Abstract
A new synthetic methodology for preparing radioactive androgen receptor binding compounds in order to determine receptor–ligands interactions has been developed.
Keywords: Bicalutamide, Iodine, Androgen, Receptor, Radioactive
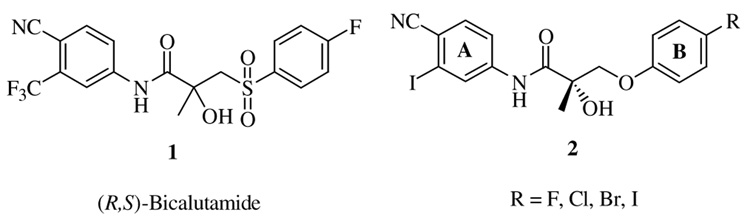
Androgen receptor is an important cellular regulatory protein, which plays a prominent role in numerous physiological processes.1,2 Compounds that bind to the androgen receptor have great therapeutic value in the treatment of various conditions ranging from regulation of male fertility to prostate cancer.3 In our continued efforts to establish the mechanism of action of the tissue selective androgen receptor modulator (SARM), which we discovered in 1998 and to develop it as a potential drug candidate for prostate cancer we synthesized a series of iodo analogs of bicalutamide.4,5 These molecules, which exhibited antagonistic activity were found to block the pharmacological effects of testosterone; and were obtained by replacing the trifluoromethyl and sulfonyl groups of bicalutamide by iodine and oxygen, respectively, and introduction of chirality at the stereogenic center; the synthesis has been reported recently.5 This investigation was carried out primarily to develop a target molecule whose interaction with the androgen receptor could be identified by having a tag or a label attached to the molecule and to follow its course of action by imaging techniques. Our attempts to label the molecule by attaching a chromophore, which exhibits fluorescence did not turn out to be successful, because of the low binding affinity to the receptor. Hence we decided to incorporate a radioactive isotopic label (125I) to the compound 2, which showed very high binding affinity to the receptor (Fig. 1).6
Figure 1.
Earlier investigations have proved that the stereochemistry at the chiral center, the nature of the functional groups attached to the aromatic rings and the heteroatom linker playa prominent role in determining the activity and affinity.7–13 An isothiocyanate moiety had been introduced at the 4th position of the aromatic Bring so as to provide an electrophilic center for an irreversibly binding interaction between the receptor and the ligand. Analogous agents have been shown to bind irreversibly providing a theoretical basis to develop these as prostate cancer therapeutics.14,15 Biological testing of the isothiocyanate analogs of bicalutamide demonstrated high binding affinity in exchange assays with a radiolabeled high affinity androgen receptor ligand [³H]mibolerone, irreversible androgen receptor binding in Scatchard analyses and potential growth inhibitory activity against LNCaP, DU-145, PC-3, PPC-1, and TSU prostate cancer cell lines.16,17 The advantages of incorporation of the radioisotope 125I into an androgen receptor ligand are threefold: (i) it allows radioimaging of prostate tumor, (ii) provides an irreversibly binding ligand that should chemically ablate androgen receptor action in prostate, blocking cancer growth, and (iii) provides a synthetic strategy to incorporate other iodine radioisotopes such as 131I, which could be investigated in radioiodine therapy for prostate cancer.
