Abstract
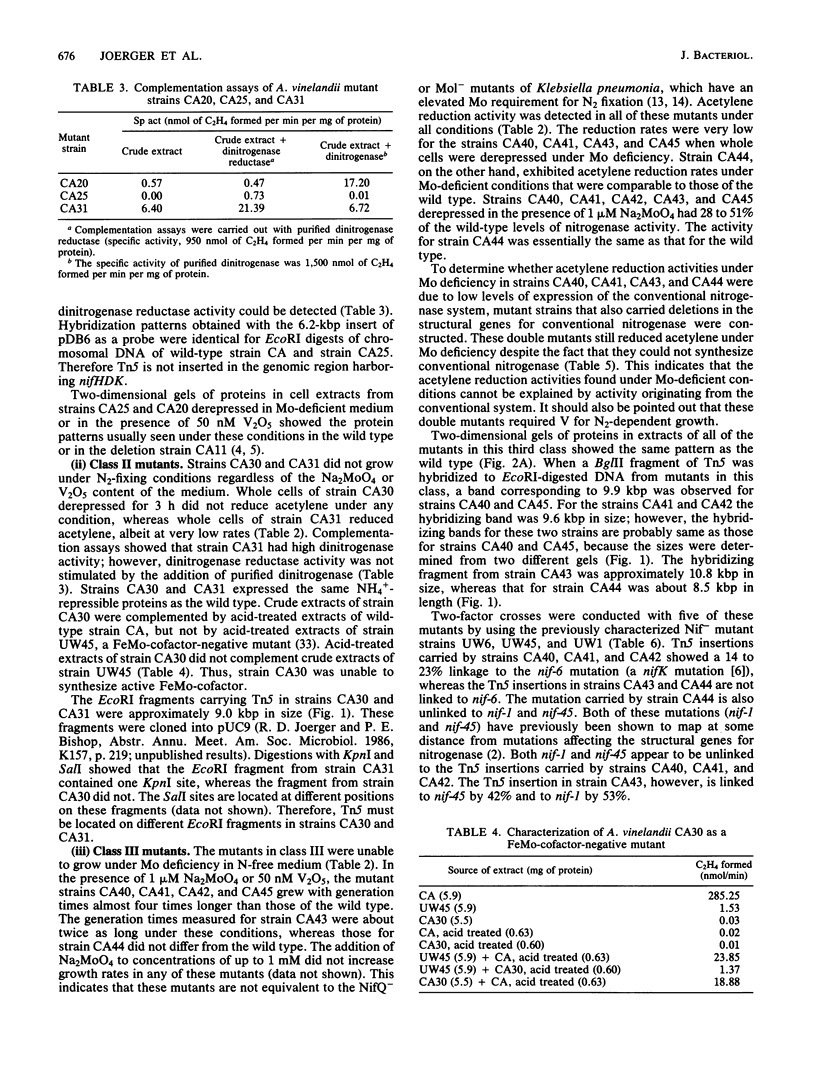
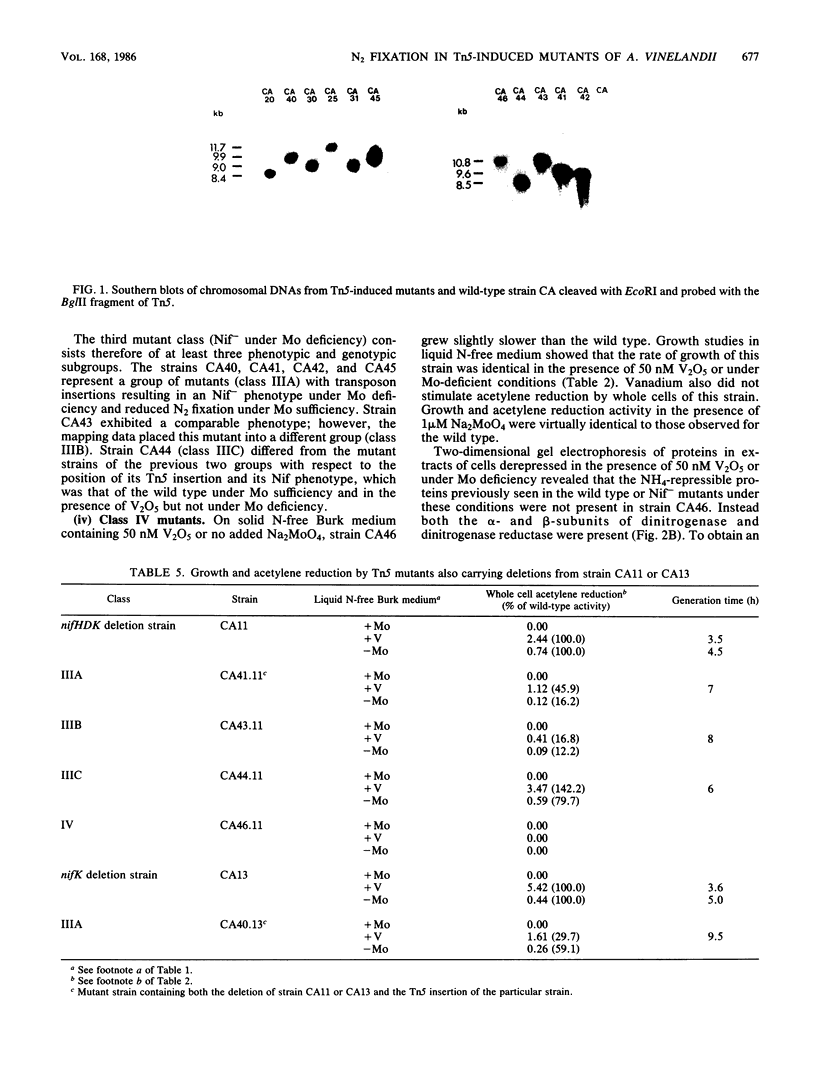
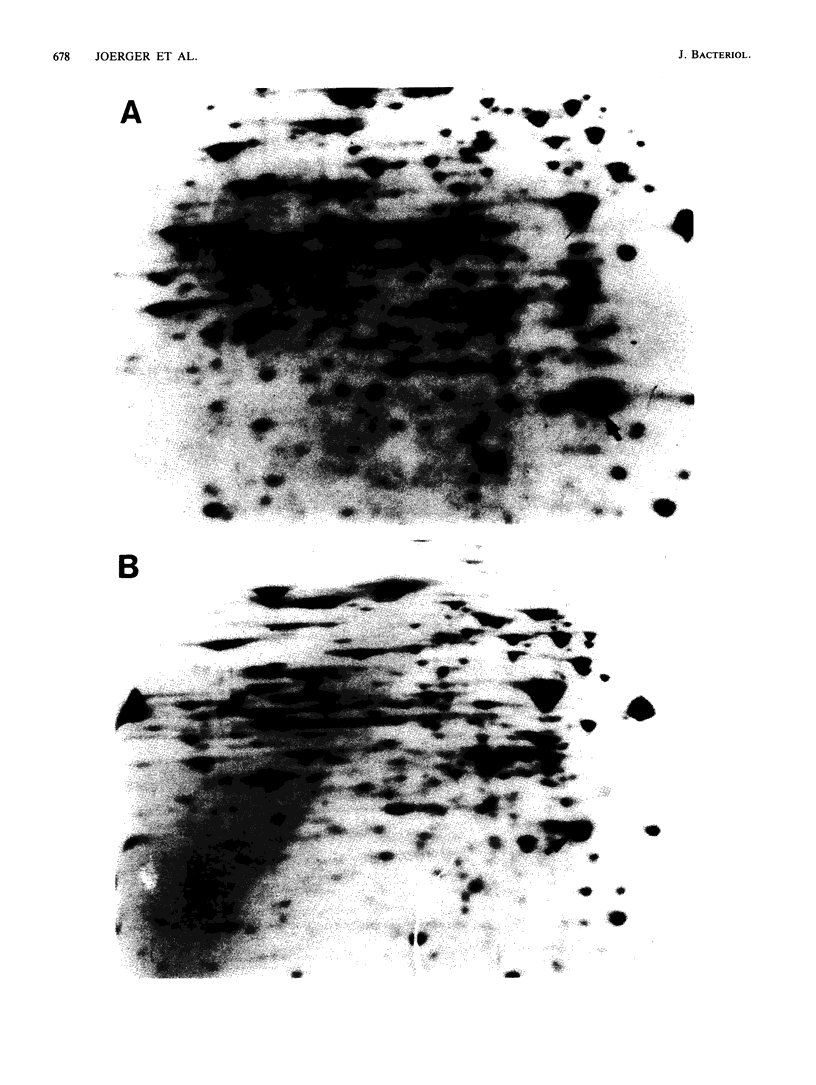
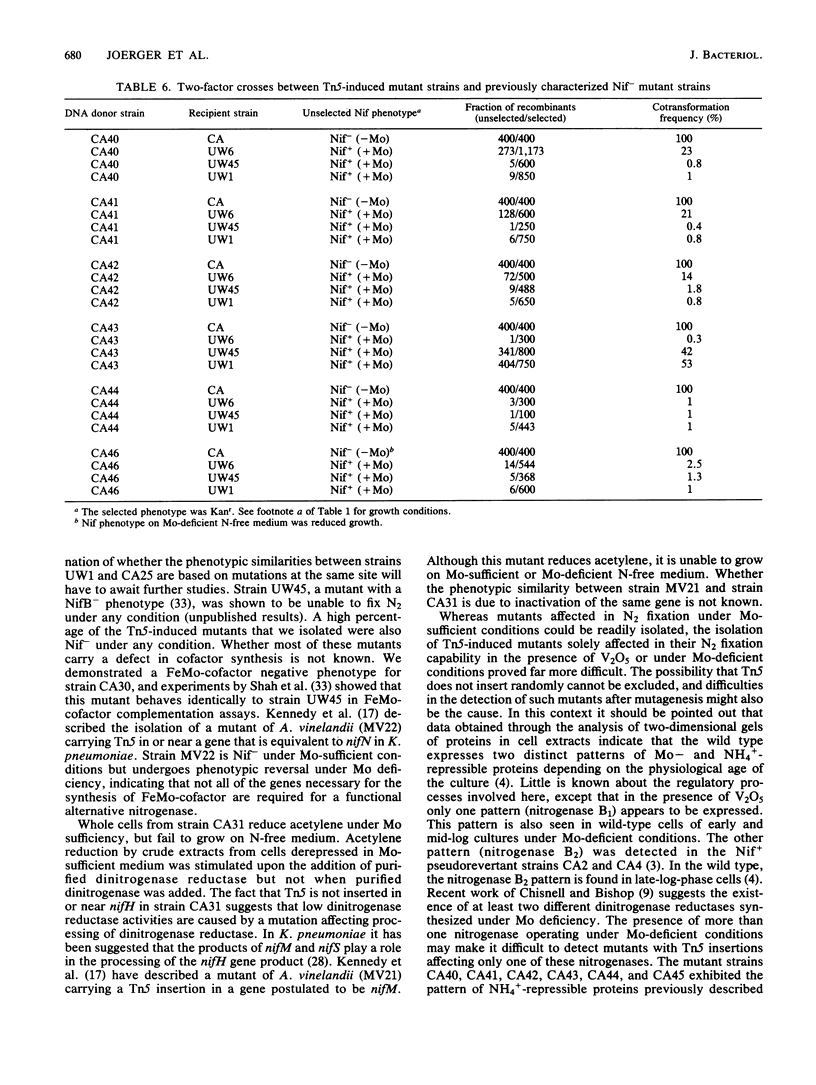
Mutants of Azotobacter vinelandii affected in N2 fixation in the presence of 1 microM Na2MoO4 (conventional system), 50 nM V2O5, or under Mo deficiency (alternative system) have been isolated after Tn5 mutagenesis with the suicide plasmid pSUP1011. These mutants can be grouped into at least four broad phenotypic classes. Mutants in the first class are Nif- under Mo sufficiency but Nif+ under Mo deficiency or in the presence of V2O5. A nifk mutant and a mutant apparently affected in regulation of the conventional system belong to this class. Mutants in the second class are Nif- under all conditions. An FeMo-cofactor-negative mutant (NifB-) belongs to this class, implying an involvement of nifB in both the conventional and the alternative N2 fixation systems. The third mutant class consists of mutants incapable of N2-dependent growth under Mo deficiency. Most of the mutants in this class are also affected in N2 fixation in the presence of 1 microM Na2MoO4, with acetylene reduction rates ranging from 28 to 51% of the rates of the wild type. Strains constructed by genetic transfer of the Kanr marker of mutants from this class into nifHDK or nifK deletion mutants showed N2-dependent growth only in the presence of V2O5, suggesting that growth in the presence of V2O5 and growth under Mo deficiency are independent phenomena. The only mutant in the fourth class shows wild-type nitrogenase activity under Mo sufficiency, but only 10% of the acetylene reduction activity of the wild type in the presence of 50 nM V2O5. The acetylene reduction rates of whole cells of this mutant are identical in Mo-deficient medium and in medium containing V2O5. The conventional nitrogenase subunits are expressed