNAs by analyzing the consequences of injecting monospecific polyclonal antibodies to xNup98 into X. laevis oocytes. We show here that nuclear injection of anti-xNup98 inhibited the export of multiple classes of RNAs, including snRNAs, 5S RNA, large ribosomal RNAs, and mRNA. In contrast, the export of tRNA was unaffected. Injection of antixNup98 into the oocyte cytoplasm had no effect on export of any of the RNAs. Significantly, nuclear injection of anti-xNup98 antibodies did not inhibit import of either karyophilic proteins or snRNPs. This latter result is in agreement with our previous finding that Nup98 is not an essential element of the protein import pathway. Thus, Nup98 plays a role specifically in RNA export from the nucleus, and it appears to be an essential component of multiple RNA export pathways.
Trafficking across the nuclear envelope occurs exclusively through the nuclear pore complex, which both imports proteins and small nuclear ribonucleoproteins (snRNPs)1 and exports RNAs and ribosomal subunits. In addition to the proteins of the pore, nucleocytoplasmic transport requires soluble factors such as the importin α/β heterodimer, which binds directly to nucleartargeted proteins, and the GTPase, Ran, with its associated stimulatory and recycling factors (for review see Moore and Blobel, 1994; Powers and Forbes, 1994; Melchior and Gerace, 1995; Görlich and Mattaj, 1996; Sazer, 1996).
The nuclear pore complex itself is a large and elaborate structure of 120 MD in vertebrates, comprising ∼100 different proteins, many of which are present in multiple copies (for review see Rout and Wente, 1994; Davis, 1995). Structurally, the pore consists of a core of eight spokes surrounding a central transporter which spans the nuclear envelope. This core structure is flanked by a cytoplasmic ring, from which fibers project into the cytoplasm, as well as a nuclear ring from which a basket-like structure extends into the nucleoplasm (for review see Pante and Aebi, 1993; Rout and Wente, 1994). Additional long fibers project from the basket into the nucleus (Cordes et al., 1993). Both the cytoplasmic fibers and the nuclear basket have been hypothesized to play roles in the initial binding of transport substrates to the pore. Indeed, scanning electron microscopy of Balbiani ring transcripts shows movement through the basket (Kiseleva et al., 1996).
Much progress has been made recently in our knowledge of the nuclear pore complex. In yeast, multiple nucleoporin genes have been identified, and mutational analysis has linked functional or structural phenotypes with specific gene products (for review see Doye and Hurt, 1995). In vertebrates, 12 of the potential ∼100 nucleoporins have been identified and localized to specific substructures of the pore (for review see Pante and Aebi, 1993). Of these 12, approximately half contain repeated peptide motifs: FXFG in the majority (for review see Fabre and Hurt, 1994; Davis, 1995), and GLFG in a single protein, Nup98 (Powers et al., 1995; Radu et al., 1995b ). Several lines of evidence implicate the FXFG family of nucleoporins as crucial to nuclear transport. In vivo, antibodies to the FXFG family impair both nuclear protein import and RNA export (Dabauvalle et al., 1988; Featherstone et al., 1988; Terns and Dahlberg, 1994; Lund and Dahlberg, manuscript in preparation). Recently, a member of this family was reported to play a role in the export of polyA+ RNA (Bastos et al., 1996). FG repeat-containing nucleoporins of the cytoplasmic fibers (Nup 358 and Nup214) and the nuclear basket (Nup153 and Nup98) have been shown to bind soluble transport factors in vitro (Moroianu et al., 1995; Radu et al., 1995a ; Radu et al., 1995b ). Moreover, binding experiments using isolated yeast nucleoporins suggest that interactions between such repeat domains and import factors may be functionally important in transport (Rexach and Blobel, 1995; Nehrbass and Blobel, 1996). Finally, in vitro nuclear reconstitution has indicated the importance of FXFG nucleoporins in nuclear import (Dabauvalle et al., 1990; Finlay and Forbes, 1990; Finlay et al., 1991; Miller and Hanover, 1994).
At present, Nup98 is the only vertebrate nucleoporin identified that contains GLFG repeats. Interestingly, reconstituted nuclei that lack this GLFG nucleoporin are competent for nuclear protein import. However, xNup98- depleted nuclei remain small and fail to replicate their DNA, indicating an essential nuclear role for Nup98, but not one in import (Powers et al., 1995). Immunofluorescence shows that xNup98 is present both at the pore and within the nuclear interior (Powers et al., 1995), and rat Nup98 has been localized within the pore to the nuclear basket by immunoelectron microscopy (Radu et al., 1995b ). In vitro binding assays indicate that rat Nup98 can interact with soluble cytoplasmic import factors (Moroianu et al., 1995; Radu et al., 1995b ). However, given the localization of Nup98 at the nuclear basket and within the nucleus, the functional significance of this latter interaction is not understood.
The GLFG family in the yeast Saccharomyces cerevisiae includes five nucleoporins: Nup49, Nup54, Nup100, Nup116, and Nup145 (Wente et al., 1992; Wimmer et al., 1992). Mutations in members of this family have pleiotropic effects on yeast nuclear function, including aberrant nuclear envelope structure, nuclear accumulation of polyA+ RNA, and impaired nuclear import (for review see Doye and Hurt, 1995). Nup49 and Nup54 are essential proteins present in a multiprotein complex that is primarily required for nuclear protein import (Schlenstedt et al., 1993; Grandi et al., 1995). Deletion of the essential Nup145 gene results in a defect not in protein import, but in poly A+ RNA export (Fabre et al., 1994). Nup100, Nup116, and Nup145 each contains a related domain that can bind homopolymeric RNA in vitro (Fabre et al., 1994). A similar domain is found in rat Nup98, which shows strong homology to this subset of the GLFG family (Radu et al., 1995b ); peptide analysis of Xenopus Nup98 indicates that this domain is conserved in Xenopus (Powers et al., 1995). In yeast, the presence of a single gene containing this putative RNA-binding domain is sufficient for cell viability; thus Nup145, Nup116, and Nup100 appear to serve a redundant function, most likely in the export of RNA.
Export of different classes of RNA, including snRNAs, mRNA, tRNA, and ribosomal RNA, occurs via distinct pathways (for review see Izaurralde and Mattaj, 1995). This conclusion is based both on kinetic analyses and on experiments demonstrating that a given RNA is able to saturate its own export but not that of the other classes of RNA (Zasloff, 1983; Bataillé et al., 1990; Terns et al., 1993a ; Jarmolowski et al., 1994; Boelens et al., 1995; Pokrywka and Goldfarb, 1995; Simons et al., 1996). These studies have led to a model in which export of different RNAs is mediated by distinct and class-specific saturable factors. In support of this model, recent studies have identified several soluble RNA binding factors in vertebrates, such as the cap binding complex (CBC), TFIIIA, and the Rev protein of HIV-1, which bind to snRNAs, 5S RNA, and unspliced HIV RNA, respectively (Guddat et al., 1990; Izaurralde and Mattaj, 1992; Fischer et al., 1995; Izaurralde et al., 1995). It is thought that each may specifically facilitate the nuclear export of the bound RNA. In contrast, the components of the vertebrate nuclear pore that constitute the export machinery have remained, for the most part, uncharacterized.
Given the contributions of GLFG nucleoporins to nuclear export in yeast, we asked whether xNup98 might be an essential component of the vertebrate RNA export machinery. For this, we have used Xenopus laevis oocytes, which allow for microinjection of transport substrates and potentially inhibitory antibodies into either the nuclear or cytoplasmic compartment. We find that affinity purified antibodies to Xenopus Nup98, when injected into oocyte nuclei, selectively inhibit the nuclear export of multiple, but not all, classes of RNAs. However, xNup98 antibodies do not significantly impair nuclear import of either snRNPs or karyophilic proteins. These results argue strongly that Xenopus Nup98 functions as a common element in multiple pathways of RNA export from the nucleus, but not as an essential component of nuclear import pathways.
Materials and Methods
DNA Templates for In Vivo and In Vitro RNA Synthesis
Templates for in vivo RNA synthesis in oocytes were plasmid DNAs containing genes for X. laevis U4 snRNA (Vankan et al., 1990), 5S rRNA (Wolffe et al., 1986), and tRNATyr (Gouilloud and Clarkson, 1986). Templates for in vitro RNA synthesis were DNA fragments generated by PCR amplification, as described previously for U1Sm−, U1Δ, U6 snRNAs (Terns et al., 1993a ), U5 snRNA (Pasquinelli et al., 1995), and NL15 RNA (Grimm et al., 1997). The template for AdML pre-mRNA was Sma1-linearized pSP64-Ad1(+/−A) plasmid DNA (Lund and Dahlberg, manuscript in preparation). pSP64-Ad1(+/−A) contains the Pst1-Sau3A1 fragment of pBSAd1 (Konarska and Sharp, 1987) cloned between the Pst1 and BamH1 sites of pSP64 Poly(A) (Promega Corp., Madison, WI).
Antibodies for Microinjection or Immunoprecipitation
Affinity purified polyclonal rabbit antibodies to the Xenopus nucleoporins xNup98 and xNup200 were prepared as previously described (Macaulay et al., 1995; Powers et al., 1995). To generate preparations suitable for oocyte injection, affinity purified antibodies were further concentrated to 0.5–1 mg immunoglobulin/ml using a microconcentrator (Microcon 50; Amicon Corp., Danvers, MA). Immunoblots were performed as described previously, using peroxidase-conjugated secondary antibodies and a chemiluminescent substrate (Powers et al., 1995). Crude sera containing the polyclonal rabbit anti-m7G antibodies (Munns et al., 1982) used for immunoprecipitation of precursor snRNAs were a generous gift of T. Munns (Washington University School of Medicine, St. Louis, MO).