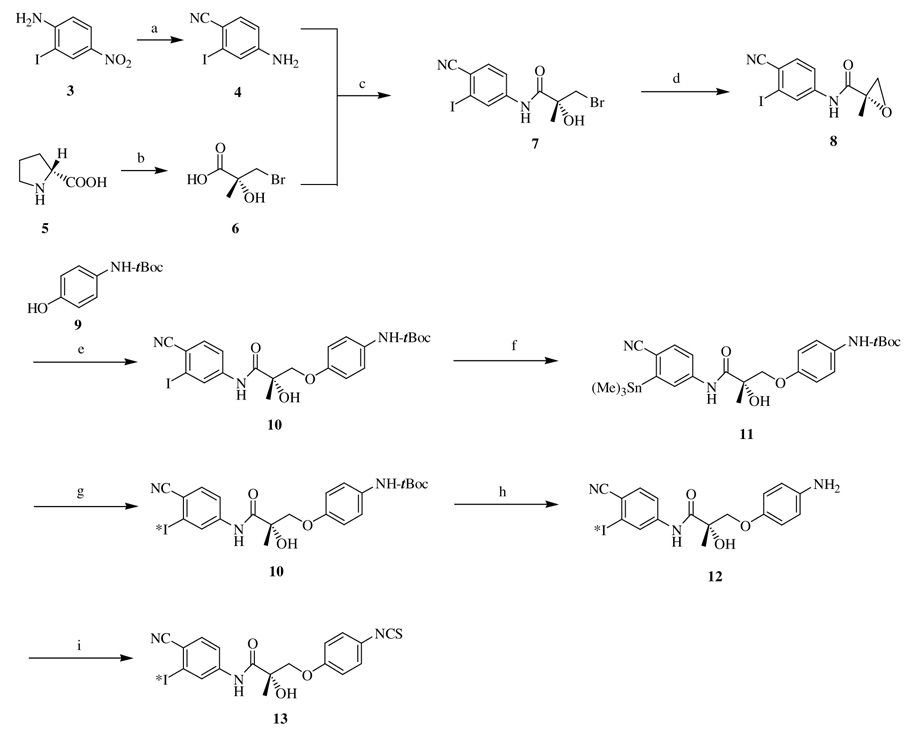
The synthetic procedure employed for the preparation of target compound is depicted in Scheme 1. We recently reported the syntheses of 4-amino-2-iodobenzonitrile 4 and (2R)-3-bromo-2-hydroxy-2-methylpropanoic acid 6 from 4-nitro-2-iodoaniline 3 and (d)-proline 5, respectively.5 A solution of 6 in tetrahydrofuran was converted to the corresponding acid chloride by thionyl chloride under argon atmosphere at 0 °C. Addition of a solution of 4 in tetrahydrofuran followed by overnight stirring at room temperature yielded the (R)-propanamide derivative 7. A solution of compound 7 in acetone when refluxed with K2CO3 for 2 h generated the corresponding epoxide 8, which was subsequently opened up by refluxing it with tert-butylcarbonate protected 4-aminophenol 9 and K2CO3 in 2-propanol medium to obtain compound 10. The conversion of the intermediate 7 to compound 10 through the epoxide formation was carried out in a two-step, one-pot process wherein after the epoxide was formed, the solvent was removed and the resulting residue was immediately carried on to the ring opening step.
Scheme 1.
Reagents and conditions: (a) (1) NaNO2, H2SO4, CuCN, NaCN, (2) HCl, SnCl2·2H2O, EtOH; (b) (1) methacryloyl chloride, NaOH, acetone, (2) NBS, DMF, (3) HBr; (c) SOCl2, THF; (d) K2CO3, acetone; (e) K2CO3, 2-propanol; (f) Pd(PPh3)4, hexamethyl ditin, toluene; (g) NaI (Na125I), chloramine T, MeOH; (h) acetyl chloride, absolute EtOH; (i) chloroform, thiophosgene, NaHCO3; *I = I or 125I.
To a mixture of compound 10 and tetrakis (triphenylphosphine) palladium in anhydrous toluene under argon atmosphere, hexamethyl ditin18 was introduced and refluxed for 5 h. Workup of the reaction mixture followed by purification using flash column chromatography afforded the trimethyl tin derivative 11. Equimolar amounts of NaI and chloramine T were added to a solution of compound 11 in methanol and stirred at room temperature for 30 min. The reaction mixture turned yellow during the course of the reaction; the completion of the reaction was determined by the absence of the starting material in the reaction mixture. Subsequent washing with sodium bicarbonate solution and extraction into ethyl acetate afforded back the compound 10. The success of this step ensured the repetition of the experiment with radioactive Na125I so as to obtain the radioactive iodine incorporated analog of compound 10. The protective group on the amino functionality was then removed by dissolving it in absolute ethanol and treating it with acetyl chloride under ice-cold conditions, followed by stirring at room temperature for 2 h; which upon concentration followed by washing in aqueous medium and extraction into ethyl acetate gave the free amine 12. Thereafter, to the solution of compound 12 in chloroform, thiophosgene, and sodium bicarbonate were added at 0 °C and stirred overnight at room temperature to yield the target compound 13.19 The synthetic strategy outlined herein provides a facile route to the development of a new class of androgen receptor ligands with a tracer that helps to identify, understand and establish the mechanism of action along with a detailed insight to the receptor–ligand binding interactions. Simultaneously computational studies using molecular modeling techniques are also being carried out by docking the ligand into the androgen receptor model that we developed, so as to provide a theoretical rationale to substantiate the experimental mechanistic observations of the binding interactions and the resulting structure activity relationships. Efforts are ongoing to confirm the binding loci by X-ray crystallographic techniques as well.