in this mutant even under Mo deficiency or in the presence of V2O5; however, the NH4+- and Mo-repressible proteins normally seen under these conditions could not be detected on two-dimensional gels. The Tn5 insertion carried by this mutant makes N2 fixation dependent solely on the conventional system and consequently abolishes the vanadium effect.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benemann J. R., McKenna C. E., Lie R. F., Traylor T. G., Kamen M. D. The vanadium effect in nitrogen fixation by azotobacter. Biochim Biophys Acta. 1972 Mar 30;264(1):25–38. doi: 10.1016/0304-4165(72)90113-4. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Brill W. J. Genetic analysis of Azotobacter vinelandii mutant strains unable to fix nitrogen. J Bacteriol. 1977 May;130(2):954–956. doi: 10.1128/jb.130.2.954-956.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Evidence for an alternative nitrogen fixation system in Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7342–7346. doi: 10.1073/pnas.77.12.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Expression of an alternative nitrogen fixation system in Azotobacter vinelandii. J Bacteriol. 1982 Jun;150(3):1244–1251. doi: 10.1128/jb.150.3.1244-1251.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Premakumar R., Dean D. R., Jacobson M. R., Chisnell J. R., Rizzo T. M., Kopczynski J. Nitrogen Fixation by Azotobacter vinelandii Strains Having Deletions in Structural Genes for Nitrogenase. Science. 1986 Apr 4;232(4746):92–94. doi: 10.1126/science.232.4746.92. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Rizzo T. M., Bott K. F. Molecular cloning of nif DNA from Azotobacter vinelandii. J Bacteriol. 1985 Apr;162(1):21–28. doi: 10.1128/jb.162.1.21-28.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigle K. E., Newton W. E., Dean D. R. Complete nucleotide sequence of the Azotobacter vinelandii nitrogenase structural gene cluster. Gene. 1985;37(1-3):37–44. doi: 10.1016/0378-1119(85)90255-0. [DOI] [PubMed] [Google Scholar]
