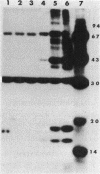
Abstract
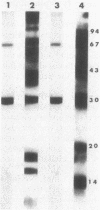
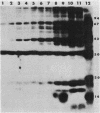
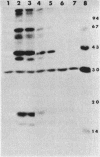
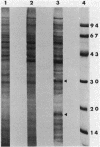
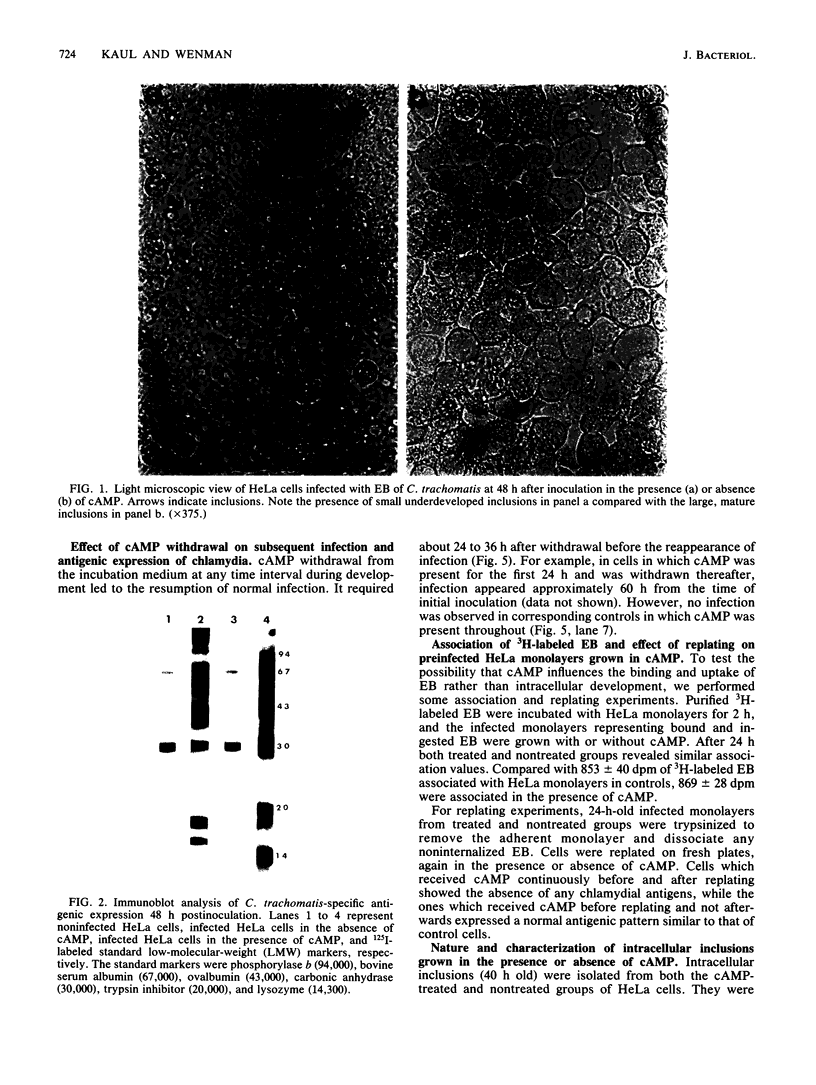
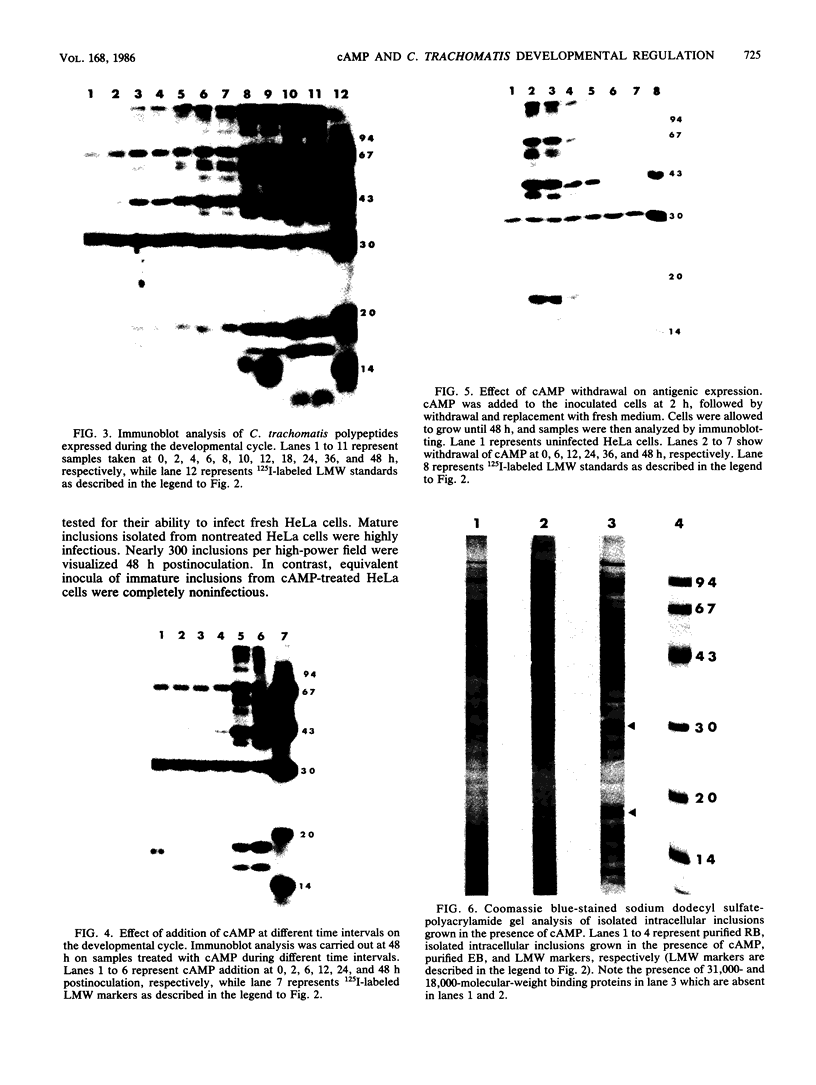
The effect of cyclic AMP (cAMP) on the chlamydial growth cycle was studied with Chlamydia trachomatis-infected HeLa cells. At concentrations of 1 mM, cAMP had a profound effect on the chlamydial developmental cycle, resulting in small, immature inclusions. Immunoblot analysis revealed the absence of elementary body (EB)-specific antigens in the cAMP-treated cells. This effect was observed only if cAMP was added within the first 12 h of incubation and continued thereafter. Its withdrawal at any time from the medium led to the reappearance of fully mature, infectious organisms. Analogs or breakdown products of cAMP exerted no inhibitory effect on chlamydial development. Intracellular inclusions from the cAMP-treated cells were unable to infect fresh HeLa monolayers, in contrast to the completely infectious nontreated inclusions. Protein profiles of the cAMP-treated organisms (at any time point) resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis very closely resembled reticulate bodies (RB) and did not possess characteristic EB-binding proteins. Collectively, these observations suggest an inhibitory role for cAMP at the RB stage of intracellular development. We also identified a cAMP receptor protein which is associated with RB and not with EB, further supporting a role for this system in the developmental regulation of chlamydiae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L., Drexler M. The cyclic 3',5'-adenosine monophosphate receptor protein and regulation of cyclic 3',5'-adenosine monophosphate synthesis in Escherichia coli. Mol Gen Genet. 1978 Sep 20;165(1):47–56. doi: 10.1007/BF00270375. [DOI] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURNESS G., GRAHAM D. M., REEVE P. The titration of trachoma and inclusion blennorrhoea viruses in cell cultures. J Gen Microbiol. 1960 Dec;23:613–619. doi: 10.1099/00221287-23-3-613. [DOI] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Gutter B., Asher Y., Cohen Y., Becker Y. Studies on the developmental cycle of Chlamydia trachomatis: isolation and characterization of the initial bodies. J Bacteriol. 1973 Aug;115(2):691–702. doi: 10.1128/jb.115.2.691-702.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T. Identification and properties of chlamydial polypeptides that bind eucaryotic cell surface components. J Bacteriol. 1986 Jan;165(1):13–20. doi: 10.1128/jb.165.