Acknowledgements
We thank the Department of Defense (DOD) for the grant ‘Novel Strategy for Prostate Cancer Imaging: Synthesis and Pharmacology of Nonsteroidal Ligands’ DAMD17-01-1-0103 and the National Institute of Health (NIH) for the grant ‘Novel Irreversible Nonsteroidal Selective Androgen Receptor Modulators (SARMs) for Prostate Cancer’ DK 065227. The American Chemical Society, Division of Medicinal Chemistry is gratefully acknowledged for a pre-doctoral fellowship for M.L.M.
References and notes
- 1.Evans RM. Science. 1988;240:889. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Dort ME, Robins DM, Wayburn B. J. Med. Chem. 2000;43:3344. doi: 10.1021/jm000163y. [DOI] [PubMed] [Google Scholar]
- 3.Tucker H, Chesterson GJ. J. Med. Chem. 1988;31:885. doi: 10.1021/jm00399a034. [DOI] [PubMed] [Google Scholar]
- 4.Dalton JT, Mukherjee A, Zhu Z, Kirkovsky L, Miller DD. Biochem. Biophys. Res. Commun. 1998;244:1. doi: 10.1006/bbrc.1998.8209. [DOI] [PubMed] [Google Scholar]
- 5.Nair VA, Mustafa SM, Mohler ML, Fisher SJ, Dalton JT, Miller DD. Tetrahedron Lett. 2004;45:9475. [Google Scholar]
- 6.Nair VA, Mustafa SM, Mohler ML, Fisher SJ, Dalton JT, Miller DD. Studies on some selective androgen receptor modulators. 228th American Chemical Society Meeting; Philadelphia: Division of Medicinal Chemistry; 2004. Unpublished Results. [Google Scholar]
- 7.Marhefka CA, Gao W, Chung K, Kim J, He Y, Yin D, Bohl C, Dalton JT, Miller DD. J. Med. Chem. 2004;47:993. doi: 10.1021/jm030336u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marhefka CA, Moore BM, II, Bishop TC, Kirkovsky LI, Mukherjee A, Dalton JT, Miller DD. J. Med. Chem. 2001;44:1729. doi: 10.1021/jm0005353. [DOI] [PubMed] [Google Scholar]
- 9.Rosen J, Day A, Jones TK, Nazdan AM, Stein RB. J. Med. Chem. 1995;38:4855. doi: 10.1021/jm00025a001. [DOI] [PubMed] [Google Scholar]
- 10.Bohl CE, Chang C, Mohler ML, Chen J, Miller DD, Swaan PW, Dalton JT. J. Med. Chem. 2004;47:3765. doi: 10.1021/JM0499007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris JJ, Hughes LR, Len AT, Taylor PJ. J. Med. Chem. 1991;34:447. doi: 10.1021/jm00105a067. [DOI] [PubMed] [Google Scholar]
- 12.James KD, Ekwuribe NN. Synthesis. 2002;7:850. [Google Scholar]
- 13.Yin D, Gao W, Kearby JD, Xu H, Chung K, He Y, Marhefka CA, Veverka KA, Miller DD, Dalton JT. J. Pharmacol. Exp. Ther. 2003;304:1334. doi: 10.1124/jpet.102.040840. [DOI] [PubMed] [Google Scholar]
- 14.Kirkovsky L, Mukherjee A, Yin D, Dalton JT, Miller DD. J. Med. Chem. 2000;43:581. doi: 10.1021/jm990027x. [DOI] [PubMed] [Google Scholar]
- 15.Yin D, Xu H, Kirkovsky LI, Miller DD, Dalton JT. J. Pharmacol. Exp. Ther. 2003;304:1323. doi: 10.1124/jpet.102.040832. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee A, Kirkovsky LI, Kimura Y, Marvel MM, Miller DD, Dalton JT. Biochem. Pharmacol. 1999;58:1259. doi: 10.1016/s0006-2952(99)00218-x. [DOI] [PubMed] [Google Scholar]
- 17.Hwang DJ, Xu H, Mustafa SM, Dalton JT, Miller DD. Synthesis of isothiocyanate derivatives of irreversible selective androgen receptor modulators (SARMs) and biological testing in prostate cancer cell lines. 229th American Chemical Society Meeting; San Diego: Division of Medicinal Chemistry; 2005. Unpublished Results. [Google Scholar]
- 18.Hexamethyl ditin is highly toxic when inhaled or in contact with skin and is also dangerous to the environment. Therefore it should be handled with adequate precautions and care. For more information: ‘Toxicity of tin and its compounds’: Winship KA. Adverse Drug React. Acute Poison. Rev. 1988;7:19.