- DE WITT C. W., ROWE J. A. N,O-Diacetylneuraminic acid and N-acetylneuraminic acid in Escherichia coli. Nature. 1959 Aug 1;184(Suppl 6):381–382. doi: 10.1038/184381b0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Fisher R. J., Brill W. J. Mutants of Azotobacter vinelandii unable to fix nitrogen. Biochim Biophys Acta. 1969 Jun 17;184(1):99–105. doi: 10.1016/0304-4165(69)90103-2. [DOI] [PubMed] [Google Scholar]
- Imperial J., Ugalde R. A., Shah V. K., Brill W. J. Mol- mutants of Klebsiella pneumoniae requiring high levels of molybdate for nitrogenase activity. J Bacteriol. 1985 Sep;163(3):1285–1287. doi: 10.1128/jb.163.3.1285-1287.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial J., Ugalde R. A., Shah V. K., Brill W. J. Role of the nifQ gene product in the incorporation of molybdenum into nitrogenase in Klebsiella pneumoniae. J Bacteriol. 1984 Apr;158(1):187–194. doi: 10.1128/jb.158.1.187-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. R., Premakumar R., Bishop P. E. Transcriptional regulation of nitrogen fixation by molybdenum in Azotobacter vinelandii. J Bacteriol. 1986 Aug;167(2):480–486. doi: 10.1128/jb.167.2.480-486.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean P. A., Dixon R. A. Requirement of nifV gene for production of wild-type nitrogenase enzyme in Klebsiella pneumoniae. Nature. 1981 Aug 13;292(5824):655–656. doi: 10.1038/292655a0. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Bott K. F. Restriction enzyme analysis of Bacillus subtilis ribosomal ribonucleic acid genes. J Bacteriol. 1979 Oct;140(1):99–105. doi: 10.1128/jb.140.1.99-105.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani H. H., Shah V. K., Brill W. J. Activation of inactive nitrogenase by acid-treated component I. J Bacteriol. 