Abstract
Prostate cancer (PCa) is the most commonly diagnosed cancer in American men with a subset inevitably presenting with metastatic disease to the bone. A well-recognized limitation in evaluating new treatments for metastatic PCa is the inability to use imaging to objectively assess response therapy. In this study, we evaluated the feasibility of clinically translating the functional diffusion map (fDM) imaging biomarker for quantifying the spatiotemporal effects of bone tumor response in a patient treated for metastatic PCa with bone metastases. A patient beginning therapy was scanned using MRI before treatment and again at 2 and 8 weeks post-treatment initiation to quantify changes in tumor diffusion values. Three metastatic lesions were identified for fDM analysis, all of which all demonstrated an early increase in diffusion values at 2 weeks, which increased further at 8 weeks post-treatment initiation. This finding correlated with a decrease in the patient's prostate-specific antigen (PSA) levels suggestive of patient response. CT, bone scans, and anatomic MRI images obtained posttreatment were found to be uninformative for the assessment of treatment effectiveness. This study presents the feasibility of fDM-measurements in osseous lesions over time and shows that changes in fDM values were consistent with therapeutic response. Thus, the fDM imaging biomarker may provide a quantifiable therapeutic endpoint to assess response in patients with metastatic bone cancer.
Keywords: Metastatic prostate cancer, diffusion MRI, functional diffusion map, imaging biomarker, androgen deprivation therapy
Introduction
In 2007, approximately 218,890 men would have been diagnosed with prostate cancer (PCa) and 27,050 men in the United States are expected to die of PCa [1]. The mortality is directly related to the development of metastatic disease, which is incurable, thus requiring the development of improved therapies [2]. One of the main limitations in evaluating new treatments for metastatic PCa is the inability to use available clinical imaging modalities to assess treatment response in bone, which is the predominant and often the only site of metastasis in 85% to 90% of patients [3,4].
Currently, assessments of tumor response in bone using criteria defined by the International Union Against Cancer [5,6], the World Health Organization [7], and the Response Evaluation Criteria in Solid Tumors (RECIST) [8] group do not meet the needs of oncologists in clinical practice [9]. In fact, the RECIST system considers bone disease to be unmeasureable. Traditional clinical assessment of bony metastases is achieved through radionuclide bone scintigraphy. Although considered to be the standard screening technique for assessing the entire skeleton for metastases, it is well recognized that this imaging technique lacks the specificity needed to accurately distinguish metastatic lesions from areas of abnormal radionuclide uptake due to inflammation, degeneration, or trauma, as well as to measure early therapeutic response. Although the use of bone scintigraphy, computed tomography (CT), and magnetic resonance imaging (MRI) plays a distinct role in identifying and characterizing the extent of disease, the use of these techniques for the assessment of treatment response is limited. To overcome these limitations, prostate-specific antigen (PSA) alterations have been explored as a screening tool for antitumor effect. A decline in PSA of at least 50% is a widely accepted measure for antitumor effect; however, the use of PSA endpoints have not been prospectively validated as fulfilling surrogacy requirements for clinical benefit in any setting [10]. Therefore, development and validation of an imaging technology which would be capable of reliably and accurately measuring antitumor effect in metastatic bone disease would provide a significant advance and aide in the timely investigation of new therapeutic agents not only for PCa but also for other malignancies common to the bone (i.e., metastatic breast cancer and primary bone cancers).
The use of diffusion MRI for assessing response to anticancer therapy is based on its ability to quantify the random or Brownian motion of water. Diffusion of water within a tumor is reduced in the presence of cellular membranes that act to impede the random motion of water molecules. During the course of successful treatment, loss of tumor cells and/or tumor cell membrane integrity occurs, which will then result in a reduction in the barriers that impede mobility of water molecules. Diffusion MRI can be used to assess the treatment effect through quantification of the amount of increased apparent diffusion coefficient (ADC) values in tumor regions experiencing a loss of cellular density. Thus, water mobility within a tumor will increase over time following effective treatment, as represented by an increase in MRI-quantified ADC values, with the magnitude of the change related to the effectiveness of the therapy. This principle was first initially demonstrated using a 1,3-bis(2-chloroethyl)-1-nitrosourea-treated 9L glioma model [11] and was successfully extended in a variety of preclinical studies assessing the response to anticancer agents including: cytotoxic and cytostatic therapies, radiation therapy, and gene therapy [12–34]. Moreover, the treatment-induced changes in tumor diffusion values were shown to be an early event as they preceded changes in tumor growth kinetics and regression providing the rationale for using this imaging biomarker as an early predictive marker of treatment response [13]. However, the response of ADC to therapy in the clinical setting was found to be more complex to quantify due to inherent pretreatment and posttreatment heterogeneity observed within human tumors requiring the development of an alternative postprocessing approach known as the functional diffusion map (fDM) [35,36]. Thus, the fDM approach was developed to standardize the processing of clinical diffusion MRI data to provide for a sensitive and quantifiable means for early assessment of cancer treatment outcome.