Oocyte Injection and Analysis of 32P-labeled RNAs Made In Vivo
Stage V and VI oocytes were obtained from X. laevis frogs as described (Lund and Dahlberg, 1989). For in vivo synthesis of RNAs, 12–15 nl of a solution containing a mixture of plasmid DNAs (Fig. 1, legend) plus blue dextran (as a marker) was injected into the oocyte nucleus. To monitor synthesis of the RNAs, ∼1.0 μCi of α-[32P] GTP (NEN-Dupont, Boston, MA) was injected into the cytoplasm of each oocyte, and the intracellular distribution of the newly made transcripts was assayed with time. When used, control IgG and anti-nucleoporin antibodies were either coinjected with the DNA into the nucleus (6–15 ng/oocyte) or injected separately into the cytoplasm (15–30 ng/oocyte) prior to the cytoplasmic injection of labeled GTP. Nuclei and cytoplasms were isolated from successfully injected oocytes (those with blue nuclei) by manual dissection under mineral oil (Lund and Paine, 1990), using at least three oocytes per time point. Isolation of RNAs from the nuclear and cytoplasmic fractions using proteinase K and phenol and analysis in denaturing 8% (30:0.8) polyacrylamide gels were done as detailed elsewhere (Pasquinelli et al., 1995). Autoradiograms were exposed for 18–72 h, and transport was quantitated by PhosphorImager (Molecular Dynamics, Inc., Sunnyvale, CA) analysis.
Figure 1.
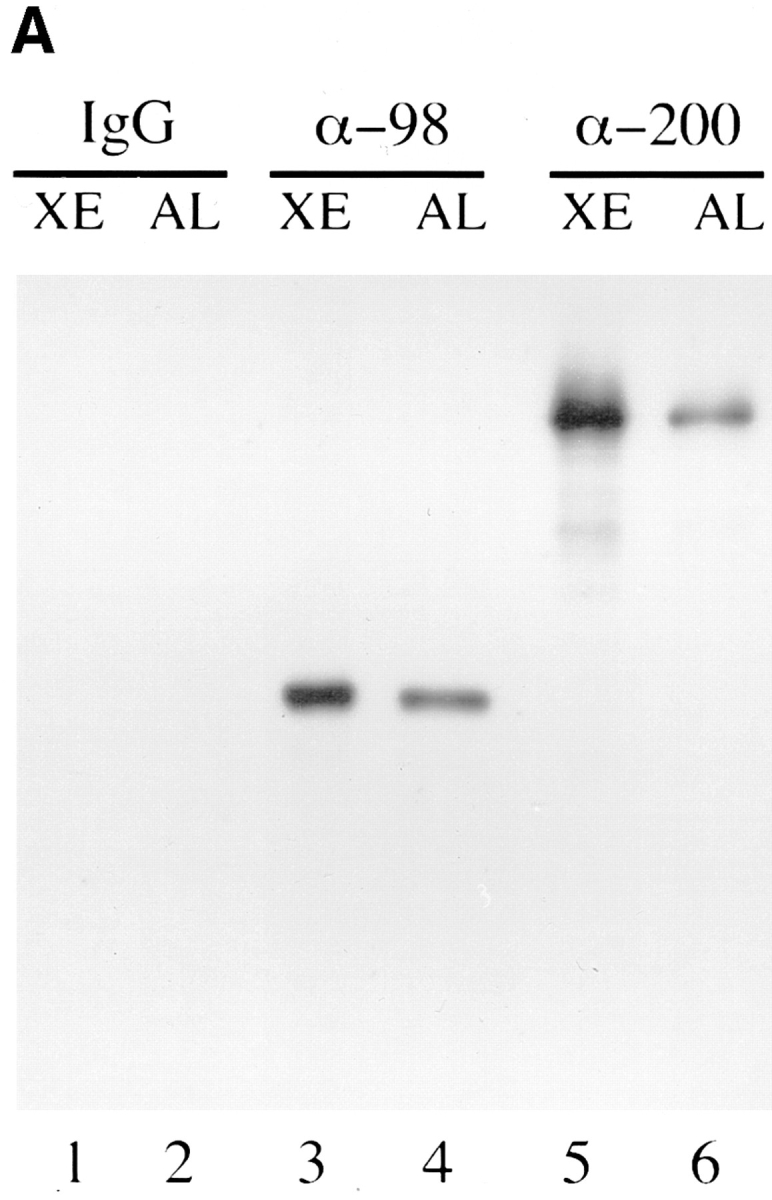
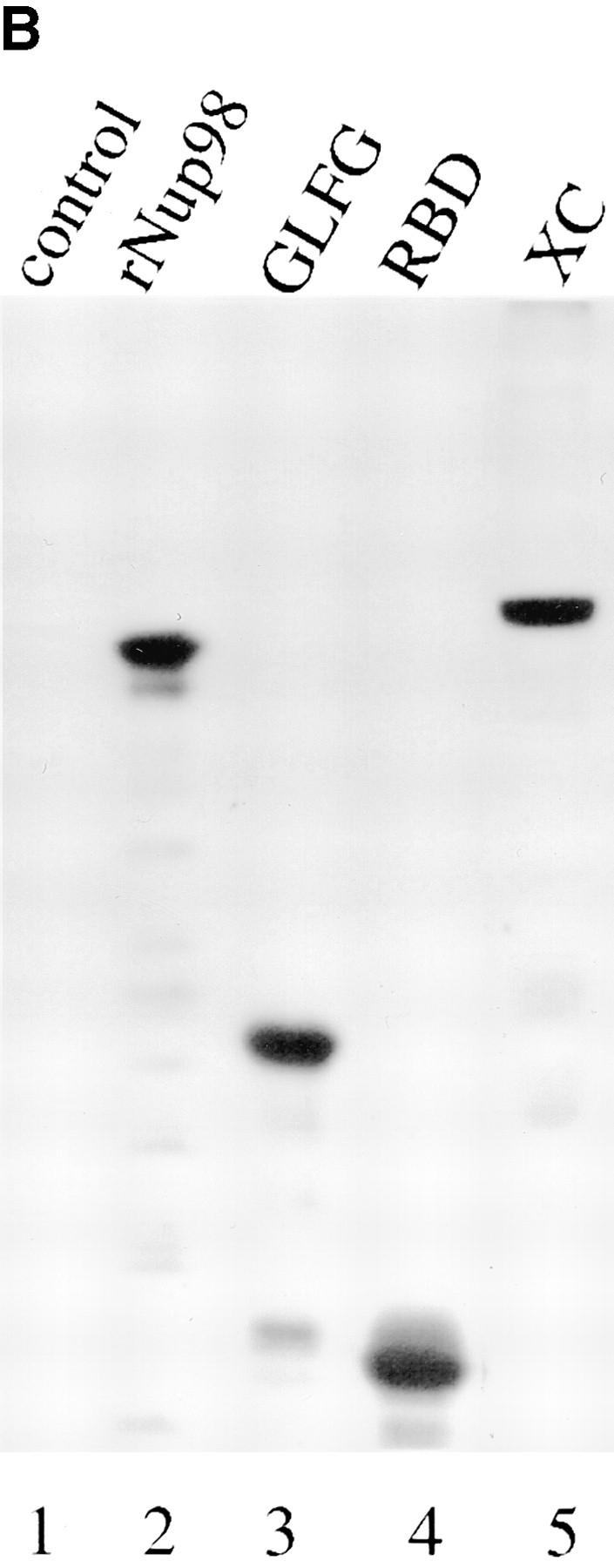
Specificity of affinity-purified anti-xNup98 antibodies. (A) Affinity purified anti-nucleoporin antibodies (α-98 and α-200) or a nonspecific rabbit IgG was used to probe immunoblots containing Xenopus WGA binding proteins (Xenopus eluate, XE) and a nuclear pore fraction from Xenopus annulate lamellae (AL). (B) Affinity purified anti-xNup98 was used to probe blots containing either E. coli lysate (control), lysate from strains expressing regions of rat Nup98, or Xenopus cytosol (XC). rNup98 corresponds to the nearly full length protein, amino acids 43–824. The GLFG repeat domain (GLFG) corresponds to amino acids 43–490. The RNA binding domain (RBD) corresponds to amino acids 618–824.
Immunoprecipitation of snRNA Precursors
Deproteinized nuclear RNAs were immunoprecipitated with anti-m7Gantibodies bound to protein A-Sepharose (Sigma Chemical Co., St. Louis, MO) as previously described (Neuman de Vegvar and Dahlberg, 1990; Terns et al., 1993b ). The RNAs in both precipitate and supernatant fractions were analyzed by PAGE, and the levels of m7G-capped snRNA precursors were quantitated by PhosphorImager analysis.
In Vitro Synthesis of 32P-labeled RNAs and Analysis of Transport
In vitro transcription with SP6 RNA polymerase (for AdML pre-mRNA and U1Sm−, U1Δ, and U5 snRNAs) or T7 RNA polymerase (for U6 snRNA and NL15 RNA) was done as described previously (Melton et al., 1984; Pasquinelli et al., 1995) using α-[32P]GTP as the label. AdML premRNA, U1Sm−, U1Δ, and U5 snRNA transcripts were synthesized with m7GpppG caps, and U6 snRNA was synthesized with a γ-mpppG cap (Terns et al., 1993a ). All RNA products were purified by electrophoresis in 8% denaturing polyacrylamide gels. For use in transport assays, 12–15 nl of a mixture of RNAs (1–10 fmol each, see Figs. 2 and 4, legends) was injected into the nucleus or cytoplasm of oocytes. For nuclear injections, U6 snRNA, which is not exported to the cytoplasm (Vankan et al., 1990; Terns et al., 1993a ), and blue dextran (Jarmolowski et al., 1994) was included as controls for the accuracy of injections and dissections. After different times of incubation, the intracellular distributions of the injected RNAs were analyzed as described above for in vivo transcribed RNAs.
Figure 2.