- 19.Compound 10: Yield: 60%; NMR (¹H, 300 MHz, CDCl3): 1.40 (m, 9H, 3CH3), 1.57 (s, 3H, CH3), 4.5 (d, 1H, CH), 4.7 (d, 1H, CH), 6.8 (m, 2H, ArH, J = 2.5 Hz, 8.8 Hz), 7.5 (m, 2H, ArH, J = 2.5 Hz, 8.8 Hz), 7.2 (m, 1H, ArH, J = 0.6 Hz, 8.5 Hz), 7.8 (m, 1H, ArH, J = 2.4 Hz, 8.5 Hz), 8.2 (m, 1H, ArH, J = 0.6 Hz, 2.4 Hz), 8.0 (br, 1H, NH), 8.7 (br, 1H, NH); MS: 536.1 (M—H); C22H24IN3O5, calcd: C, 49.17; H, 4.50; N, 7.82. Found: C, 49.10; H, 4.39; N, 7.76; −25.4 (c 1.0, MeOH) 11: Yield: 53%; NMR (¹H, 300 MHz, CDCl3): 0.9 (m, 9H, 3CH3), 1.40 (m, 9H, 3CH3), 1.52 (s, 3H, CH3), 4.6 (d, 1H, CH), 4.8 (d, 1H, CH), 6.8 (m, 2H, ArH, J = 2.7 Hz, 8.6 Hz), 7.6 (m, 2H, ArH, J = 2.7 Hz, 8.6 Hz), 7.4 (m, 1H, ArH, J = 0.7 Hz, 8.2 Hz), 7.8 (m, 1H, ArH, J = 2.3 Hz, 8.2 Hz), 7.9 (m, 1H, ArH, J = 0.7 Hz, 2.3 Hz), 8.1 (br, 1H, NH), 8.7 (br, 1H, NH); MS: 572.2 (M—H); C25H33N3O5Sn, calcd: C, 52.29; H, 5.79; N, 7.32. Found: C, 52.15; H, 5.70; N, 7.21; −37.5 (c 1.0, MeOH) 12: Yield: 42%; NMR (¹H, 300 MHz, CDCl3): 1.48 (s, 3H, CH3), 4.4 (d, 1H, CH), 4.6 (d, 1H, CH), 6.4 (d, 2H, ArH, J = 2.8 Hz, 8.9 Hz), 6.6 (d, 2H, ArH, J = 2.8 Hz, 8.9 Hz), 7.2 (m, 1H, ArH, J = 0.5 Hz, 8.5 Hz), 7.8 (m, 1H, ArH, J = 2.4 Hz, 8.5 Hz), 8.2 (m, 1H, ArH, J = 0.5 Hz, 2.4 Hz), 8.1 (br, 2H, NH2), 8.3 (br, 1H, NH); MS: 460.1 (M+Na+); C17H16IN3O3, calcd: C, 46.70; H, 3.69; N, 29.02. Found: C, 46.65; H, 3.57; N, 29.1; +54.3 (c 1.0, MeOH) 13: Yield: 35%; NMR (¹H, 300 MHz, CDCl3): 1.5 (s, 3H, CH3), 4.5 (d, 1H, CH), 4.7 (d, 1H, CH), 6.8 (m, 2H, ArH, J = 2.7 Hz, 9.0 Hz), 7.2 (m, 2H, ArH, J = 2.7 Hz, 9.0 Hz), 7.3 (m, 1H, ArH, J = 0.5 Hz, 8.5 Hz), 7.8 (m, 1H, ArH, J = 2.4 Hz, 8.5 Hz), 8.2 (m, 1H, ArH, J = 0.5 Hz, 2.4 Hz), 8.5 (br, 1H, NH); MS: 502.3 (M+Na+); C18H14IN3O3S, calcd: C, 45.11; H, 2.94; N, 8.77. Found: C, 45.15; H, 2.85; N, 8.69; +65.8 (c 1.0, MeOH).