The fDM approach of monitoring anticancer therapy allows spatial, voxel by voxel tracking of changes in tumor water diffusion values over time. Changes in diffusion values are depicted in fDM images by color encoding of tumor diffusion voxels that were altered due to therapy (either increased or decreased ADC value), thereby allowing for a spatially resolved analysis of ADC within an individual lesion. The initial demonstration of this novel technique was achieved in a study involving patients with primary malignant brain tumors where the amount of fDM-detected change in diffusion values was found to correlate with overall clinical response [35]. In a more recent study, we analyzed patients with grade III/IV gliomas using fDM, which demonstrated that this technique was able to stratify patients as responsive or nonresponsive to therapy as early as 3 weeks into a 6- to 7-week fractionated therapy schedule, which was later confirmed using traditional outcome measures including radiologic response, time to progression, and overall survival [37]. In this study, patients identified by fDM as nonresponsive had significantly shorter survival and time to progression compared to patients identified as responsive. Moreover, further investigation of the fDM approach in preclinical dose escalation studies revealed that this approach was indeed predictive of therapeutic efficacy and, more importantly, highly correlative with traditional outcome measures (cell kill and overall survival) [36]. As such, the fDM technique has shown significant promise as an early imaging-based biomarker for treatment response.
We have recently extended and evaluated the fDM approach outside of CNS tumors for providing an early indication of treatment response in a preclinical model of metastatic PCa to the bone. Using PC3 PCa xenografts with confirmed bone metastases, a correlation between changes in fDM and tumor response to docetaxel treatment was found as early as 7 days post-treatment initiation [16]. This preclinical finding provided the rationale for a pilot clinical trial to assess the feasibility and the ability of the fDM biomarker in predicting response to systemic therapy in patients with metastatic hormone sensitive and hormone refractory PCa. Our hypothesis is that the fDM biomarker can be successfully translated into the clinical setting for early assessment of bone tumor response arising from metastatic PCa. In this report, we summarize data from the first registered patient, which indicate that this approach is clinically feasible. Briefly, our findings demonstrate a correlation between diffusion changes, as determined by fDM analysis, and a decrease in PSA levels consistent with a positive treatment response. Moreover, traditional analysis of diffusion MRI data by mean ADC changes failed to provide a clear indication of diffusion changes attributable to positive treatment response, whereas the fDM biomarker provided a sensitive and quantitative readout of diffusion changes within the lesions over time. This study demonstrates for the first time the feasibility and potential use of a standardized, quantifiable imaging biomarker for assessing early treatment response of skeletal tumors.
Materials and Methods
Patient Information
The clinical trial was approved by the University of Michigan Institutional Review Board. A hormone-naïve 68-year-old male with newly diagnosed metastatic PCa was consented and was enrolled into the study. He was initiated on combined androgen blockade with bicalutamide and goserelin acetate. Magnetic resonance imaging scans were acquired at baseline, 2 and 8 weeks post-treatment initiation. Due to pain and pathologic fracture, the patient was also treated with palliative radiation therapy encompassing the sacrum and iliumat 2 weeks post initiation of androgen deprivation therapy.
Clinical Correlates
Serum samples were obtained at weeks 0 (baseline), 2, and 8 following treatment initiation. Prostate-specific antigen levels were quantified and expressed in grams per milliliter (g/ml) of plasma.
Bone Scintigraphy
A whole-body bone scan (BS) was performed by administering 25.8 mCi of Tc-99m methylene diphosphonate (MDP) and gamma camera images of the entire skeleton obtained 4 hours later (Siemens Medical Solutions, Malvern, PA). Nuclear imaging was performed 2 weeks before and 2 months after initiation of androgen deprivation therapy.
Computed Tomography
Computed tomography (CT) of the pelvis was performed without IV contrast on a 16-slice helical scanner (General Electric Medical Systems, Milwaukee, WI). Two hundred two axial sections of 2.5 mm thickness were acquired using 140 kVp, 590 mA, 0.8 sec/revolution, and a 512 matrix over a 36-cm field of view.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) examinations including diffusion-weighted and standard anatomic sequences were performed before and 2 weeks after initiation of therapy. MR imaging was performed on a 3-T scanner (Achieva model; Philips Medical Systems, Bothell, WA) using a quadrature body coil for transmission and a six-channel cardiac coil for reception. Standard sequences for depiction of anatomy and tumor extent included axial proton density (repetition time [TR]/echo time [TE] = 2700/30 msec, two averages) turbo spin-echo and axial T2-weighted turbo spin-echo (TR/TE = 7800/60 msec, two averages) with fat suppression through a short inversion time (TI = 200 msec). Geometry for these sequences was as follows: field of view, 281 mm right/left by 200 mm anterior/posterior; thirty-eight 4-mm sections with a 1-mm gap; 256 x 232 acquisition matrix for proton density; and 248 x 165 for T2-weighted scans. Diffusion-weighted scans were acquired using fat-suppressed, single-shot, spin-echo, echo-planar imaging to reduce motion artifact and parallel imaging (SENSE factor = 2) to reduce spatial distortion. Geometry of the diffusion-weighted scans was as follows: 350 mm right/left by 302 mm anterior/posterior; thirty 5-mm sections with a 1-mm gap; 200 x 172 acquisition matrix; TR/TE = 2000/58 msec; and 8 averages for low (b = 0 sec/mm2) and 16 averages for high (b = 800 sec/mm2) diffusion-sensitivity scans. Apparent diffusion coefficient (ADC) maps were calculated in the routine manner given by the logarithm of the ratio of low-b and high-b images, then scaled by the inverse of b-value difference.
