Abstract
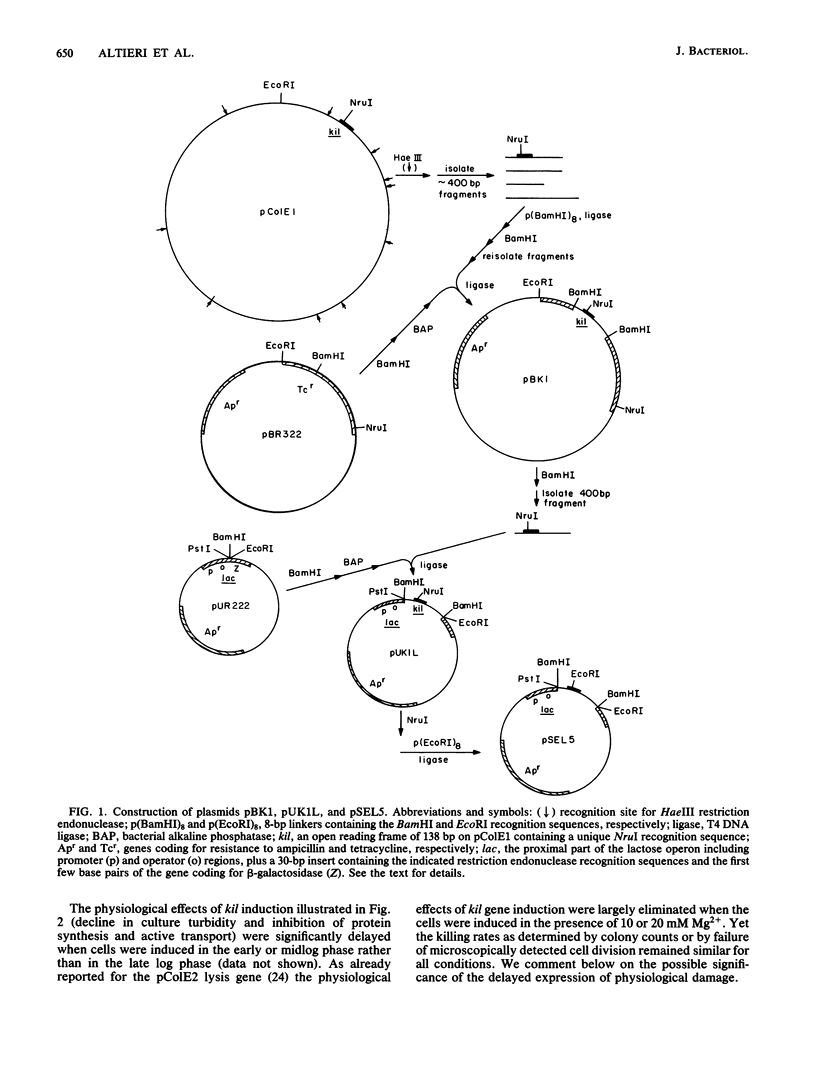
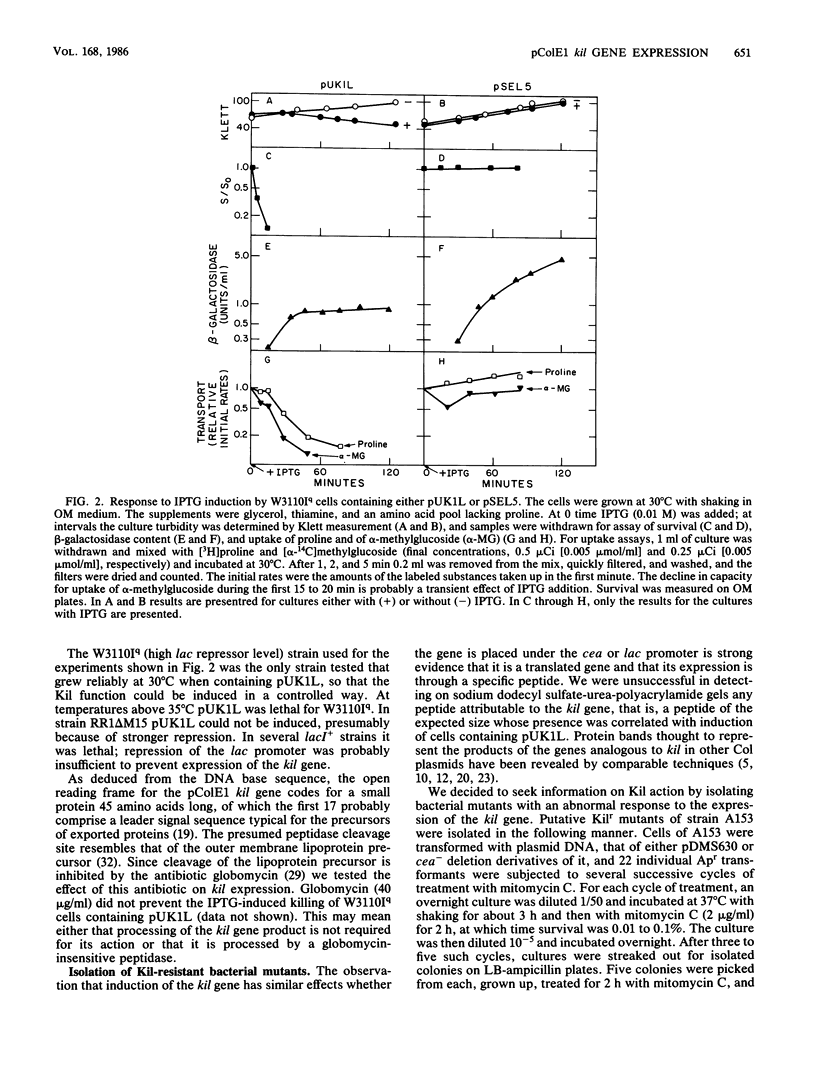
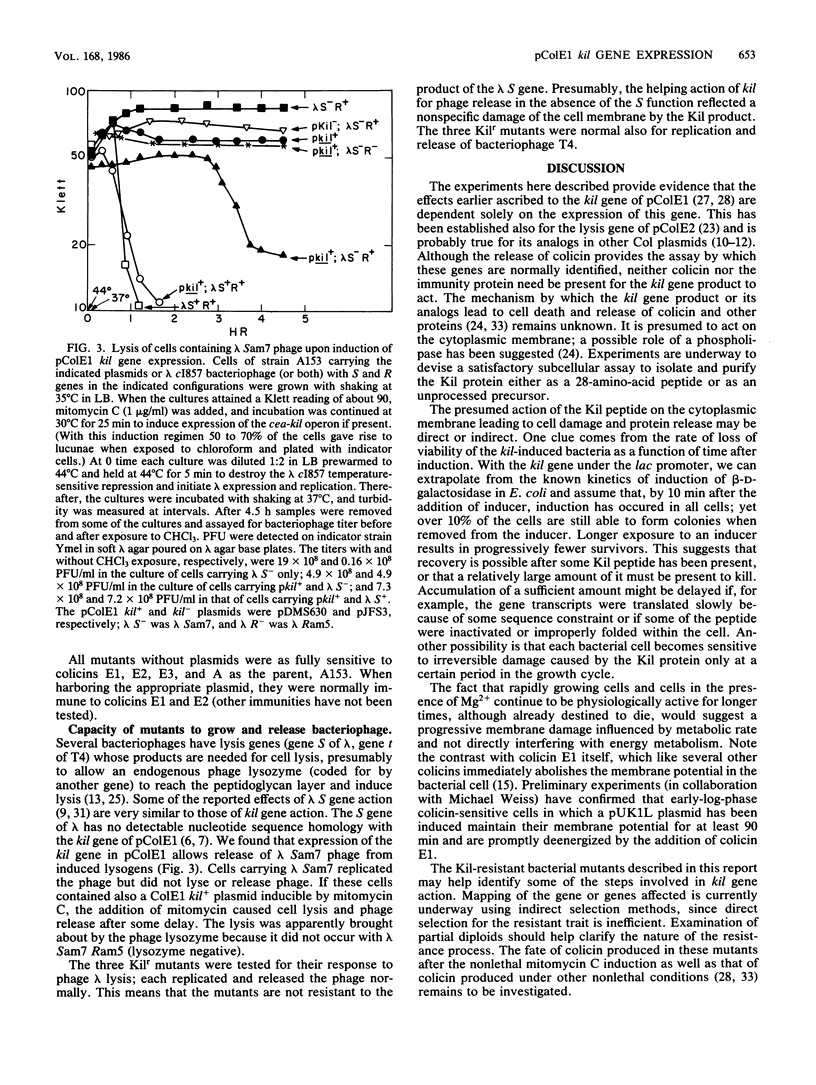
The kil gene of the ColE1 plasmid was cloned under control of the lac promoter. Its expression under this promoter gave rise to the same pattern of bacterial cell damage and lethality as that which accompanies induction of the kil gene in the colicin operon by mitomycin C. This confirms that cell damage after induction is solely due to expression of kil and is independent of the cea or imm gene products. Escherichia coli derivatives resistant to the lethal effects of kil gene expression under either the normal or the lac promoter were isolated and found to fall into several classes, some of which were altered in sensitivity to agents that affect the bacterial envelope.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. T., Ohmori H., Tomizawa J., Lebowitz J. Nucleotide sequence and gene organization of ColE1 DNA. J Biol Chem. 1985 Jul 25;260(15):8925–8935. [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. T., Saint-Joanis B., Pugsley A. P. Molecular characterisation of the colicin E2 operon and identification of its products. Mol Gen Genet. 1985;198(3):465–472. doi: 10.1007/BF00332940. [DOI] [PubMed] [Google Scholar]
- Garrett J. M., Young R. Lethal action of bacteriophage lambda S gene. J Virol. 1982 Dec;44(3):886–892. doi: 10.1128/jvi.44.3.886-892.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Protein H encoded by plasmid Clo DF13 involved in lysis of the bacterial host. I. Localisation of the gene and identification and subcellular localisation of the gene H product. Mol Gen Genet. 1981;183(2):318–325. doi: 10.1007/BF00270635. [DOI] [PubMed] [Google Scholar]
- Hakkaart M. J., Veltkamp E., Nijkamp H. J. Protein H encoded by plasmid Clo DF13 involved in lysis of the bacterial host. II. Functions and regulation of synthesis of the gene H product. Mol Gen Genet. 1981;183(2):326–332. doi: 10.1007/BF00270636. [DOI] [PubMed] [Google Scholar]