1974 Nov;120(2):697–701. doi: 10.1128/jb.120.2.697-701.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Optimal conditions for transformation of Azotobacter vinelandii. J Bacteriol. 1979 Sep;139(3):1058–1061. doi: 10.1128/jb.139.3.1058-1061.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S. S., Bott K. F., Newbold J. E. Two-dimensional restriction analysis of the Bacillus subtilis genome: gene purification and ribosomal ribonucleic acid gene organization. J Bacteriol. 1977 Jan;129(1):492–500. doi: 10.1128/jb.129.1.492-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Nitrogenase. IV. Simple method of purification to homogeneity of nitrogenase components from Azotobacter vinelandii. Biochim Biophys Acta. 1973 May 30;305(2):445–454. doi: 10.1016/0005-2728(73)90190-4. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis I. C., Gordon J. K., Orme-Johnson W. H., Brill W. J. Nitrogenase. 3. Nitrogenaseless mutants of Azotobacter vinelandii: activities, cross-reactions and EPR spectra. Biochim Biophys Acta. 1973 Jan 18;292(1):246–255. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Davis L. C., Brill W. J. Nitrogenase. I. Repression and derepression of the iron-molybdenum and iron proteins of nitrogenase in Azotobacter vinelandii. Biochim Biophys Acta. 1972 Feb 28;256(2):498–511. doi: 10.1016/0005-2728(72)90078-3. [DOI] [PubMed] [Google Scholar]
- Shah V. K., Imperial J., Ugalde R. A., Ludden P. W., Brill W. J. In vitro synthesis of the iron-molybdenum cofactor of nitrogenase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1636–1640. doi: 10.1073/pnas.83.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Sorger G. J., Trofimenkoff D. Nitrogenaseless mutants of Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1970 Jan;65(1):74–80. doi: 10.1073/pnas.65.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]