Diffusion Analysis
Lesions in MR images acquired at weeks 2 and 8 were coregistered to their corresponding pretreatment MR images using an automatic algorithm based on maximizing mutual information. Registration was accomplished for each individual tumor located in the sacrum, ilium, and femoral head regions after the images were cropped to localize the registration to a limited region-of-interest (ROI) containing a single lesion. Lesions were manually contoured by a radiologist who defined the ROI by inspection of all available image data. Diffusion-average data were generated for weeks 0, 2 and 8 using the ROI contours of each of the three lesions and the mean change in diffusion values were calculated. Computation of fDMs for each lesion at weeks 2 and 8 was accomplished by comparison of voxels within the tumor at weeks 2 and 8 with the pretreatment values (week 0) as previously described [35,37] using MIAMI Fuse (University of Michigan, Ann Arbor, MI) [38]. The tumor was segmented into three different categories wherein the red voxels represent regions within the tumor where ADC values increased (>26 x 10-5 mm2/sec), the blue voxels represent a decreased ADC (<26 x 10-5 mm2/sec), and the green regions represent tumor diffusion values that were within these thresholds (e.g., unchanged). These thresholds were determined to be the 95% confidence intervals that were calculated using the variation in the adjacent muscle tissue as a test region, which should have unaltered diffusion values following registration at weeks 2 and 8 posttreatment. The percentage of tumor within each of the three categories was then calculated as VI (% red voxels), VO (% green voxels), and VD (% blue voxels). In this feasibility study, all fDM values reported represent the values computed using VI.
Statistical Analysis
Statistical analysis was accomplished using Microsoft Excel using Student's t test to compare changes in diffusion at different time points and between fDM and average ADC measurements. As this is a feasibility study wherein we are reporting the data analysis of three lesions from a single patient, the significance of the fDM changes in terms of correlation with clinical outcome measures will require additional patients.
Results
Identification of Metastatic Disease and Characterization of Osseous Lesions
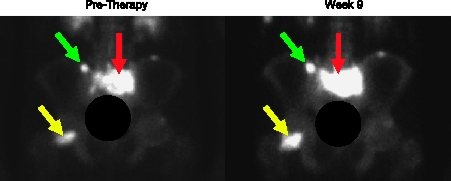
At the time of initial diagnosis, the patient underwent Tc-99m MDP BS to ascertain the extent of skeletal involvement. As shown in Figure 1A, posterior BS revealed two large areas of increased uptake in the sacrum (red arrow) and left femoral head (yellow arrow), with two additional smaller foci of uptake in each ilium (green arrow denotes lesion analyzed by fDM). Nine weeks after initiation of androgen deprivation therapy, a follow-up BS was obtained (Figure 1B) revealing the continuing presence of previous identified lesions; however, the uptake in these regions appeared more intense with greater uptake of the radionuclide. Visualization by CT revealed mixed lucency and sclerosis within the left femoral head lesion (Figure 2A, yellow arrow). After receiving androgen deprivation therapy for 7 weeks, increased sclerosis was observed by CT (Figure 2B, yellow arrow). Computed tomographic images of the sacral lesion before treatment (Figure 2C, red arrow) revealed a predominantly lytic morphology within the sacrum; however, interval-increased sclerosis was observed after therapy (Figure 2D, red arrow). Computed tomographic images of the right ilium lesion (Figure 2C, green arrow) before treatment revealed similar morphology to the lesion in the sacrum with increased sclerosis observed following treatment (Figure 2D, green arrow). Further characterization of the lesions was achieved by MRI. Proton density-weighted images of the femoral head lesion revealed the presence of heterogeneous marrow replacement (Figure 3A, yellow arrow), with an apparent increase of low signal after 7 weeks of treatment (Figure 3B, yellow arrow). Similarly, the sacral and ilium lesions from proton density-weighted MR images exhibited heterogeneous marrow replacement (Figure 3C, red and green arrows, respectively), with increased low signal after treatment (Figure 3D).
Figure 1.
Bone scintigraphy. Posterior bone scintigraphic image of the pelvis shows increased uptake of the sacrum and left femoral head lesions, with two additional foci of uptake in each ilium. Follow-up bone scintigraphy 9 weeks after therapy shows increased intensity of the uptake.
Figure 2.
Computed tomography. Axial CT images (A) before and (B) 7 weeks after treatment show a mixed lucent and sclerotic metastasis (yellow arrow) of the left femoral head. Axial CT images reveal the sacral lesion (red arrow) and ilium lesion (green arrow) (C) before and (D) 7 weeks after treatment.
Figure 3.
T1-weighted MRI. Axial T1-weighted MR images of the femoral head (A) before and (B) 7 weeks after treatment show heterogeneous marrow replacement (yellow arrow). Axial T1-weighted MR images of the sacrum (red arrow) and ilium (green arrow) (C) before and (D) 7 weeks after treatment.
Tumor volumes were quantified using ROI analysis of each of the three tumors evaluated using T2-weighted MR images at weeks 0, 2, and 8 posttreatment initiation. As shown in Table 1, tumor volumes were similar at the pre-treatment time point (week 0) and at weeks 2 and 8 post-treatment. Overall, tumor volume measurements were unable to detect significant perturbations in tumor size in the 8-week timeframe following treatment initiation.
Table 1.