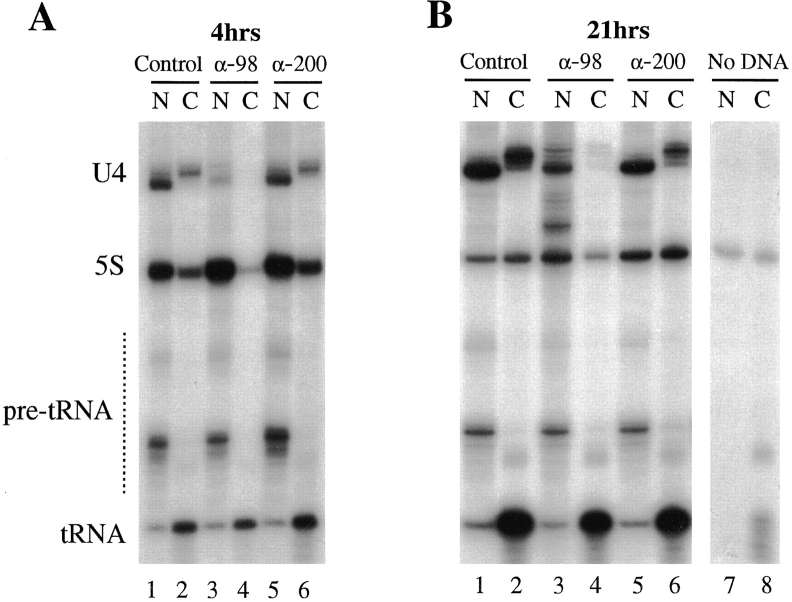
Differential effects of anti-xNup98 antibodies on transport of in vivo synthesized RNA polymerase II and III transcripts. A mixture of DNA templates encoding U4 snRNA, 5S rRNA, and tRNATyr was injected into oocyte nuclei together with buffer (Control) or monospecific antibodies against nucleoporins xNup98 (α-98) or xNup200 (α200). 2 h later, α[32P]GTP (∼1.0 μCi/oocyte) was injected into the cytoplasms of all oocytes. After 4 (A) or 21 h of labeling (B), oocytes (3–6/timepoint) were manually dissected, and the intracellular distributions of the newly made RNAs were determined by analyses of 0.5 oocyte equivalents of the nuclear (N) and cytoplasmic (C) RNAs in 8% denaturing polyacrylamide gels. The RNAs made from the injected genes are indicated. Endogenous oocyte RNAs made in the absence of injected genes (No DNA) are shown in B, lanes 7 and 8. The level of 5S DNA templates was ∼10-fold higher in A than in B.
Figure 4.
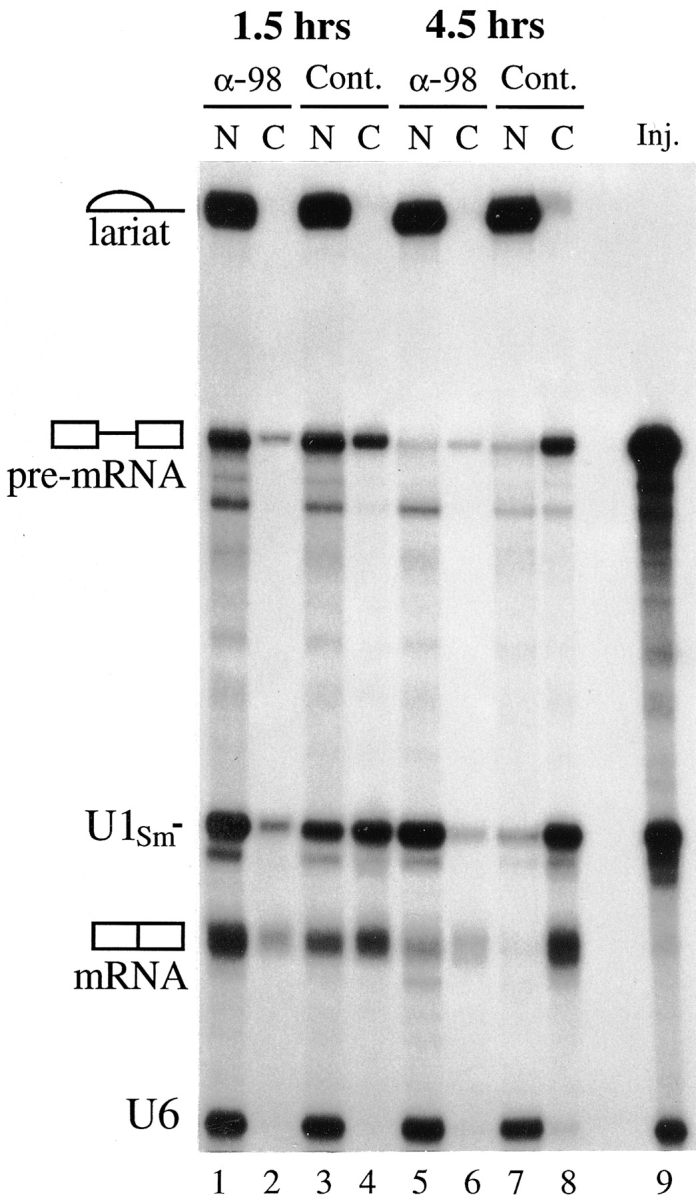
Inhibition of mRNA export by anti-xNup98 antibodies. Anti-xNup98 antibodies (α-98) or buffer (Cont.) was injected into oocyte nuclei and 2 h later the same oocytes received a second nuclear injection with a mixture of 32P-labeled AdML premRNA, U1Sm− RNA, and U6 RNA that had been synthesized in vitro using SP6 or T7 RNA polymerases. At 1.5 (lanes 1–4) and 4.5 h (lanes 5–8) after RNA injection, splicing of the AdML premRNA and export of the spliced mRNA and U1Sm− RNA were monitored by analyses of nuclear (N) and cytoplasmic (C) RNAs isolated from individual oocytes. The injected mixture of RNAs (Inj.) is shown in lane 9. U1Sm− RNA, which lacks a functional Sm binding site, is exported to the cytoplasm but cannot be imported back into the nucleus. U6 RNA is retained in the nucleus and serves as a marker for the accuracy of the second nuclear injection and oocyte dissections.
In Vivo Synthesis of 35S-labeled Nuclear Proteins and Analysis of Protein Import
To produce 35S-labeled Xenopus karyophilic proteins, 100–150 stage V and VI oocytes were incubated for 24 h in 1 ml of MBS-H medium (Gurdon and Wickens, 1983) containing 0.5–1.0 mCi of [35S]methionine (TRAN35SLABELTM; ICN Radiochemicals, Irvine, CA). Labeled nuclei were isolated by manual dissection of oocytes under mineral oil (Lund and Paine, 1990) and collected in a 0.5-ml microfuge tube on ice. After removal of excess mineral oil, 50–100 nuclei were homogenized in 10–20 μl of protein buffer (10 mM MOPS, pH 7.2, 75 mM KCl, 25 mM NaCl containing 1 mM DTT, and 1 μg/ml each of aprotinin, leupeptin, and pepstatin) by repeated pipetting. The homogenate was centrifuged at 14,000 rpm for 4 min in a microfuge; the clarified supernatant was collected and either used immediately or stored at −70°C for later injection. For protein import assays, 35–50 nl of 35S-labeled nuclear extract (∼0.25 oocyte equivalents) was injected into each oocyte cytoplasm. Where used, control IgG and either anti-nucleoporin antibodies or wheat germ agglutinin (WGA; Vector Labs, Inc., Burlingame, CA) was injected separately into the nuclei or cytoplasms before cytoplasmic injection of the import substrate. For WGA, 8–10 nl or 15–30 nl of a saturated solution (∼20 mg/ml in 88 mM NaCl, 10 mM TrisHCl, pH 8.0) was injected per nucleus or cytoplasm, respectively. After overnight incubation, oocytes (at least 5/sample) were manually dissected, and the intracellular distribution of labeled proteins was determined by analysis of 0.5–1.0 oocyte equivalents of the nuclear and cytoplasmic fractions in 10% polyacrylamide gels containing SDS (Adolph et al., 1977). Autoradiograms of the dried gels were exposed for 2–14 d at room temperature.
Results
Anti-xNup98 Antibodies Inhibit Nuclear Export of snRNA and 5S RNA, but Not tRNA
Previously, we showed that the Xenopus nucleoporin xNup98, which is located both in the nuclear interior and at the nucleoplasmic side of the nuclear pore, is not essential for protein import (Powers et al., 1995). To determine if Nup98 is required for transport out of the nucleus, we examined the effect of anti-xNup98 antibodies on the nuclear export of different classes of RNA.
Anti-xNup98 antibodies, prepared and affinity purified, as previously described, recognized a single WGA binding protein from Xenopus egg extracts (Fig. 1 A, lane 3; Powers et al., 1995). This antibody also specifically recognized a single protein of identical size in the enriched nuclear pores of Xenopus annulate lamellae, indicating that the antibody does not cross-react with any other component of the pore (Fig. 1 A, lane 4; Meier et al., 1995). In addition, the antibody was seen to cross-react with both the GLFG repeat and RNA binding domains of the homologous rat Nup98 (Fig. 1 B, lanes 2–4). A nonspecific rabbit IgG as well as antibodies to the Xenopus nucleoporin p200, which is the homolog of the mammalian nucleoporin Nup214/CAN and is localized to the cytoplasmic face of the pore (Miller and Hanover, 1994; Kraemer et al., 1994; Macaulay et al., 1995), were used for control antibody injections.
To examine their effect on RNA export, xNup98 specific antibodies were injected into Xenopus oocyte nuclei together with a mixture of plasmid DNAs encoding Xenopus U4 snRNA, 5S rRNA, and tRNATyr (Fig. 2, α-98). The DNA templates were also injected with either buffer alone (Fig. 2, Control) or with affinity purified antibodies against the Xenopus nuclear pore protein, Nup200 (α–200). AntixNup200 antibodies injected into the nucleus have no access to their cytoplasmic antigen and serve as controls for nonspecific effects of antibody injection. After a short incubation to allow for the binding of the antibodies and formation of transcription complexes, [32P]GTP was injected into the oocyte cytoplasm. At various time points, oocytes were manually dissected into nuclear and cytoplasmic fractions, and the newly synthesized, labeled RNAs in each fraction were isolated and resolved by PAGE.
In sharp contrast to the control injections, injection of antibodies to xNup98 strongly inhibited the export of U4 snRNA from the oocyte nuclei (Fig. 2, A and B, lanes 3 and 4). At both the 4 and 21 h timepoints, the level of U4 RNA in the cytoplasm of oocytes injected with antixNup98 was greatly reduced relative to the level in oocytes injected with either buffer or the control antibody (Fig. 2, A and B; lanes 2, 4, and 6). This inhibition of export was apparent even at incubation times as short as 2 h (data not shown).
