Abstract
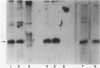
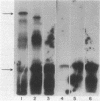
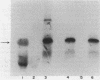
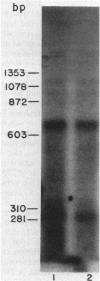
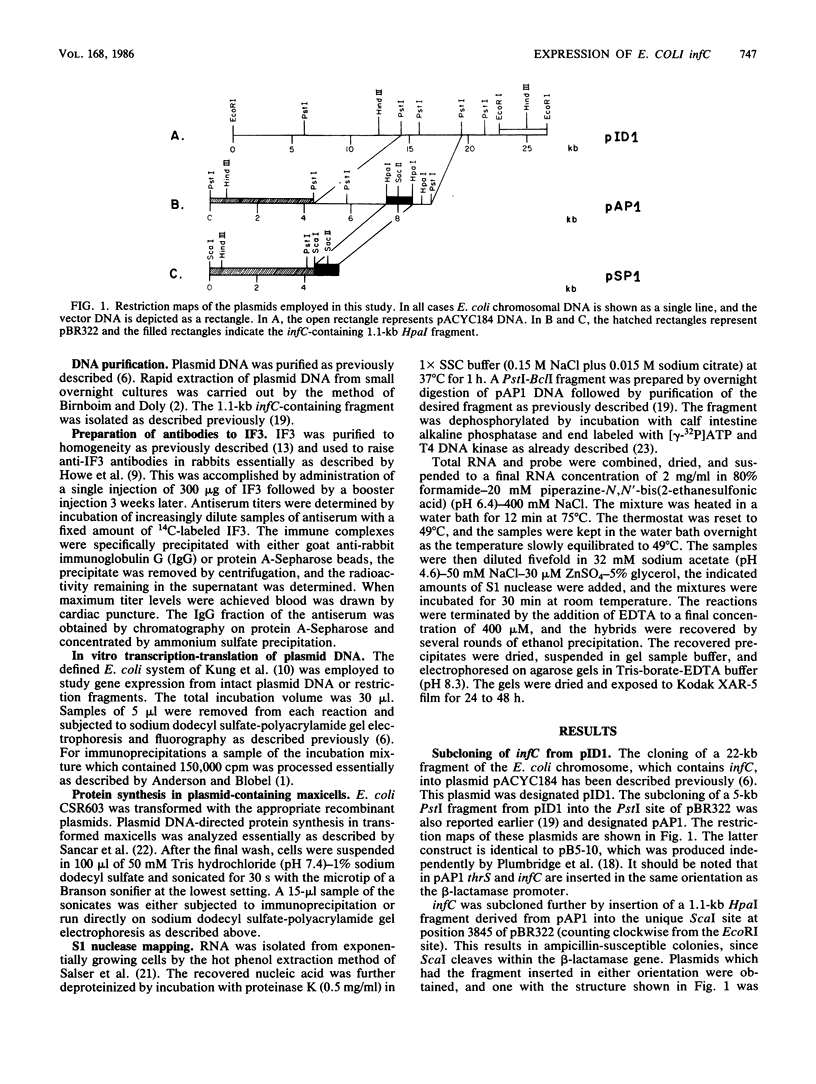
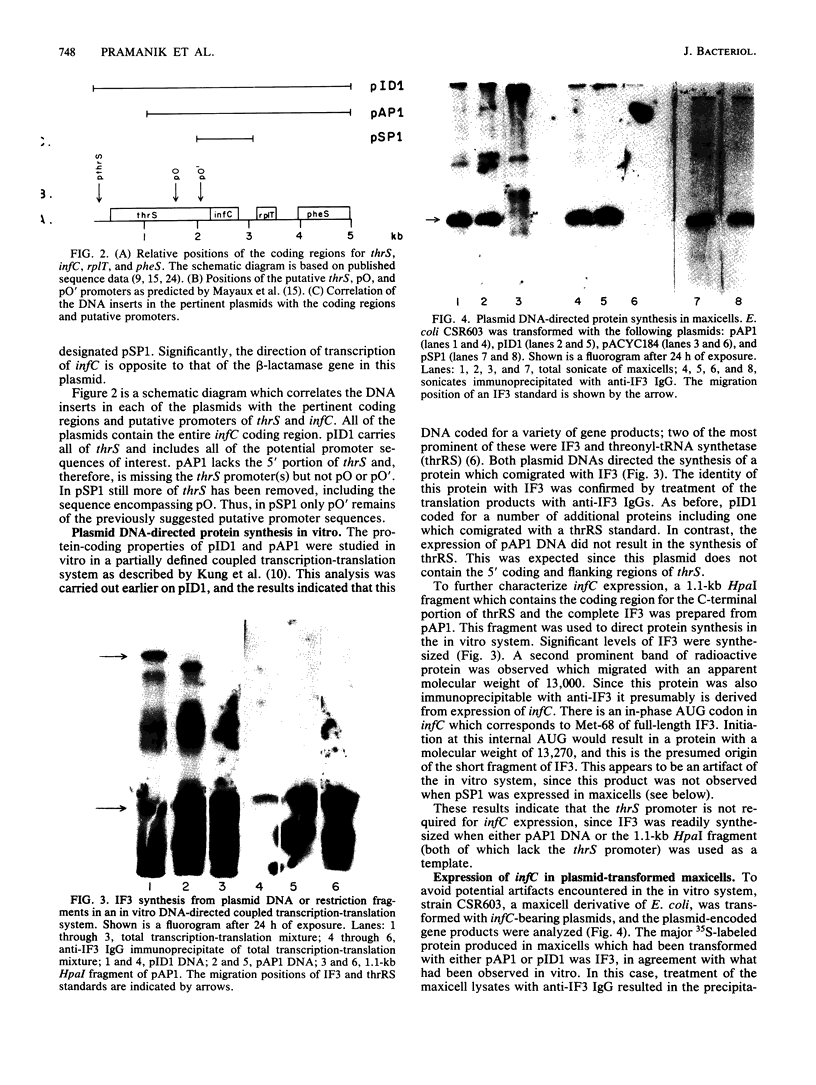
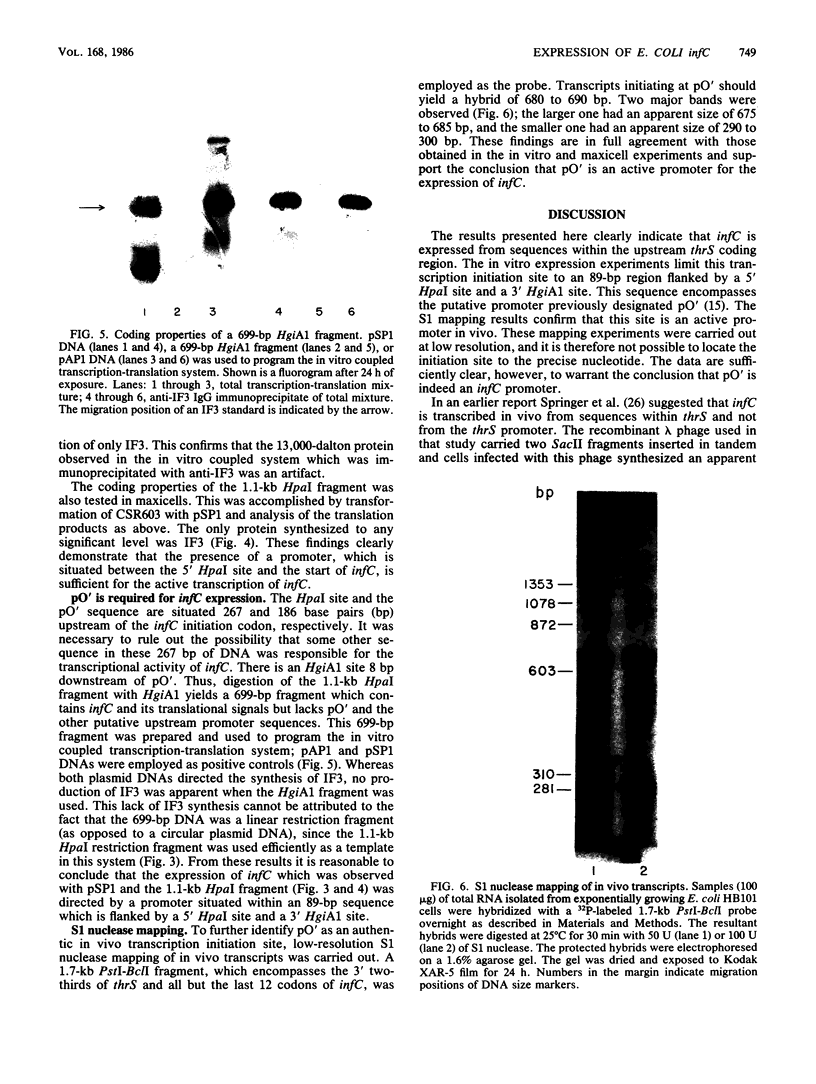
infC, the gene which codes for translation initiation factor 3, is situated in a cluster in the genome of Escherichia coli with genes for several other components of the translation apparatus. Only three nucleotides separate the termination codon of thrS from the initiation codon of infC. This implies that infC is either cotranscribed with thrS from a thrS promoter or that the transcriptional signals for infC are embedded within the upstream thrS coding region. In the present work, several plasmids have been constructed which encompass infC and various amounts of the upstream thrS sequence. The ability of the plasmid DNA, or derived restriction fragments, to direct the synthesis of initiation factor 3 was tested in an in vitro DNA-dependent coupled transcription-translation system and in plasmid-transformed maxicells. The results indicate that initiation factor 3 is synthesized in the absence of the thrS promoter. A promoter whose presence is sufficient for the expression of infC has been localized to an 89-base-pair region which lies 178 to 267 base pairs upstream of the infC initiation codon. S1 nuclease mapping of in vivo transcripts confirms that a transcription initiation site is located in this region. These studies demonstrate that infC can be transcribed from a promoter within the upstream thrS coding sequence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Comer M. M. Gene organization around the phenylalanyl-transfer ribonucleic acid synthetase locus in Escherichia coli. J Bacteriol. 