Tumor Volume Measurements over Time.
| Tumor Site | Pretreatment (cm3) | Week 2 (cm3) | Week 3 (cm3) |
| Femoral Head | 52.97 | 51.52 | 51.35 |
| Sacrum | 68.29 | 69.46 | 72.21 |
| Ilium | 2.34 | 2.76 | 2.56 |
fDM Analysis of Osseous Lesions Reveal Changes in Tumor Diffusion Subsequent to Therapy
Using the fDM approach, diffusion MRI data from the femoral head, sacral, and ilium lesions were analyzed to detect spatial changes in tumor diffusion. As shown in Figure 4A, fDM analysis of tumor diffusion after 2 weeks of therapy revealed regions within the femoral head lesion (yellow arrow) that had significant increases in diffusion (depicted as red voxels). Analysis revealed that 21.1% of the total analyzed volume had significant increase in ADC at week 2 posttreatment. A small region of tumor was not analyzed in this lesion due to overlap with abdominal fat signal due to inadequate fat suppression that occurred on that time interval. The sacral and ilium lesions also exhibited distinct areas of increased ADC (red voxels) (Figure 4, C and E, respectively), which were found to be 26.4% and 24.5% of the tumor volume at 2 weeks posttreatment. After 8 weeks of therapy, fDM analysis of the femoral head, sacral, and ilium lesions again identified regions of increased diffusion values (encoded as red voxels on fDM), signifying areas of increased diffusion within these three lesions (Figure 4, B, D, and F, respectively). Quantification of fDM scatter plots revealed regions of increased ADC to be 36.4% (femoral head lesion), 29.0% (sacral lesion), and 47.1% (ilium lesion) of the tumor volume at 8 weeks posttreatment.
Figure 4.
Functional diffusion maps. Regional changes of ADC are plotted on the image to provide a visual representation of areas with increased ADC (red voxels), decreased ADC (blue voxels), and areas where ADC did not change significantly (green voxels). fDM analysis of the femoral head lesion (yellow arrows) at (A) 2 and (B) 8 weeks after treatment initiation revealed distinct regions of red voxels signifying areas with significant increases in ADC (>26 x 10-6 mm2/s). fDM analysis of the sacral lesion (red arrows) at (C) 2 and (D) 8 weeks after treatment revealed significant regions of increased ADC as depicted by the red voxels. fDM analysis of the ilium lesion (green arrows) at (E) 2 and (F) 8 weeks after treatment show large regions of increased ADC values (red voxels).
Analysis of Tumor-Average Diffusion MRI Data
Lesion-mean ADC values were generated by the average of ADC values within a volume of interest defined on each lesion. Lesion volumes of interest were defined for the femoral head, sacral, and ilium lesions. Before treatment initiation, baseline mean ADC values of the femoral head, sacral, and ilium lesions were determined to be 74.5 x 10-6, 110.8 x 10-6, and 78.0 x 10-6 mm2/sec, respectively. As the patient underwent treatment, the femoral head lesion exhibited little change in tumor mean diffusion where mean ADC values were determined to be 77.8 x 10-6 mm2/sec at 2 weeks and 76.8 x 10-6 mm2/sec at 8 weeks posttreatment. The sacral lesion revealed an approximate 10% decrease in mean ADC values to 99.8 x 10-6 mm2/sec at 2 weeks post-treatment initiation, which later increased by nearly 5% above baseline at 8 weeks (116.2 x 10-6 mm2/sec). The ilium lesion revealed very little change in mean ADC at week 2 (80.7 x 10-6 mm2/sec) and a 16.7%increase in mean ADC to 91.1 x 10-6 mm2/sec at 8 weeks post-treatment initiation. Comparison of the fDM and mean ADC analysis approaches was accomplished and the results are displayed in Figure 5. For each tumor, the percentage change of mean ADC values from baseline was calculated at 2 and 8 weeks post-treatment initiation. The average fDM percent increase in diffusion fDM values, which represents the percent of tumor volume with increased diffusion values, was 24 ± 1.6% and 38 ± 5.2% at weeks 2 and 8, respectively. However, the mean percentage change from baseline for the three lesions obtained from the mean ADC analysis was -0.7 ± 4.7% and 8.3 ± 4.3% at 2 and 8 weeks, respectively.
Figure 5.
Comparison of mean ADC versus fDM with respect to pre-therapy baseline. At 2 weeks post-therapy, a decrease of 0.7 ± 4.8% was observed by comparing mean ADC whereas fDM demonstrated a 24 ± 1.6% increase in ADC. At 8 weeks, mean ADC increased by 8.3 ± 4.3% whereas fDM demonstrated a 38 ± 5.2% increase in ADC.
Clinical Response Assessment
PSA levels were monitored over the course of treatment. At week 0, PSA levels were 10.7 ng/ml and were significantly reduced at weeks 2 and 8 posttreatment to 1.3 ng/ml and 0.3 ng/ml, respectively. The PSA levels indicated that the patient was responding to androgen deprivation, which is also reflected in the fDM but not in the mean-derived ADC values.
Discussion
Bony metastases are the leading cause of morbidity and mortality from PCa. A continuing challenge for the clinical management of this disease is the lack of imaging tools that can assess response in bone accurately [39]. This has negatively impacted drug development in this disease [40]. Although imaging modalities such as BS, CT, and MRI play important diagnostic and staging roles, the complex nature of osseous lesions limit the utility of these imaging technologies for accurately measuring response [41]. Part of the difficulty in using conventional anatomic imaging (CT and MRI) for assessing tumor volumetric changes as is typically accomplished in nonskeletal tumor sites for treatment response assessment is the fact that the bone undergoes constant remodeling with a strict coordination in the dynamic interaction between osteoclasts and osteoblasts to maintain proper homeostasis. Lesions residing in the bone deregulate this dynamic process and thus can present as osteolytic lesions, osteoblastic lesions, or mixed lesions when visualized by imaging, complicating interpretation and potentially confounding assessment of treatment-specific effects. Thus, current recommendations on the use of these imaging techniques for monitoring treatment response widely differ depending on recommendations established from various studies, hence no consensus has been established for the validity of using BS, CT, or MRI for assessing treatment response in bone cancer patients.
