Abstract
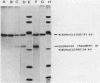
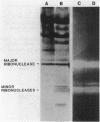
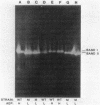
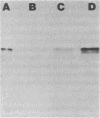
Production of extracellular RNase(s) by Yarrowia lipolytica CX161-1B was examined in media between pHs 5 and 7. RNase production occurred during the exponential growth phase. High-molecular-weight nitrogen compounds supported the highest levels of RNase production. Several RNases were detected in the supernatant medium. Based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the RNases had estimated molecular weights of 45,000, 43,000, and 34,000. It was found that Y. lipolytica secretes only one RNase (the 45,000-molecular-weight RNase) and that the 43,000 and 34,000-molecular-weight RNases are degradation products of this RNase. The alkaline extracellular protease secreted by Y. lipolytica was shown to have a major role in the 45,000- to 43,000-molecular-weight conversion, and it was demonstrated that the 45,000-molecular-weight RNase could be purified from a mutant which does not produce the alkaline extracellular protease. Purification of the RNase from a wild-type strain resulted in purification of the 43,000-molecular-weight RNase. This RNase was a glycoprotein with a molecular weight of 44,000 as estimated by gel filtration, an isoelectric point of pH 4.8, and a pH optimum between 6.5 and 7.0.
Full text
PDF

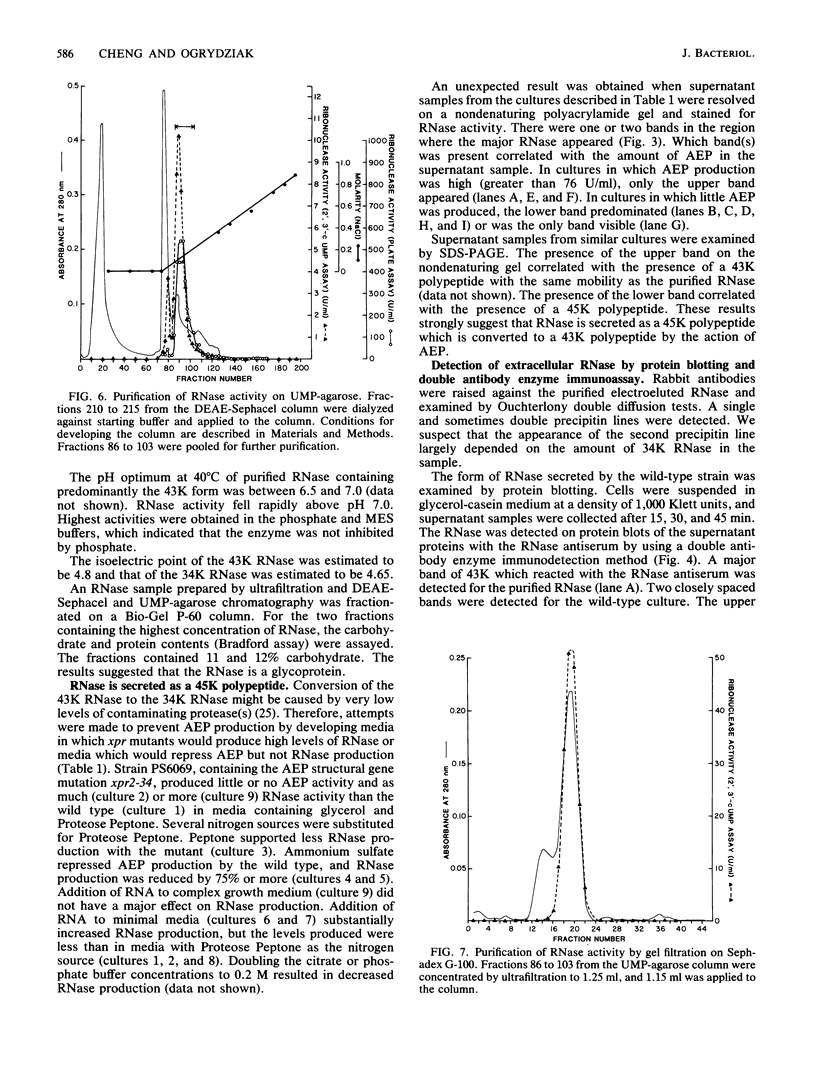



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., REDFIELD R. R., CHOATE W. L., PAGE J., CARROLL W. R. Studies on the gross structure, cross-linkages, and terminal sequences in ribonuclease. J Biol Chem. 1954 Mar;207(1):201–210. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burt W. R., Cazin J., Jr Production of extracellular ribonuclease by yeasts and yeastlike fungi, and its repression by orthophosphate in species of Cryptococcus and Tremella. J Bacteriol. 1976 Mar;125(3):955–960. doi: 10.1128/jb.125.3.955-960.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S., Hansen W., Schauer I., Schekman R. Genes required for completion of import of proteins into the endoplasmic reticulum in yeast. J Cell Biol. 1984 Jan;98(1):44–53. doi: 10.1083/jcb.98.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillardin C. M., Charoy V., Heslot H. A study of copulation, sporulation and meiotic segregation in Candida lipolytica. Arch Mikrobiol. 1973;92(1):69–83. doi: 10.1007/BF00409513. [DOI] [PubMed] [Google Scholar]
- Imada A., Hunt J. W., Van De Sande H., Sinskey A. J., Tannenbaum S. R. Puridication and properties of an intracellular ribonuclease from Candida lipolytica. Biochim Biophys Acta. 1975 Jul 23;395(4):490–500. doi: 10.1016/0005-2787(75)90072-6. [DOI] [PubMed] [Google Scholar]
- Imada A., Sinskey A. J., Tannenbaum S. R. A new macromolecular inhibitor of yeast ribonuclease mediated through purine nucleotides. Biochim Biophys Acta. 1972 Jun 16;268(3):674–679. doi: 10.1016/0005-2744(72)90271-9. [DOI] [PubMed] [Google Scholar]