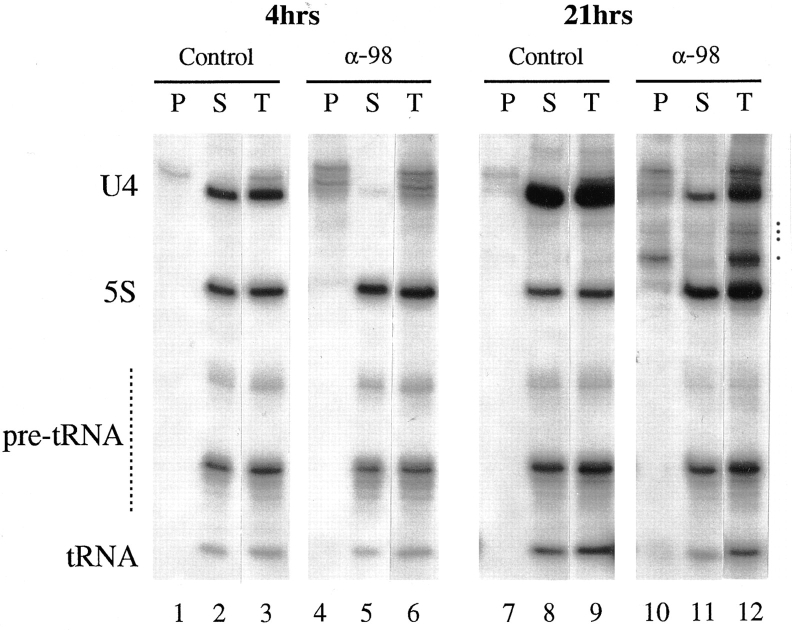
Normally, m7G-capped precursor snRNA is rapidly exported to the cytoplasm. There, it becomes complexed with cytoplasmic Sm proteins, and its cap is hypermethylated to the mature m2,2,7G form. The fully matured snRNP can then be imported back into the nucleus (Mattaj, 1986). It was thus important to determine whether the U4 RNA in the nuclei of anti-xNup98 injected oocytes had been exported to the cytoplasm and reimported, or had remained in the nucleus due to a block in export. To determine this, we immunoprecipitated nuclear RNAs with an antibody that specifically recognizes the m7G-cap common to RNA polymerase II transcripts (Munns et al., 1982). In control oocytes at both 4 and 21 h, the vast majority of U4 RNA in the nucleus consisted of the mature, nonprecipitable m2,2,7G-capped form, indicating it had been reimported (Fig. 3, lanes 1–3 and 7–9; Neuman de Vegvar and Dahlberg, 1990). In contrast, the U4 RNA present in nuclei of anti-xNup98 treated oocytes at 4 h was almost exclusively the m7G-capped, precursor forms (Fig. 3, lanes 4–6). Thus, export of newly synthesized snRNAs had been effectively blocked by microinjection of anti-xNup98 antibodies into the nucleus. Even at 21 h, only a small amount of mature U4 RNA had accumulated in the nuclei of antixNup98 treated oocytes (Fig. 3, lane 11), showing that the inhibitory effect on snRNA export was persistent. When anti-xNup98 antibodies were injected into the cytoplasm of oocytes, no deficiency in RNA transport was observed, consistent with the nuclear localization of the target Nup98 nucleoporin (data not shown). The reduced level of U4 snRNA in anti-xNup98 injected oocytes was not due to a defect in U4 transcription (data not shown) but to the inherent instability of m7G-capped precursor U4 snRNA, an instability also seen in other cases where pre-U4 is retained in the nucleus (Lund, E., and J.E. Dahlberg, manuscript in preparation; Her, L.-S., and E. Lund, unpublished results).
Figure 3.
Failure of snRNA precursors to leave the nucleus of anti-xNup98 treated oocytes. Total nuclear RNAs (T), corresponding to those in lanes 1 and 3 of Fig. 2, A and B, were immunoprecipitated with anti-m7G antibodies, which are specific for the cap structure of the nonexported, nuclear precursor snRNA. The RNAs in the precipitate (P) and supernatant (S) fractions were analyzed as in Fig. 2. Dots by lane 12 indicate m7G-capped degradation products of pre-U4 RNA that also are observed when nuclear export is inhibited by other reagents.
The export of both 5S RNA and tRNA occurs by different pathways than U4 snRNA, as determined by competition studies (Jarmolowski et al., 1994). When the export of 5S rRNA was examined, it was found to be substantially reduced in the presence of nuclear xNup98 antibodies (Fig. 2, lanes 2, 4, and 6). A reduced amount of 5S DNA was injected for the 21-h timepoint to avoid overwhelming the signals from other transcripts (Fig. 2, legend). Strikingly, the export of tRNA was completely unaffected by anti-xNup98 antibodies at all times. The persistance of tRNA export in the presence of anti-xNup98 antibodies makes it unlikely that the inhibition of U4 and 5S RNA export resulted from physical obstruction of the nuclear pore. Instead, we conclude that the binding of antibody to xNup98 prevents a specific function of this protein, a function required for multiple pathways of RNA export.
Anti-xNup98 Antibodies Impair mRNA Export
Although both mRNA and pre-snRNAs contain 5′ m7Gcaps which serve as signals for export from the nucleus (Hamm and Mattaj, 1990; Izaurralde et al., 1992; Terns et al., 1993a ; Izaurralde et al., 1995), transport of these RNAs apparently occurs via different pathways (Jarmolowski et al., 1994; Fischer et al., 1995). To determine whether the export of mRNA is also dependent on Nup98 function, we monitored the export of an mRNA that was generated by splicing of injected 32P-labeled adenovirus major late (AdML) pre-mRNA in oocyte nuclei (Hamm and Mattaj, 1990; Lund and Dahlberg, manuscript in preparation). Oocytes were injected in the nucleus twice, first with either xNup98-specific antibodies or buffer, and then, after a short incubation to allow for antibody binding, with a mixture of the 32P-labeled pre-mRNA and control RNAs. As a control for the accuracy of the second nuclear injection, we included labeled U6 snRNA, which is not exported from the nucleus. As a further test for inhibition of snRNA export by anti-xNup98, we also included U1Sm− RNA, a mutant U1RNA which would normally be exported, but which lacks the Sm binding site required for import back into the nucleus (Hamm and Mattaj, 1990).
In control oocytes, the spliced AdML mRNA was exported in a time-dependent manner (Fig. 4, mRNA, lanes 3 and 4, 7 and 8). In contrast, in anti-xNup98 injected oocytes, the majority of spliced mRNA remained in the nucleus (Fig. 4, lanes 1 and 2, 5 and 6). The level of nuclear mRNA at 4.5 h in anti-xNup98 injected oocytes (Fig. 4, lane 5) was reduced relative to the level at 1.5 h (Fig. 4, lane 1), due to the RNA turnover that occurs in the nucleus when export is inhibited; still, the majority of the mRNA observed was nuclear, not cytoplasmic (Fig. 4, lanes 5 and 6). U1Sm− RNA also was exported to the cytoplasm in control oocytes, but was largely retained in the nucleus in antixNup98 injected oocytes. Control U6 snRNA and the lariat of the pre-mRNA, RNAs normally not exported, stayed localized in the nucleus in both control and antixNup98 injected oocytes. A certain amount of unspliced pre-mRNA export was observed in control injected oocytes, most likely due to initial saturation of the splicing machinery (Lund and Dahlberg, unpublished results). Strikingly, however, this export was also sensitive to inhibition by anti-xNup98 antibodies.
In parallel experiments, we found that the simultaneous injection of anti-xNup98 antibodies with snRNA and premRNA resulted in little or no inhibition of export (data not shown). The necessity for preinjection to allow binding of antibody to antigen supports our conclusion that inhibition of RNA export results from specific interaction of the antibodies with xNup98. Taken together, the results of Figs. 2, 3, and 4 indicate that both mRNA and snRNA pathways for export are effectively inhibited by the binding of antibodies to xNup98.
Anti-xNup98 Antibodies Decrease Ribosomal RNA Export
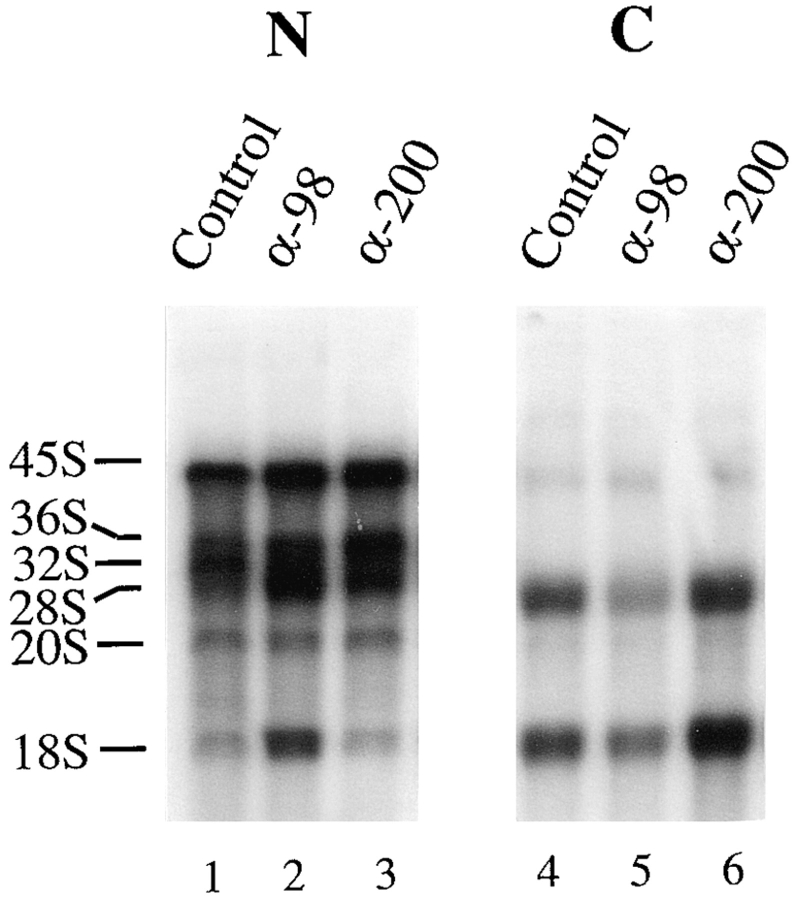
We next asked whether xNup98 function was also required for export of the large 18 and 28S ribosomal RNAs, which occurs by yet another distinct RNA export pathway (Bataillé et al., 1990; Fischer et al., 1995; Pokrywka and Goldfarb, 1995). In Xenopus oocytes, the rRNA genes are highly amplified, so it is possible to monitor expression of the endogenous rRNA genes simply by injection of [32P]GTP (Peculis and Steitz, 1993). We found that anti-xNup98 antibodies significantly retarded export of the large 18 and 28S ribosomal RNAs, as shown by the reduction in levels of these newly made rRNAs in the cytoplasm (Fig. 5, lanes 4 and 5). The concomitant accumulation of excess mature 18 and 28S rRNAs in the nucleus (Fig. 5, lane 2) showed that anti-xNup98 antibodies inhibited export but not the synthesis or maturation of ribosomal RNAs. We also note that export of rRNAs, in the form of large preribosomal subunits, is inhibited to a lesser extent than is the export of the much smaller 5S RNA particles. This is a further indication that inhibition does not result simply from nonspecific obstruction of the nuclear pore. Additionally, maturation of the nascent labeled rRNA transcripts requires the assembly of the ribosomal subunits in the nucleus which, in turn, depends on the import of ribosomal proteins (for review see Hadjiolov, 1985). Thus, the results in Fig. 5 suggested that protein import is not influenced by the antixNup98 antibodies.