Although the data presented here are limited to one patient with multifocal disease, the results presented clearly establish the feasibility of acquiring and processing fDM data for metastatic bone lesions. The results confirm previous findings, which have reported that bone scintigraphy following treatment can yield false-positive images termed the flare phenomenon where an apparent increase in radiotracer uptake after treatment occurs [42,43]. The increased uptake can occur with increased sclerosis of the abnormality; however, whether the increased sclerosis is due to bone healing of a lytic tumor or a potentially blastic component of a tumor cannot be differentiated, thus making its use in early treatment response assessment problematic. In fact, data presented in Figure 1 show that at 8 weeks following treatment, an increased uptake of the radiotracer probe (Tc-99m MDP) was observed. Similar lack of treatment response information was also encountered in the anatomic CT (Figure 2) and MR (Figure 3) scans, where increased density from CT and increased low signal on MRI could represent a positive response to therapy from necrosis and healing. However, a blastic component of a negative response could not be clearly ruled out, thereby resulting in false-positive assessments. Furthermore, radiologic response of tumors outside of the skeletal system is currently quantified in terms of the magnitude of reduction in tumor volume after a specific time interval following conclusion of treatment. As shown in Table 1, tumor volumes for each of the lesions evaluated in this patient were not significantly reduced at 2 or 8 weeks post-treatment initiation revealing the lack of prognostic information obtained from this imaging metric. In most solid tumors, volume is proportional to the radiographic observed lesion size. However, this fact is not necessarily the case for bone metastasis following cytotoxic treatment as most radiologists rely on the extent of bone destruction, which is at best an indirect assessment of tumor extent [9]. The killing of tumor cells located in bony tumors may not result in a detectable loss of tumor size as the lesion volume can be contained within a bone structural deficit and, because bone regrowth does not take place, a decrease in the size of the radiographic lesion does not occur. In fact, this is consistent with the findings in the patient's images shown in Figures 2 and 3, which do not reveal a reduction in tumor volume over time.
Quantification of the Brownian motion of water molecules within the tumor tissue can be accomplished using MRI by using an image acquisition sequence which makes the MR signal intensity dependent on water mobility [44]. Clinical translation of diffusion MRI for cancer treatment assessment was initially accomplished in brain tumor patients [13] with other tumor sites reported in subsequent additional studies [45,46]. However, the analysis of diffusion data in clinical cancer trials has been hampered by the tremendous heterogeneity of pretreatment diffusion values and therapeutic-induced changes over time. Whereas analysis of mean ADC has proved useful for syngeneic and human xenograft tumor models, the sensitivity of this approach for the detection of treatment-induced changes in tumor diffusion values has been more limited in human trials due to the underlying histologic heterogeneity commonly exhibited in human cancers.
To better illustrate this point in this study, we also compared the mean ADC approach to evaluate the sensitivity of this method for the detection of treatment response in osseous lesions from serial diffusion ADC maps. The fDM approach has been proposed as a means to standardize the analysis of clinical diffusion data that relies on a voxel by voxel comparison of diffusion changes over time. This is accomplished by acquiring a pretreatment ADC map of the tumor and digitally registering the same tumor acquired at an additional time point post-treatment initiation. The fDM analysis provides an opportunity to quantify spatiotemporal alterations in tumor diffusion values in an individual tumor in terms of total change in diffusion values as a function of percent of total tumor volume [16,35–37]. The feasibility of applying the fDM imaging biomarker to assess treatment response in multifocal metastatic PCa to the bone in the clinical setting was evaluated in this study. MRI data from three lesions arising from the femoral head, sacral, and ilium were analyzed at 2 and 8 weeks post-treatment initiation to evaluate for changes in tumor fDM values. fDM analysis of each of the three lesions revealed that as early as 2 weeks, significant increases in diffusion values were found for each tumor site. The fDM analysis revealed a very interesting finding that an average 24.0% (range 21.1% to 26.4%) of the total analyzed tumor volume had a significant increase in ADC at as early as the 2-week measurement (Figure 5). At 8 weeks following treatment initiation, fDM analysis of the femoral head, sacral, and ilium lesions revealed an average of 37.5% (range 29% to 47.1%) of the total tumor volume was found to exhibit increased diffusion values over pretreatment baseline measurements. Although PSA levels are not definitive measures of patient outcome, PSA measurements from this patient revealed a decline of 88% and 97% at weeks 2 and 8, respectively. This is a consistent but not definitive proof of an overall positive response [10]. Although the data presented herein are from only a single patient, the results obtained raise very interesting opportunities for future studies. For example, it is interesting to note that in a patient with multifocal metastatic disease, each of the tumors evaluated was significantly impacted by the treatment as detected by the fDM biomarker readout. This is consistent with current clinical data, which have shown that hormone naive patients have a high (85%) response rate [47].