1981 Apr;146(1):269–274. doi: 10.1128/jb.146.1.269-274.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhardt D., Wirth R., Böck A. Regulation of formation of threonyl-tRNA synthetase, phenylalanyl-tRNA synthetase and protein synthesis initiation factor 3 from Escherichia coli in vivo and in vitro. Eur J Biochem. 1982 Apr;123(3):477–482. doi: 10.1111/j.1432-1033.1982.tb06555.x. [DOI] [PubMed] [Google Scholar]
- Elseviers D., Gallagher P., Hoffman A., Weinberg B., Schwartz I. Molecular cloning and regulation of expression of the genes for initiation factor 3 and two aminoacyl-tRNA synthetases. J Bacteriol. 1982 Oct;152(1):357–362. doi: 10.1128/jb.152.1.357-362.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayat G., Mayaux J. F., Sacerdot C., Fromant M., Springer M., Grunberg-Manago M., Blanquet S. Escherichia coli phenylalanyl-tRNA synthetase operon region. Evidence for an attenuation mechanism. Identification of the gene for the ribosomal protein L20. J Mol Biol. 1983 Dec 15;171(3):239–261. doi: 10.1016/0022-2836(83)90092-x. [DOI] [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. Initiation factor and ribosome levels are coordinately controlled in Escherichia coli growing at different rates. J Biol Chem. 1983 Feb 10;258(3):1954–1959. [PubMed] [Google Scholar]
- Howe J. G., Yanov J., Meyer L., Johnston K., Hershey J. W. Determination of protein synthesis initiation factor levels in crude lysates of Escherichia coli by a sensitive radioimmune assay. Arch Biochem Biophys. 1978 Dec;191(2):813–820. doi: 10.1016/0003-9861(78)90424-1. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Redfield B., Treadwell B. V., Eskin B., Spears C., Weissbach H. DNA-directed in vitro synthesis of beta-galactosidase. Studies with purified factors. J Biol Chem. 1977 Oct 10;252(19):6889–6894. [PubMed] [Google Scholar]