Figure 5.
Reduced export of ribosomal RNAs in the presence of anti-xNup98 antibodies. Buffer (Control) or antibodies specific for xNup98 (α-98) or xNup200 (α-200) were injected into oocyte nuclei. 2 h later α[32P]GTP (∼1.0 μCi/oocyte) was injected into the cytoplasm of all oocytes. After 20 h of labeling, the intracellular distribution of newly made endogenous ribosomal RNAs was determined by analyses of 0.5 oocyte equivalents of nuclear (lanes 1–3) and cytoplasmic RNAs (lanes 4–6) in a 1.2% formaldehyde–agarose gel. The electrophoretic mobilities of precursor (45, 36, 32, and 20S) and mature (18 and 28S) ribosomal RNAs are indicated.
Anti-xNup98 Antibodies Have No Effect on Nuclear Protein Import
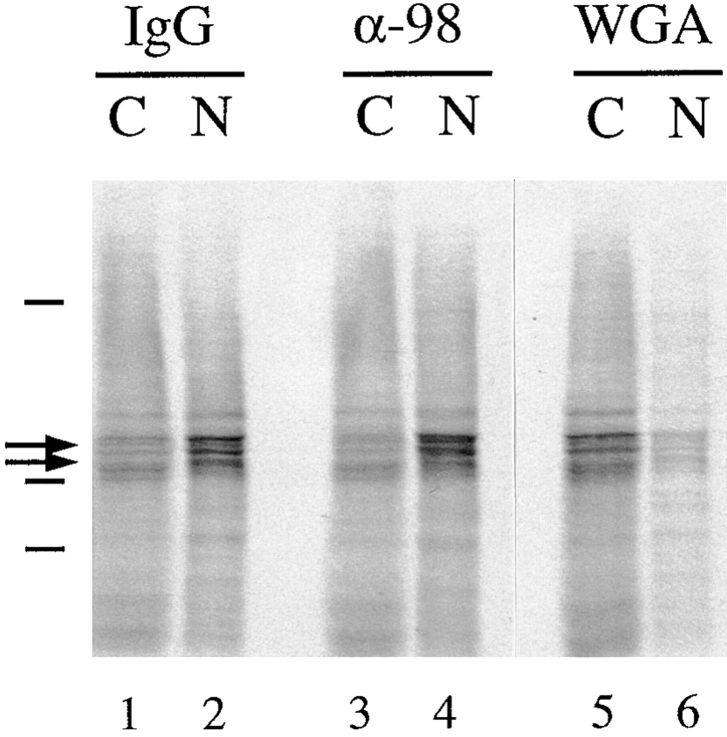
We have previously shown that xNup98 is not required for nuclear protein import in reconstituted nuclei (Powers et al., 1995). To test whether Nup98 is required for import in vivo, we compared the nuclear import of karyophilic proteins in oocytes pre-injected with anti-xNup98 antibodies, control IgG antibodies, or WGA, a potent inhibitor of protein import (Forbes, 1992). After antibody injection, total 35S-labeled nuclear proteins (Dabauvalle et al., 1988) were injected into the cytoplasm of the oocytes. 24 h later, the oocytes were dissected into nuclear and cytoplasmic fractions for determination of the intracellular distribution of labeled proteins. Injection of anti-xNup98 antibodies into the nucleus had no detectable effect on nuclear import, as shown by the efficient uptake of the major labeled nuclear proteins, N1/N2, as well as other nuclear proteins (Fig. 6, lanes 2 and 4. Cytoplasmic injection of anti-xNup98 also had no effect on nuclear protein import (data not shown). As expected, the import of proteins containing nuclear localization signals (NLS) was strongly inhibited in oocytes that were injected with WGA (Fig. 6, lanes 5 and 6; Finlay et al., 1987; Dabauvalle et al., 1988; Fischer et al., 1991). Thus, antibodies to xNup98 do not act as a physical block to protein transit through the nuclear pore, but rather, define a specific and essential role for this nucleoporin in RNA export.
Figure 6.
Lack of effect of anti-xNup98 antibodies on import of karyophilic proteins. Normal IgG (IgG), anti-xNup98 (α-98), or WGA was injected into oocyte nuclei (IgG, α-98) or cytoplasms (WGA). 2 h later, 0.25 oocyte equivalents of 35S-labeled total soluble nuclear proteins were injected into the cytoplasm of all oocytes. After 24 h incubation, oocytes were manually dissected, and total nuclear (N) and cytoplasmic (C) extracts were prepared from pools of 3–5 oocytes. 0.5 oocyte equivalents of the extracts were analyzed on a 10% polyacrylamide gel containing SDS and 35S-labeled proteins were detected by autoradiography. Arrows indicate the strongly labeled karyophilic proteins N1/N2 (Dabauvalle and Francke, 1982). Lines indicate the positions of molecular weight markers of 200, 97, and 66 kD.
Anti-xNup98 Antibodies Do Not Prevent snRNP Import
Certain small RNAs, like U6 snRNA (Vankan et al., 1990; Terns et al., 1993a ; Boelens et al., 1995) and the synthetic NL-15 RNA (Grimm et al., 1997), are not normally exported from the nucleus. However, if injected into the cytoplasm, these RNAs can be imported into the nucleus via the same WGA-sensitive pathway as NLS-containing proteins (Fischer et al., 1991; Grimm et al., 1997). Other RNAs, like U1 and U5 snRNAs, which normally undergo maturation in the cytoplasm, are imported via a separate, snRNP-specific pathway (Mattaj, 1986) that is less sensitive to WGA inhibition (Fischer et al., 1991; Michaud and Goldfarb, 1991; Lund and Dahlberg, manuscript in preparation). We found that nuclear import of cytoplasmically injected NL-15 RNA was completely unaffected by the presence of anti-xNup98 antibodies (Fig. 7), in agreement with the results for protein import (Fig. 6). Likewise, antixNup98 antibodies had little if any effect on the import of U5 or U1 snRNAs via the snRNP pathway (Fig. 7, lanes 2 and 3). In contrast, WGA completely blocked NL15 import and impaired U1 and U5 import (Fig. 7, lane 4). Taken together, these results strongly argue that Nup98 is not essential for nuclear import of RNA.
Figure 7.
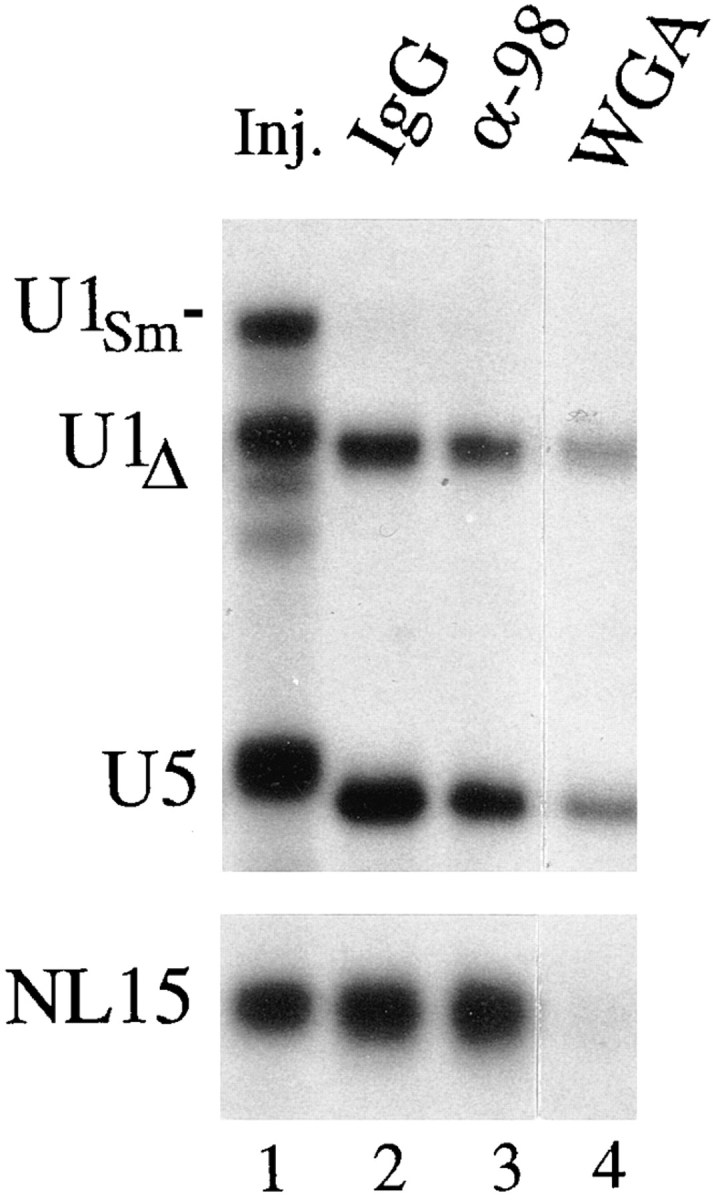
Unimpaired import of snRNPs and small RNAs in the presence of anti-xNup98 antibodies. Oocytes that had been pre-injected with antibodies or WGA, as in Fig. 6, were injected in the cytoplasm with a mixture of 32P-labeled U1Sm−, U1Δ, U5, and NL15 RNAs that were made by in vitro transcription. After 7 h of incubation, import of the RNAs was monitored by polyacrylamide gel analysis of 0.5 oocyte equivalents of nuclear RNAs isolated from pools of 3–5 oocyte nuclei. The mixture of injected RNAs (Inj.) is shown in lane 1; the slightly faster gel mobilities of U1Δ (a mutant U1 RNA lacking the U1A protein binding site) and U5 RNAs in lanes 2–4 are due to 3′ end trimming which occurs upon nuclear import of snRNPs (Neuman de Vegvar and Dahlberg, 1990). U1Sm− RNA lacks the Sm binding site required for import and serves as a control for cytoplasmic contamination of the nuclear samples. Cytoplasmic fractions are not shown, as RNA levels were in excess, and differences among them were not significant.