The preliminary data presented in Figure 5 also reveal that the fDM analysis of bone tumors was significantly different from mean ADC analysis (P = .004 and P = .006 at 2 and 8 weeks, respectively) and provided for amuchmore sensitive and robust detection of treatment-induced diffusion changes versus the conventional mean ADC approach. In fact, the lack of significant change in tumor diffusion based on mean ADC values obtained from data at 2 and 8 weeks (P = .116) would suggest that androgen deprivation therapy had little effect on the femoral head, sacral, and ilium lesions potentially leading to the conclusion that the treatment was ineffective. In stark contrast, the fDM analysis of the same lesions revealed that large changes in tumor diffusion values had in fact occurred and significantly increased over time (P = .034). Although further clinical data need to be acquired to verify this fact, it appears that fDM measurements from this patient with PCa and with evidence of bone metastases are consistently more sensitive to treatment response than the traditional mean ADC values, as has been reported in the mouse PCa study [16] and clinical brain tumor studies [35,37]. Overall, these data show the feasibility of pursuing the use and validation of fDM for assessment of metastatic PCa treatment monitoring as it provides a potential, standardized approach for the analysis of diffusion clinical tumor response data.
Optimally, routine implementation of an imaging biomarker for treatment assessment should not be overly time consuming, cost-prohibitive, or difficult to access or implement in the myriad of clinical settings worldwide. In these regards, acquisition of diffusion MRI data in the clinical setting requires only a few additional minutes of scan time which can be accomplished without the need for contrast. Calculation of diffusion ADC maps is available on commercial scanners and fDM analysis will soon be accomplished using commercially available software (I-Response, Cedara Software, Mississauga, ON, Canada). Moreover, as diffusion MRI measurements are a biophysical measurement of water mobility, diffusion values are considered to be independent of magnet field strength and scanner type. Therefore, implementation of this technology within a multicenter trial would be relatively straightforward, allowing for validation of this approach in a large-scale clinical trial. Finally, the ability to conduct whole-body diffusion MRI interrogation of disseminated skeletal disease will offer a unique opportunity to assess overall tumor response [48–50]. As shown in this present study, three lesions were followed using fDM over time, thus revealing the overall feasibility of using this imaging biomarker for whole-body response assessment.
Validation of fDM in further studies as a quantitative and early imaging biomarker for assessment of treatment response in patients with metastatic PCa would be a major leap forward for designing clinical trials and for overall patient management. Ultimately, acceptance of the fDM biomarker would require multicenter trials and a consensus to revise the International Union Against Cancer, World Health Organization, and RECIST response criteria to incorporate this imaging biomarker. The successful validation and inclusion of fDM into clinical response criteria could provide for an integrated consensus regarding how to evaluate most accurately the treatment response of osseous lesions.
Acknowledgements
The authors thank Susan Rohrer for assistance with the acquisition of the clinical image data sets. T. L. C., A. R., and B. D. R. have a financial interest in the underlying diffusion technology. Technology has been licensed to a company in which A. R. and B. D. R. have a financial interest.
Abbreviations
- ADC
apparent diffusion coefficient
- BS
bone scan
- CT
computed tomography
- fDM
functional diffusion map
- MRI
magnetic resonance imaging
- PCa
prostate cancer
- PSA
prostate-specific antigen
- RECIST
Response Evaluation Criteria in Solid Tumors
- ROI
region-of-interest
Footnotes
This work was supported in part by the National Institutes of Health grants P01CA85878, P01CA87634, and P50CA93990 and by a seed grant from the University of Michigan Comprehensive Cancer Center. D. B. is supported by T32CA009357. K. J. P. is supported as an American Cancer Society Clinical Research Professor and by P50CA69568 and P01CA093900.
References
- 1.American Cancer Society, author. Cancer Facts & Figures 2007. Atlanta: American Cancer Society; 2007. [Google Scholar]
- 2.Taichman RS, Loberg RD, Mehra R, Pienta KJ. The evolving biology and treatment of prostate cancer. J Clin Invest. 2007;117(9):2351–2361. doi: 10.1172/JCI31791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Hayward JL, Carbone PP, Heusen JC, Kumaoka S, Segaloff A, Rubens RD. Assessment of response to therapy in advanced breast cancer. Br J Cancer. 1977;35(3):292–298. doi: 10.1038/bjc.1977.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayward JL, Carbone PP, Rubens RD, Heuson JC, Kumaoka S, Segaloff A. Assessment of response to therapy in advanced breast cancer (an amendment) Br J Cancer. 1978;38(1):201. doi: 10.1038/bjc.1978.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization, author. WHO Handbook for Reporting Results of Cancer Treatment. Atlanta: World Health Organization Offset Publication; 1979. [Google Scholar]