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mallick U., Herrlich P. Regulation of synthesis of a major outer membrane protein: cyclic AMP represses Escherichia coli protein III synthesis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5520–5523. doi: 10.1073/pnas.76.11.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Intracellular parasitism: life in an extreme environment. J Infect Dis. 1974 Sep;130(3):300–306. doi: 10.1093/infdis/130.3.300. [DOI] [PubMed] [Google Scholar]
- Movva R. N., Green P., Nakamura K., Inouye M. Interaction of cAMP receptor protein with the ompA gene, a gene for a major outer membrane protein of Escherichia coli. FEBS Lett. 1981 Jun 15;128(2):186–190. doi: 10.1016/0014-5793(81)80077-4. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Adenosine 3',5'-phosphate in fungi. Microbiol Rev. 1981 Sep;45(3):462–480. doi: 10.1128/mr.45.3.462-480.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampeno C., Krakow J. S. Cross-linking of the cAMP receptor protein of Escherichia coli by o-phenylenedimaleimide as a probe of conformation. Biochemistry. 1979 Apr 17;18(8):1519–1525. doi: 10.1021/bi00575a020. [DOI] [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Rickenberg H. V. Cyclic AMP in prokaryotes. Annu Rev Microbiol. 1974;28(0):353–369. doi: 10.1146/annurev.mi.28.100174.002033. [DOI] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (third of three parts). N Engl J Med. 1978 Mar 9;298(10):540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- Sheppard H., Wiggan G. Analogues of 4-(3,4-dimethoxybenzyl)-2-imidazolidinone as potent inhibitors of rat erythrocyte adenosine cyclic 3',5'-phosphate phosphodiesterase. Mol Pharmacol. 1971 Jan;7(1):111–115. [PubMed] [Google Scholar]
- Sneddon J. M., Wenman W. M. The effect of ions on the adhesion and internalization of Chlamydia trachomatis by HeLa cells. Can J Microbiol. 1985 Apr;31(4):371–374. doi: 10.1139/m85-071. [DOI] [PubMed] [Google Scholar]
- Solaiman D., Uffen R. L. Influence of cyclic AMP on photosynthetic development in Rhodospirillum rubrum. J Bacteriol. 1984 Aug;159(2):790–792. doi: 10.1128/jb.159.2.790-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi R., Tanabe H., Nakamoto Y., Kawamukai M., Sakai H., Himeno M., Komano T., Hirota Y. Inhibitory effect of adenosine 3',5'-phosphate on cell division of Escherichia coli K-12 mutant derivatives. J Bacteriol. 1981 Sep;147(3):1105–1109. doi: 10.1128/jb.147.3.1105-1109.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Salari H. Control mechanisms governing the infectivity of Chlamydia trachomatis for hela cells: modulation by cyclic nucleotides, prostaglandins and calcium. J Gen Microbiol. 1982 Mar;128(3):639–650. doi: 10.1099/00221287-128-3-639. [DOI] [PubMed] [Google Scholar]