Discussion
By monitoring nuclear transport in Xenopus oocytes in the presence of monospecific antibodies to xNup98, we have demonstrated that this GLFG nucleoporin functions in the export of multiple classes of RNA, but not in the export of tRNA. Further, our results show that Nup98 does not have an essential role in nuclear protein import, supporting our previous conclusion from nuclear reconstitution studies. Similarly, we find that Nup98 is not required for the import of spliceosomal snRNPs. Thus, Nup98 represents the first vertebrate nuclear pore component for which a unidirectional role in multiple RNA export pathways has been established.
The existence of distinct pathways of nuclear export for different classes of RNA is suggested both by kinetic analyses (Zasloff, 1983; Jarmolowski et al., 1994; Lund and Dahlberg, manuscript in preparation) and by competition experiments, which demonstrate that various RNAs (e.g., snRNA, mRNA, and tRNA) are unable to compete with one another for export (Jarmolowski et al., 1994; Boelens et al., 1995; Pokrywka and Goldfarb, 1995; Simons et al., 1996). These latter results indicate the existence of at least one specific limiting factor in the export of each class of RNA. Since it is believed that RNA exits the nucleus as an RNA–protein complex, it is likely that specific RNA binding proteins represent these limiting factors, and indeed candidate proteins have been identified. TFIIIA, which binds to 5S RNA, contains a nuclear export signal (NES) that may mediate interaction with the export machinery (Guddat et al., 1990; Fridell et al., 1996). CBC, which binds to m7G-capped RNAs, is thought to promote export of snRNA precursors (Izaurralde et al., 1992). Antibodies to the CBP20 protein, a CBC subunit, are capable of reducing the export of snRNA (Izaurralde et al., 1995). The CBC complex may also be involved in mRNA export (Visa et al., 1996), although antibodies to CBP20 have no effect on mRNA export (Izaurralde et al., 1995). The hnRNP A1 protein may fulfill the role of a class-specific export factor for mRNA, since this protein binds to mRNA, shuttles between nucleus and cytoplasm, and contains a unique NES sequence (Michael et al., 1995). The retrovirus HIV-1 encodes its own specific export factor, the Rev protein, which recognizes a sequence present only in incompletely spliced viral RNAs (for review see Hope and Pomerantz, 1995) and, via an NES sequence, mediates the export of these RNAs to the cytoplasm (Fischer et al., 1995). No class-specific factor has yet been identified for tRNA.
The next question is what then recognizes the different class-specific export factors. One possibility is that a general export receptor recognizes NES signals present in the individual class-specific factors and ferries the RNA–protein complexes to the pore. Alternatively, different RNAs, via their class-specific factors, may bind directly to a protein or proteins of the nuclear pore. Nup98, localized both on the nucleoplasmic side of the pore and within the nucleus, potentially on intranuclear filaments, is ideally positioned to mediate either the binding of a general export receptor, or the binding of individual class-specific factors. Antibodies to xNup98 impair snRNA, mRNA, 5S RNA, and ribosomal RNA export, indicating involvement of this nucleoporin in all of these pathways (Figs. 2, 4, and 5). The saturability of tRNA export indicates that this class of RNA also has a specific export system (Zasloff, 1983), but the lack of inhibition by anti-xNup98 antibodies (Fig. 2) implies that such export bypasses the need for Nup98. Thus, the export of tRNA might involve binding to a different nucleoporin, or tRNA may enter the export machinery at a point downstream of Nup98. Interestingly, we have previously found that tRNA export, unlike that of most other RNAs, occurs independently of the Ran GTPase cycle (Cheng et al., 1995).
All classes of RNA export, including tRNA export, can be blocked by certain inhibitors; however, because these componds also block protein import, it is unclear if they affect RNA export directly. For example, injection of the lectin WGA inhibits export of all classes of RNA (Featherstone et al., 1988; Bataillé et al., 1990; Neuman de Vegvar and Dahlberg, 1990), but it binds to N-acetylglucosamine residues present on multiple glycoproteins of the nuclear pore (Nup200, Nup98, and p62 in Xenopus; Forbes, 1992), making conclusions about the role of any given nucleoporin difficult to interpret. Similarly, nuclear injection of the monoclonal antibody, 414, which binds to the FXFG repeat sequences found in some nucleoporins (Nup200, Nup153, and p62), blocks numerous classes of RNA export but not that of tRNA (Terns and Dahlberg, 1994; Lund and Dahlberg, manuscript in preparation). However, because this antibody recognizes both Nup153 on the basket and p62 in or near the central transporter (Pante and Aebi, 1993), its inhibition of export can not be attributed to any specific nucleoporin. Both these reagents, WGA and mAb 414, also block the import of proteins into the nucleus (Finlay et al., 1987; Featherstone et al., 1988; Lund and Dahlberg, manuscript in preparation). Thus, only now in the case of Nup98 has a monospecific reagent implicated a single vertebrate nucleoporin in a specific transport process, RNA export.
If a general export receptor carries the different RNAs to the pore, what might that export receptor be? It is presently not known whether either of the components of the heterodimeric import receptor, importins α and β, might play a role in export through the pore. In nuclear import, importin α binds to the NLS of a nuclear-targeted protein; importin β then acts to dock the import receptor complex to proteins of the nuclear pore (for review see Melchior and Gerace, 1995; Görlich and Mattaj, 1996). The observations that importin β can bind to numerous repeat-containing nucleoporins on blots, including Nup98, led to a proposal that Nup98 has a role in nuclear protein import (Moroianu et al., 1995; Radu et al., 1995b ). However, in our earlier studies, we found that nuclei reconstituted in the absence of Nup98 were competent for protein import, indicating that binding of importin β to Nup98 is not essential for this process (Powers et al., 1995). Consistent with this, we find here that anti-xNup98 antibodies inhibit export but have no significant effect on import of either proteins or snRNPs. Thus, while there may be an interaction between Nup98 and importin β, we have found no functional evidence supporting a role for Nup98 in nuclear import.
If importin β has a function in RNA export, it might be mediated through the interaction of this factor with the GLFG domain of Nup98 (Radu et al., 1995b ). Indeed, in yeast, importin β interacts with the GLFG domain of Nup116, a homolog of Nup98, in a two hybrid assay. Moreover, the GLFG domain of Nup116 is the only GLFG domain of any yeast nucleoporin essential for cell viability, and overexpression of this domain in yeast results in loss of poly A+ RNA export (Iovine et al., 1995). However, in a separate study using recombinant subdomains of nucleoporins, yeast importin β was found to interact with the yeast Nup1 FXFG domain but not with the GLFG domains of either Nup145 or Nup54 (Nup116 was not tested in this assay; Rexach and Blobel, 1995). These conflicting results raise questions about the specificity of interactions between purified, recombinant proteins in vitro and emphasize the importance of conducting parallel functional assays, as was done with Nup116 (Iovine et al., 1995). During protein import, vertebrate importin β traverses the pore but remains bound to the nuclear side of the pore and is not released into the nucleoplasm (Görlich et al., 1995). One possibility is that the GLFG domain of Nup98 may provide a final docking site for this import factor before it is recycled to the cytoplasm, thus explaining the interaction of Nup98 with importin β.
Several other regions of Nup98 present interesting possibilities for a potential function in export. A distinct sequence of 40 amino acids devoid of any repeat motifs is present near the NH2 terminus of Nup98, within the GLFG repeat domain (amino acids 170–192; Radu et al., 1995b ). A segment of this region, rich in charged residues, is identical in Xenopus and rat Nup98, highly conserved in yeast Nup116, and absent from other members of the GLFG family (Nup49, Nup54, Nup100, and Nup145; Wente et al., 1992; Wimmer et al., 1992; Fabre et al., 1994; Wente and Blobel, 1994). In Nup116, a somewhat larger region containing this sequence is essential for function, as indicated by the loss of cell viability when it is deleted (Iovine et al., 1995). Because of its strong evolutionary conservation, this small domain may well be important for the RNA export function of Nup98.