- 8.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22(14):2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 10.Bubley GJ, Carducci M, Dahut W, Dawson M, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17(11):3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 11.Ross BD, Chenevert TL, Kim B, Ben-Yoseph O. Magnetic resonance imaging and spectroscopy: application to experimental neuro-oncology. Q Magn Reson Biol Med. 1994;1:89–106. [PMC free article] [PubMed] [Google Scholar]
- 12.Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res. 1997;3(9):1457–1466. [PubMed] [Google Scholar]
- 13.Chenevert TL, Stegman LD, Taylor JM, Robertson PL, Greenberg HS, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92(24):2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 14.Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, Ross BD, Rehemtulla A. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA. 2000;97(4):1754–1759. doi: 10.1073/pnas.030545097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KC, Moffat BA, Schott AF, Layman R, Ellingworth S, Juliar R, Khan AP, Helvie M, Meyer CR, Chenevert TL. Prospective early response imaging biomarker for neoadjuvant breast cancer chemotherapy. Clin Cancer Res. 2007;13(2 Pt 1):443–450. doi: 10.1158/1078-0432.CCR-06-1888. [DOI] [PubMed] [Google Scholar]
- 16.Lee KC, Sud S, Meyer CR, Moffat BA, Chenevert TL, Rehemtulla A, Pienta KJ, Ross BD. An imaging biomarker of early treatment response in prostate cancer that has metastasized to the bone. Cancer Res. 2007;67(8):3524–3528. doi: 10.1158/0008-5472.CAN-06-4236. [DOI] [PubMed] [Google Scholar]
- 17.Moffat BA, Hall DE, Stojanovska J, McConville PJ, Moody JB, Chenevert TL, Rehemtulla A, Ross BD. Diffusion imaging for evaluation of tumor therapies in preclinical animal models. Magma. 2004;17(3–6):249–259. doi: 10.1007/s10334-004-0079-z. [DOI] [PubMed] [Google Scholar]
- 18.Ross BD, Moffat BA, Lawrence TS, Mukherji SK, Gebarski SS, Quint DJ, Johnson TD, Junck L, Robertson PL, Muraszko KM. Evaluation of cancer therapy using diffusion magnetic resonance imaging. Mol Cancer Ther. 2003;2(6):581–587. [PubMed] [Google Scholar]
- 19.Stegman LD, Rehemtulla A, Hamstra DA, Rice DJ, Jonas SJ, Stout KL, Chenevert TL, Ross BD. Diffusion MRI detects early events in the response of a glioma model to the yeast cytosine deaminase gene therapy strategy. Gene Ther. 2000;7(12):1005–1010. doi: 10.1038/sj.gt.3301199. [DOI] [PubMed] [Google Scholar]
- 20.Zhao M, Pipe JG, Bonnett J, Evelhoch JL. Early detection of treatment response by diffusion-weighted 1H-NMR spectroscopy in a murine tumour in vivo. Br J Cancer. 1996;73(1):61–64. doi: 10.1038/bjc.1996.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamstra DA, Lee KC, Tychewicz JM, Schepkin VD, Moffat BA, Chen M, Dornfeld KJ, Lawrence TS, Chenevert TL, Ross BD. The use of 19F spectroscopy and diffusion-weighted MRI to evaluate differences in gene-dependent enzyme prodrug therapies. Mol Ther. 2004;10(5):916–928. doi: 10.1016/j.ymthe.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Lee KC, Hall DE, Hoff BA, Moffat BA, Sharma S, Chenevert TL, Meyer CR, Leopold WR, Johnson TD, Mazurchuk RV. Dynamic imaging of emerging resistance during cancer therapy. Cancer Res. 2006;66(9):4687–4692. doi: 10.1158/0008-5472.CAN-05-3205. [DOI] [PubMed] [Google Scholar]
- 23.Lee KC, Hamstra DA, Bhojani MS, Khan AP, Ross BD, Rehemtulla A. Noninvasive molecular imaging sheds light on the synergy between 5-fluorouracil and TRAIL/Apo2L for cancer therapy. Clin Cancer Res. 2007;13(6):1839–1846. doi: 10.1158/1078-0432.CCR-06-1657. [DOI] [PubMed] [Google Scholar]
- 24.Lee KC, Hamstra DA, Bullarayasamudram S, Bhojani MS, Moffat BA, Dornfeld KJ, Ross BD, Rehemtulla A. Fusion of the HSV-1 tegument protein vp22 to cytosine deaminase confers enhanced bystander effect and increased therapeutic benefit. Gene Ther. 2006;13(2):127–137. doi: 10.1038/sj.gt.3302631. [DOI] [PubMed] [Google Scholar]
- 25.Schepkin VD, Lee KC, Kuszpit K, Muthuswami M, Johnson TD, Chenevert TL, Rehemtulla A, Ross BD. Proton and sodium MRI assessment of emerging tumor chemotherapeutic resistance. NMR Biomed. 2006;19(8):1035–1042. doi: 10.1002/nbm.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chenevert TL, Meyer CR, Moffat BA, Rehemtulla A, Mukherji SK, Gebarski SS, Quint DJ, Robertson PL, Lawrence TS, Junck L. Diffusion MRI: a new strategy for assessment of cancer therapeutic efficacy. Mol Imaging. 2002;1(4):336–343. doi: 10.1162/15353500200221482. [DOI] [PubMed] [Google Scholar]
- 27.Hall DE, Moffat BA, Stojanovska J, Johnson TD, Li Z, Hamstra DA, Rehemtulla A, Chenevert TL, Carter J, Pietronigro D. Therapeutic efficacy of DTI-015 using diffusion magnetic resonance imaging as an early surrogate marker. Clin Cancer Res. 2004;10(23):7852–7859. doi: 10.1158/1078-0432.CCR-04-1218. [DOI] [PubMed] [Google Scholar]