- Kocková-Kratochvílová A. Urease and extracellular nucleases of yeasts. Folia Microbiol (Praha) 1982;27(6):404–412. doi: 10.1007/BF02876451. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg R. A., Drucker H. Regulation of a Neurospora crassa extracellular RNase by phosphorus, nitrogen, and carbon derepressions. J Bacteriol. 1984 Feb;157(2):380–384. doi: 10.1128/jb.157.2.380-384.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrydziak D. M., Demain A. L., Tannenbaum S. R. Regulation of extracellular protease production in Candida lipolytica. Biochim Biophys Acta. 1977 Apr 27;497(2):525–538. doi: 10.1016/0304-4165(77)90209-4. [DOI] [PubMed] [Google Scholar]
- Ogrydziak D. M., Mortimer R. K. Genetics of Extracellular Protease Production in SACCHAROMYCOPSIS LIPOLYTICA. Genetics. 1977 Dec;87(4):621–632. doi: 10.1093/genetics/87.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrydziak D. M., Scharf S. J. Alkaline extracellular protease produced by Saccharomycopsis lipolytica CX161-1B. J Gen Microbiol. 1982 Jun;128(6):1225–1234. doi: 10.1099/00221287-128-6-1225. [DOI] [PubMed] [Google Scholar]
- Pringle J. R. Methods for avoiding proteolytic artefacts in studies of enzymes and other proteins from yeasts. Methods Cell Biol. 1975;12:149–184. doi: 10.1016/s0091-679x(08)60956-5. [DOI] [PubMed] [Google Scholar]
- Reese E. T., Maguire A. Surfactants as stimulants of enzyme production by microorganisms. Appl Microbiol. 1969 Feb;17(2):242–245. doi: 10.1128/am.17.2.242-245.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Uchida T., Egami F. Action of ribonuclease T2 on 2',3'-cyclic nucleotides and related compounds. Arch Biochem Biophys. 1966 Jul;115(1):48–52. doi: 10.1016/s0003-9861(66)81036-6. [DOI] [PubMed] [Google Scholar]
- Simms P. C., Ogrydziak D. M. Structural gene for the alkaline extracellular protease of Saccharomycopsis lipolytica. J Bacteriol. 1981 Jan;145(1):404–409. doi: 10.1128/jb.145.1.404-409.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Wierenga R. K., Huizinga J. D., Gaastra W., Welling G. W., Beintema J. J. Affinity chromatography of porcine pancreatic ribonuclease reinvestigation of the N-terminal amino acid sequence. FEBS Lett. 1973 Apr 15;31(2):181–185. doi: 10.1016/0014-5793(73)80098-5. [DOI] [PubMed] [Google Scholar]
- Wilson C. M. A rapid staining technique for detection of RNase after polyacrylamide gel electrophoresis. Anal Biochem. 1969 Oct 1;31(1):506–511. doi: 10.1016/0003-2697(69)90294-2. [DOI] [PubMed] [Google Scholar]
- Yamada T., Ogrydziak D. M. Extracellular acid proteases produced by Saccharomycopsis lipolytica. J Bacteriol. 1983 Apr;154(1):23–31. doi: 10.1128/jb.154.1.23-31.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Walt J. P., von Arx J. A. The yeast genus Yarrowia gen. nov. Antonie Van Leeuwenhoek. 1980;46(6):517–521. doi: 10.1007/BF00394008. [DOI] [PubMed] [Google Scholar]