Alternatively, the COOH-terminal domain of Nup98 contains a sequence of ∼150 amino acids; this domain has no GLFG repeats but exhibits weak homology to certain RNA binding proteins (amino acids 737–892; Radu et al., 1995b ). A homologous region is present in the yeast nucleoporins Nup100, Nup116, and Nup145, but presumably serves a redundant function in the three yeast nucleoporins, since the presence of any one copy of the sequence is sufficient for cell viability (Fabre et al., 1994). This sequence may be involved in RNA export, but in no case has the defect in yeast poly A+ RNA export been linked directly to deletion of this putative RNA-binding sequence. In vitro, the region was found to bind preferentially to poly rG homopolymers (Fabre et al., 1994). Under similar conditions, we find that Nup98 from Xenopus egg extracts also exhibits preferential binding to poly rG over other homopolymers (Powers and Forbes, unpublished results). However, as was the case for the yeast GLFG proteins (Fabre et al., 1994), only a very small fraction of the input xNup98 bound to the homopolymer. Since many other proteins of the egg extract also bind preferentially to poly rG, the significance of such in vitro interactions should be interpreted with caution. When poly rG and poly rI are injected into oocyte nuclei, inhibition of RNA export has been observed (Jarmolowski et al., 1994); but it remains to be determined whether such inhibition of export is due to binding and inhibition of Nup98 or to inhibition of another component of the RNA export machinery. Because our polyclonal antibody strongly interacts with both the GLFG and RNA-binding domains of rNup98 (Fig. 1 B), we can not precisely distinguish whether both or only one domain is involved in RNA export. It will be interesting to test even more specific antibody tools as they are developed.
In summary, we have demonstrated that the GLFG nucleoporin Nup98 plays a central role in the export of multiple classes of RNAs. The future identification of the nuclear export factors that specifically interact with Nup98 and Nup153 (Bastos et al., 1996), either alone or complexed with an RNA export substrate, will be important for understanding the role of this and other nucleoporins in RNA export.
Acknowledgments
The authors wish to thank Drs. Katharine Ullman, Christian Grimm, and Amy Pasquinelli for helpful discussions, Dr. Sanjay Vasu for rNup98 peptide fragments, Dr. Brian Miller for annulate lamellae preparations, Dr. Ted Munns for anti-m7G-antibodies, and Dr. Arianne Grandjean and Ms. Michelle Barr for preparation of oocytes for injection.
Abbreviations used in this paper
- AdML
adenovirus major late
- CBC
cap binding complex
- NES
nuclear export signal
- NLS
nuclear localization signal
- snRNPs
small nuclear ribonucleoproteins
- WGA
wheat germ agglutinin
Footnotes
This research was funded by grants from the National Institutes of Health to D.J. Forbes (GM 33279) and to J.E. Dahlberg and E. Lund (GM 30220).
Please address all correspondence to Elsebet Lund, Department of Biomolecular Chemistry, University of Wisconsin, Madison, WI 53706. Tel.: 608.262.1359, Fax.: 608.262.8704. E-mail: elund@facstaff.wisc.edu
References
- Adolph KW, Cheng SM, Laemmli UK. Role of nonhistone proteins in metaphase chomosome structure. Cell. 1977;12:805–816. doi: 10.1016/0092-8674(77)90279-3. [DOI] [PubMed] [Google Scholar]
- Bastos R, Lin A, Enarson M, Burke B. Targeting and function in mRNA export of nuclear pore complex protein Nup153. J Cell Biol. 1996;134:1141–1156. doi: 10.1083/jcb.134.5.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataillé N, Helser T, Fried HM. Cytoplasmic transport of ribosomal subunits microinjected into the Xenopus laevisoocyte nucleus: a generalized, facilitated process. J Cell Biol. 1990;111:1571–1582. doi: 10.1083/jcb.111.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelens WC, Palacios I, Mattaj IW. Nuclear retention of RNA as a mechanism for localization. RNA. 1995;1:273–283. [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dahlberg JE, Lund E. Diverse effects of the guanine nucleotide exchange factor RCC1 on RNA transport. Science (Wash DC) 1995;267:1807–1810. doi: 10.1126/science.7534442. [DOI] [PubMed] [Google Scholar]
- Cordes VC, Reidenbach S, Kohler A, Stuurman N, van Driel R, Franke WW. Intranuclear filaments containing a nuclear pore complex protein. J Cell Biol. 1993;123(6):1333–1344. doi: 10.1083/jcb.123.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabauvalle MC, Franke WW. Karyophilic proteins: polypeptides synthesized in vitro accumulate in the nucleus on microinjection into the cytoplasm of amphibian oocytes. Proc Natl Acad Sci USA. 1982;79:5302–5306. doi: 10.1073/pnas.79.17.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabauvalle MC, Benavente R, Chaly N. Monoclonal antibodies to a Mr 68,000 pore complex glycoprotein interfere with nuclear protein uptake in Xenopusoocytes. Chromosoma (Berl) 1988;97:193–197. doi: 10.1007/BF00292960. [DOI] [PubMed] [Google Scholar]
- Dabauvalle MC, Schulz B, Scheer U, Peters R. Inhibition of nuclear accumulation of karyophilic proteins in living cells by microinjection of the lectin wheat germ agglutinin. Exp Cell Res. 1988;174:291–296. doi: 10.1016/0014-4827(88)90163-2. [DOI] [PubMed] [Google Scholar]
- Dabauvalle MC, Loos K, Scheer U. Identification of a soluble precursor complex essential for nuclear pore assembly in vitro. Chromosoma (Berl) 1990;100:56–66. doi: 10.1007/BF00337603. [DOI] [PubMed] [Google Scholar]
- Davis L. The nuclear pore complex. Annu Rev Biochem. 1995;64:865–896. doi: 10.1146/annurev.bi.64.070195.004245. [DOI] [PubMed] [Google Scholar]
- Doye V, Hurt EC. Genetic approaches to nuclear pore structure and function. Trends Genet. 1995;11:235–241. doi: 10.1016/s0168-9525(00)89057-5. [DOI] [PubMed] [Google Scholar]
- Fabre E, Hurt EC. Nuclear transport. Curr Opin Cell Biol. 1994;6:335–342. doi: 10.1016/0955-0674(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Fabre E, Boelens WC, Wimmer C, Mattaj I, Hurt EC. Nup145p is required for nuclear export of mRNA and binds homopolymeric RNA in vitro via a novel conserved motif. Cell. 1994;78:275–289. doi: 10.1016/0092-8674(94)90297-6. [DOI] [PubMed] [Google Scholar]
- Featherstone C, Darby MK, Gerace L. A monoclonal antibody against the nuclear pore complex inhibits nucleocytoplasmic transport of protein and RNA in vivo. J Cell Biol. 1988;107:1289–1297. doi: 10.1083/jcb.107.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay DR, Forbes DJ. Reconstitution of biochemically altered nuclear pores: transport can be eliminated and restored. Cell. 1990;60:17–29. doi: 10.1016/0092-8674(90)90712-n. [DOI] [PubMed] [Google Scholar]
- Finlay DR, Newmeyer DD, Price TM, Forbes DJ. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987;104:189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay DR, Meier E, Bradley P, Horecka J, Forbes DJ. A complex of nuclear pore proteins required for pore function. J Cell Biol. 1991;114:169–183. doi: 10.1083/jcb.114.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Darzynkiewicz E, Tahara SM, Dathan NA, Lührmann R, Mattaj IW. Diversity in the signals required for nuclear accumulation of U snRNPs and variety in the pathways of nuclear transport. J Cell Biol. 1991;113:705–714. doi: 10.1083/jcb.113.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Huber J, Boelens WC, Mattaj IW, Lührmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- Forbes DJ. Structure and function of the nuclear pore complex. Annu Rev Cell Biol. 1992;8:495–527. doi: 10.1146/annurev.cb.08.110192.002431. [DOI] [PubMed] [Google Scholar]
- Fridell RA, Fischer U, Luhrmann R, Meyer BE, Meinkoth JL, Malim MH, Cullen BR. Amphibian transcription factor IIIA proteins contain a sequence element functionally equivalent to the nuclear export signal of human immunodeficiency virus type 1 Rev. Proc Natl Acad Sci USA. 1996;93:2936–2940. doi: 10.1073/pnas.93.7.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Mattaj IW. Protein kinesis: Nucleocytoplasmic transport. Science (Wash DC) 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- Görlich D, Vogel F, Mills AD, Hartmann E, Laskey RA. Distinct functions for the two importin subunits in nuclear protein import. Nature (Lond) 1995;377:246–248. doi: 10.1038/377246a0. [DOI] [PubMed] [Google Scholar]
- Gouilloud E, Clarkson SG. A dispersed tyrosine tRNA gene from Xenopus laeviswith high transcriptional activity in vitro. J Biol Chem. 1986;261:486–494. [PubMed] [Google Scholar]
- Grandi P, Schlaich N, Tekotte H, Hurt EC. Functional interaction of Nic96p with a core nucleoporin complex consisting of Nsp1p, Nup49p, and a novel protein Nup57p. EMBO (Eur Mol Biol Organ) J. 1995;14:76–87. doi: 10.1002/j.1460-2075.1995.tb06977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, C., E. Lund, and J.E. Dahlberg. 1997. EMBO (Eur. Mol. Biol. Organ.) J. In Press.
- Guddat U, Bakken AH, Pieler T. Protein-mediated nuclear export of RNA: 5S rRNA containing small RNPs in Xenopusoocytes. Cell. 1990;60:619–628. doi: 10.1016/0092-8674(90)90665-2. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Wickens MP. The use of Xenopusoocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Hadjiolov, A.A. 1985. The Nucleolus and Ribosome Biogenesis. In Cell Biology Monographs. Springer-Verlag Inc., New York. 125:133–165.