- 28.Jennings D, Hatton BN, Guo J, Galons JP, Trouard TP, Raghunand N, Marshall J, Gillies RJ. Early response of prostate carcinoma xenografts to docetaxel chemotherapy monitored with diffusion MRI. Neoplasia. 2002;4(3):255–262. doi: 10.1038/sj.neo.7900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan BF, Runquist M, Raghunand N, Baker A, Williams R, Kirkpatrick L, Powis G, Gillies RJ. Dynamic contrast-enhanced and diffusion MRI show rapid and dramatic changes in tumor microenvironment in response to inhibition of HIF-1alpha using PX-478. Neoplasia. 2005;7(5):475–485. doi: 10.1593/neo.04628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theilmann RJ, Borders R, Trouard TP, Xia G, Outwater E, Ranger-Moore J, Gillies RJ, Stopeck A. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia. 2004;6(6):831–837. doi: 10.1593/neo.03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galons JP, Altbach MI, Paine-Murrieta GD, Taylor CW, Gillies RJ. Early increases in breast tumor xenograft water mobility in response to paclitaxel therapy detected by non-invasive diffusion magnetic resonance imaging. Neoplasia. 1999;1(2):113–117. doi: 10.1038/sj.neo.7900009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hakumaki JM, Poptani H, Puumalainen AM, Loimas S, Paljarvi LA, Yla-Herttuala S, Kauppinen RA. Quantitative 1H nuclear magnetic resonance diffusion spectroscopy of BT4C rat glioma during thymidine kinase-mediated gene therapy in vivo: identification of apoptotic response. Cancer Res. 1998;58(17):3791–3799. [PubMed] [Google Scholar]
- 33.Poptani H, Puumalainen AM, Grohn OH, Loimas S, Kainulainen R, Yla-Herttuala S, Kauppinen RA. Monitoring thymidine kinase and ganciclovir-induced changes in rat malignant glioma in vivo by nuclear magnetic resonance imaging. Cancer Gene Ther. 1998;5(2):101–109. [PubMed] [Google Scholar]
- 34.Ross BD, Zhao YJ, Neal ER, Stegman LD, Ercolani M, Ben-Yoseph O, Chenevert TL. Contributions of cell kill and posttreatment tumor growth rates to the repopulation of intracerebral 9L tumors after chemotherapy: an MRI study. Proc Natl Acad Sci USA. 1998;95(12):7012–7017. doi: 10.1073/pnas.95.12.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moffat BA, Chenevert TL, Lawrence TS, Meyer CR, Johnson TD, Dong Q, Tsien C, Mukherji S, Quint DJ, Gebarski SS. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci USA. 2005;102(15):5524–5529. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moffat BA, Chenevert TL, Meyer CR, McKeever PE, Hall DE, Hoff BA, Johnson TD, Rehemtulla A, Ross BD. The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia. 2006;8(4):259–267. doi: 10.1593/neo.05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamstra DA, Chenevert TL, Moffat BA, Johnson TD, Meyer CR, Mukherji SK, Quint DJ, Gebarski SS, Fan X, Tsien CI. Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc Natl Acad Sci USA. 2005;102(46):16759–16764. doi: 10.1073/pnas.0508347102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer CR, Boes JL, Kim B, Bland PH, Zasadny KR, Kison PV, Koral K, Frey KA, Wahl RL. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Med Image Anal. 1997;1(3):195–206. doi: 10.1016/s1361-8415(97)85010-4. [DOI] [PubMed] [Google Scholar]
- 39.Scher HI, Morris MJ, Kelly WK, Schwartz LH, Heller G. Prostate cancer clinical trial end points: “RECIST”ing a step backwards. Clin Cancer Res. 2005;11(14):5223–5232. doi: 10.1158/1078-0432.CCR-05-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scher HI, Warren M, Heller G. The association between measures of progression and survival in castrate-metastatic prostate cancer. Clin Cancer Res. 2007;13(5):1488–1492. doi: 10.1158/1078-0432.CCR-06-1885. [DOI] [PubMed] [Google Scholar]
- 41.Fogelman I, Cook G, Israel O, Van der Wall H. Positron emission tomography and bone metastases. Semin Nucl Med. 2005;35(2):135–142. doi: 10.1053/j.semnuclmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Cook GJ, Fogelman I. The role of nuclear medicine in monitoring treatment in skeletal malignancy. Semin Nucl Med. 2001;31(3):206–211. doi: 10.1053/snuc.2001.23527. [DOI] [PubMed] [Google Scholar]
- 43.Koizumi M, Matsumoto S, Takahashi S, Yamashita T, Ogata E. Bone metabolic markers in the evaluation of bone scan flare phenomenon in bone metastases of breast cancer. Clin Nucl Med. 1999;24(1):15–20. doi: 10.1097/00003072-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 45.Hayashida Y, Yakushiji T, Awai K, Katahira K, Nakayama Y, Shimomura O, Kitajima M, Hirai T, Yamashita Y, Mizuta H. Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: initial results. Eur Radiol. 2006;16(12):2637–2643. doi: 10.1007/s00330-006-0342-y. [DOI] [PubMed] [Google Scholar]
- 46.Uhl M, Saueressig U, Koehler G, Kontny U, Niemeyer C, Reichardt W, Ilyasof K, Bley T, Langer M. Evaluation of tumour necrosis during chemotherapy with diffusion-weighted MR imaging: preliminary results in osteosarcomas. Pediatr Radiol. 2006;36(12):1306–1311. doi: 10.1007/s00247-006-0324-x. [DOI] [PubMed] [Google Scholar]
- 47.Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, Wilding G, Akdas A, Small EJ, Donnelly B. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24(24):3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 48.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188(6):1622–1635. doi: 10.2214/AJR.06.1403. [DOI] [PubMed] [Google Scholar]
- 49.Komori T, Narabayashi I, Matsumura K, Matsuki M, Akagi H, Ogura Y, Aga F, Adachi I. 2-[Fluorine-18]-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography versus whole-body diffusion-weighted MRI for detection of malignant lesions: initial experience. Ann Nucl Med. 2007;21(4):209–215. doi: 10.1007/s12149-007-0010-6. [DOI] [PubMed] [Google Scholar]
- 50.Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22(4):275–282. [PubMed] [Google Scholar]