- Jakes K. S., Zinder N. D. Plasmid ColE3 specifies a lysis protein. J Bacteriol. 1984 Feb;157(2):582–590. doi: 10.1128/jb.157.2.582-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josslin R. Physiological studies on the t gene defect in T4-infected Escherichia coli. Virology. 1971 Apr;44(1):101–107. doi: 10.1016/0042-6822(71)90157-7. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Nomura N., Morita M., Sugisaki H., Sugimoto K., Takanami M. Nucleotide sequence of small ColE1 derivatives: structure of the regions essential for autonomous replication and colicin E1 immunity. Mol Gen Genet. 1979 May 4;172(2):151–159. doi: 10.1007/BF00268276. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Plate C. A. Mutant of Escherichia coli defective in response to colicin K and in active transport. J Bacteriol. 1976 Feb;125(2):467–474. doi: 10.1128/jb.125.2.467-474.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. A genetic approach to the study of mitomycin-induced lysis of Escherichia coli K-12 strains which produce colicin E2. Mol Gen Genet. 1983;190(3):366–372. doi: 10.1007/BF00331060. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase. EMBO J. 1984 Oct;3(10):2393–2397. doi: 10.1002/j.1460-2075.1984.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Expression of a gene in a 400-base-pair fragment of colicin plasmid ColE2-P9 is sufficient to cause host cell lysis. J Bacteriol. 1983 Oct;156(1):109–114. doi: 10.1128/jb.156.1.109-114.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader R. W., Siminovitch L. Lysis defective mutants of bacteriophage lambda: genetics and physiology of S cistron mutants. Virology. 1971 Mar;43(3):607–622. doi: 10.1016/0042-6822(71)90286-8. [DOI] [PubMed] [Google Scholar]