- Hamm J, Mattaj IW. Monomethylated cap structures facilitate RNA export from the nucleus. Cell. 1990;63:109–118. doi: 10.1016/0092-8674(90)90292-m. [DOI] [PubMed] [Google Scholar]
- Hope T, Pomerantz RJ. The human immunodeficiency virus type 1 Rev protein: a pivotal protein in the viral life cycle. Curr Top Microbiol Immunol. 1995;193:91–105. doi: 10.1007/978-3-642-78929-8_5. [DOI] [PubMed] [Google Scholar]
- Iovine MK, Watkins JL, Wente SR. The GLFG repetitive region of the nucleoporin Nup116p interacts with Kap95p, an essential yeast nuclear import factor. J Cell Biol. 1995;131:1699–1713. doi: 10.1083/jcb.131.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E, Mattaj IW. Transport of RNA between nucleus and cytoplasm. Semin Cell Biol. 1992;3:279–288. doi: 10.1016/1043-4682(92)90029-u. [DOI] [PubMed] [Google Scholar]
- Izaurralde E, Mattaj IW. RNA export. Cell. 1995;81:153–159. doi: 10.1016/0092-8674(95)90323-2. [DOI] [PubMed] [Google Scholar]
- Izaurralde E, Stepinski J, Darzynkiewicz E, Mattaj IW. A cap binding protein that may mediate nuclear export of RNA polymerase IItranscribed RNAs. J Cell Biol. 1992;118:1287–1295. doi: 10.1083/jcb.118.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E, Lewis J, Gamberi C, Jarmolowski A, McGuigan C, Mattaj IW. A cap-binding protein complex mediating U snRNA export. Nature (Lond) 1995;376:709–712. doi: 10.1038/376709a0. [DOI] [PubMed] [Google Scholar]
- Jarmolowski A, Boelens WC, Izaurralde E, Mattaj IW. Nuclear export of different classes of RNA is mediated by specific factors. J Cell Biol. 1994;124:627–635. doi: 10.1083/jcb.124.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisleva E, Goldberg MW, Daneholt B, Allen TD. RNP export is mediated by structural reorganization of the nuclear pore basket. J Mol Biol. 1996;260:304–311. doi: 10.1006/jmbi.1996.0401. [DOI] [PubMed] [Google Scholar]
- Konarska MM, Sharp PA. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987;49:763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- Kraemer D, Wozniak RW, Blobel G, Radu A. The human CAN protein, a putative oncogene product associated with myeloid leukemogenesis, is a nuclear pore complex protein that faces the cytoplasm. Proc Natl Acad Sci USA. 1994;91:1519–1523. doi: 10.1073/pnas.91.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E, Dahlberg JE. In vitro synthesis of vertebrate U1 snRNA. EMBO (Eur Mol Biol Organ) J. 1989;8:287–292. doi: 10.1002/j.1460-2075.1989.tb03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E, Paine PL. Nonaqueous isolation of transcriptionally active nuclei from Xenopusoocytes. Methods Enzymol. 1990;181:36–43. doi: 10.1016/0076-6879(90)81110-g. [DOI] [PubMed] [Google Scholar]
- Macaulay C, Meier E, Forbes DJ. Differential mitotic phosphorylation of proteins of the nuclear pore complex. J Biol Chem. 1995;207:254–262. doi: 10.1074/jbc.270.1.254. [DOI] [PubMed] [Google Scholar]
- Mattaj IW. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell. 1986;46:905–911. doi: 10.1016/0092-8674(86)90072-3. [DOI] [PubMed] [Google Scholar]
- Meier E, Miller B, Forbes DJ. Nuclear pore complex assembly studied with a biochemical assay for annulate lamellae formation. J Cell Biol. 1995;129:1459–1472. doi: 10.1083/jcb.129.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior F, Gerace L. Mechanisms of nuclear protein import. Curr Opin Cell Biol. 1995;7:310–318. doi: 10.1016/0955-0674(95)80084-0. [DOI] [PubMed] [Google Scholar]
- Melton DA, Krieg PA, Rebagliati MR, Maniatis T, Zinn K, Green MR. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984;12:7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael MW, Choi M, Dreyfuss G. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell. 1995;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- Michaud N, Goldfarb DS. Multiple pathways in nuclear transport: the import of U2 snRNP occurs by a novel kinetic pathway. J Cell Biol. 1991;112:215–223. doi: 10.1083/jcb.112.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Hanover JA. Functional nuclear pores reconstituted with β1-4 galactose-modified O-linked N-acetylglucosamine modified glycoproteins. J Biol Chem. 1994;269:9289–9297. [PubMed] [Google Scholar]
- Moore MS, Blobel G. A G protein involved in nucleocytoplasmic transport: the role of Ran. Trends Biochem Sci. 1994;19:211–216. doi: 10.1016/0968-0004(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Moroianu J, Hijikata M, Blobel G, Radu A. Mammalian karyopherin a1b and a2b heterodimers: a1 or a2 subunit binds nuclear localization signal and b subunit interacts with peptide repeat-containing nucleoporins. Proc Natl Acad Sci USA. 1995;92:6532–6536. doi: 10.1073/pnas.92.14.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns TW, Liszewski MK, Tellam JT, Sims HF, Rhoads RE. Antibody–nucleic acid complexes. Immunospecific retention of globin messenger ribonucleic acid with antibodies specific for 7-methylguanosine. Biochemistry. 1982;21:2922–2928. doi: 10.1021/bi00541a018. [DOI] [PubMed] [Google Scholar]
- Nehrbass U, Blobel G. Role of the nuclear transport factor p10 in nuclear import. Science (Wash DC) 1996;272:120–122. doi: 10.1126/science.272.5258.120. [DOI] [PubMed] [Google Scholar]
- Neuman de Vegvar HE, Dahlberg E. Nucleocytoplasmic transport and processing of small nuclear RNA precursors. Mol Cell Biol. 1990;10:3365–3375. doi: 10.1128/mcb.10.7.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pante N, Aebi U. The nuclear pore complex. J Cell Biol. 1993;122:977–984. doi: 10.1083/jcb.122.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquinelli AE, Dahlberg JE, Lund E. Reverse 5′ caps in RNAs made in vitro by phage RNA polymerases. RNA. 1995;1:957–967. [PMC free article] [PubMed] [Google Scholar]
- Peculis BA, Steitz JA. Disruption of U8 nucleolar snRNA inhibits 5.8S and 28S rRNA processing in the Xenopusoocyte. Cell. 1993;73:1233–1245. doi: 10.1016/0092-8674(93)90651-6. [DOI] [PubMed] [Google Scholar]
- Pokrywka NJ, Goldfarb DS. Nuclear export pathways of tRNA and 40 S ribosomes include both common and specific intermediates. J Biol Chem. 1995;270:3619–3624. doi: 10.1074/jbc.270.8.3619. [DOI] [PubMed] [Google Scholar]
- Powers MA, Forbes DJ. Cytosolic factors in nuclear import: what's importin? . Cell. 1994;79:931–934. doi: 10.1016/0092-8674(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Powers MA, Macaulay C, Masiarz F, Forbes DJ. Reconstituted nuclei depleted of a vertebrate GLFG nuclear pore protein, p97, import but are defective in nuclear growth and replication. J Cell Biol. 1995;128:721–736. doi: 10.1083/jcb.128.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu A, Blobel G, Moore MS. Identification of a protein complex that is required for nuclear protein import and mediates docking of import substrate to distinct nucleoporins. Proc Natl Acad Sci USA. 1995a;92:1769–1773. doi: 10.1073/pnas.92.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu A, Moore MS, Blobel G. The peptide repeat domain of nucleoporin Nup98 functions as a docking site in transport across the nuclear pore complex. Cell. 1995b;81:215–222. doi: 10.1016/0092-8674(95)90331-3. [DOI] [PubMed] [Google Scholar]
- Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- Rout MP, Wente SR. Pores for thought: nuclear pore complex proteins. Trends Cell Biol. 1994;4:357–365. doi: 10.1016/0962-8924(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Sazer S. The search for the primary function of the Ran GTPase continues. Trends Cell Biol. 1996;6:81–85. doi: 10.1016/0962-8924(96)80992-5. [DOI] [PubMed] [Google Scholar]
- Schlenstedt G, Hurt E, Doye V, Silver PA. Reconstitution of nuclear protein transport with semi-intact yeast cells. J Cell Biol. 1993;123:785–798. doi: 10.1083/jcb.123.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons FH, Rutjes SA, van Venrooij WJ, Pruijn GJ. The interactions with Ro60 and La differentially affect nuclear export of hY1 RNA. RNA. 1996;2:264–273. [PMC free article] [PubMed] [Google Scholar]
- Terns MP, Dahlberg JE. Retention and 5′ cap trimethylation of U3 snRNA in the nucleus. Science (Wash DC) 1994;264:959–961. doi: 10.1126/science.8178154. [DOI] [PubMed] [Google Scholar]
- Terns MP, Dahlberg JE, Lund E. Multiple cis-acting signals for export of pre-U1 snRNA from the nucleus. Genes Dev. 1993a;7:1898–1908. doi: 10.1101/gad.7.10.1898. [DOI] [PubMed] [Google Scholar]
- Terns MP, Lund E, Dahlberg JE. A pre-export U1 snRNP in Xenopus laevisoocyte nuclei. Nucleic Acids Res. 1993b;21:4569–4573. doi: 10.1093/nar/21.19.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankan P, McGuigan C, Mattaj IW. Domains of U4 and U6 snRNAs required for snRNP assembly and splicing complementation in Xenopusoocytes. EMBO (Eur Mol Biol Organ) J. 1990;9:3397–3404. doi: 10.1002/j.1460-2075.1990.tb07541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visa N, Izaurralde E, Ferreira J, Daneholt B, Mattaj IW. A nuclear CAP-binding complex binds balbiani ring pre-mRNA cotranscriptionally and accompanies the ribonucleoprotein particle during nuclear export. J Cell Biol. 1996;133:5–14. doi: 10.1083/jcb.133.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente SR, Blobel G. NUP145 encodes a novel yeast glycine–leucine–phenylalanine–glycine (GLFG) nucleoporin required for nuclear envelope structure. J Cell Biol. 1994;125:955–969. doi: 10.1083/jcb.125.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente SR, Rout MP, Blobel G. A new family of yeast nuclear pore complex proteins. J Cell Biol. 1992;119:705–723. doi: 10.1083/jcb.119.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer C, Doye V, Grandi P, Nehrbass U, Hurt EC. A new subclass of nucleoporins that functionally interact with nuclear pore protein NSP1. EMBO (Eur Mol Biol Organ) J. 1992;11:5051–5061. doi: 10.1002/j.1460-2075.1992.tb05612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe AP, Jordan E, Brown DD. A bacteriophage RNA polymerase transcribes through a Xenopus5S RNA gene transcription complex without disrupting it. Cell. 1986;44:381–389. doi: 10.1016/0092-8674(86)90459-9. [DOI] [PubMed] [Google Scholar]
- Zasloff M. tRNA transport from the nucleus in a eukaryotic cell: carriermediated translocation process. Proc Natl Acad Sci USA. 1983;80:6436–6440. doi: 10.1073/pnas.80.21.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]