- Rüther U., Koenen M., Otto K., Müller-Hill B. pUR222, a vector for cloning and rapid chemical sequencing of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4087–4098. doi: 10.1093/nar/9.16.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabik J. F., Suit J. L., Luria S. E. cea-kil operon of the ColE1 plasmid. J Bacteriol. 1983 Mar;153(3):1479–1485. doi: 10.1128/jb.153.3.1479-1485.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suit J. L., Fan M. L., Sabik J. F., Labarre R., Luria S. E. Alternative forms of lethality in mitomycin C-induced bacteria carrying ColE1 plasmids. Proc Natl Acad Sci U S A. 1983 Jan;80(2):579–583. doi: 10.1073/pnas.80.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Wu H. C. Post-translational modification and processing of Escherichia coli prolipoprotein in vitro. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2255–2259. doi: 10.1073/pnas.79.7.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J., Lau P. C., Vernet T., Visentin L. P. Characterization and nucleotide sequence of a colicin-release gene in the hic region of plasmid ColE3-CA38. Gene. 1984 Jul-Aug;29(1-2):175–184. doi: 10.1016/0378-1119(84)90178-1. [DOI] [PubMed] [Google Scholar]
- Wilson D. B. Effect of the lambda S gene product on properties of the Escherichia coli inner membrane. J Bacteriol. 1982 Sep;151(3):1403–1410. doi: 10.1128/jb.151.3.1403-1410.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M., Tokunaga H., Hayashi S., Giam C. Z. Posttranslational modification and processing of membrane lipoproteins in bacteria. J Cell Biochem. 1983;22(3):161–171. doi: 10.1002/jcb.240220305. [DOI] [PubMed] [Google Scholar]
- Zhang S. P., Faro A., Zubay G. Mitomycin-induced lethality of Escherichia coli cells containing the ColE1 Plasmid: involvement of the kil gene. J Bacteriol. 1985 Jul;163(1):174–179. doi: 10.1128/jb.163.1.174-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