- Lestienne P., Dondon J., Plumbridge J. A., Howe J. G., Mayaux J. F., Springer M., Blanquet S., Hershey J. W., Grunberg-Manago M. Expression of the gene for Escherichia coli initiation factor IE-3 in vivo and in vitro. Eur J Biochem. 1982 Apr;123(3):483–488. doi: 10.1111/j.1432-1033.1982.tb06556.x. [DOI] [PubMed] [Google Scholar]
- Lestienne P., Plumbridge J. A., Grunberg-Manago M., Blanquet S. Autogenous repression of Escherichia coli threonyl-tRNA synthetase expression in vitro. J Biol Chem. 1984 Apr 25;259(8):5232–5237. [PubMed] [Google Scholar]
- MacKeen L. A., Kahan L., Wahba A. J., Schwartz I. Photochemical cross-linking of initiation factor-3 to Escherichia coli 30 S ribosomal subunits. J Biol Chem. 1980 Nov 10;255(21):10526–10531. [PubMed] [Google Scholar]
- Maitra U., Stringer E. A., Chaudhuri A. Initiation factors in protein biosynthesis. Annu Rev Biochem. 1982;51:869–900. doi: 10.1146/annurev.bi.51.070182.004253. [DOI] [PubMed] [Google Scholar]
- Mayaux J. F., Fayat G., Fromant M., Springer M., Grunberg-Manago M., Blanquet S. Structural and transcriptional evidence for related thrS and infC expression. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6152–6156. doi: 10.1073/pnas.80.20.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechulam Y., Fayat G., Blanquet S. Sequence of the Escherichia coli pheST operon and identification of the himA gene. J Bacteriol. 1985 Aug;163(2):787–791. doi: 10.1128/jb.163.2.787-791.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Pedersen S., Reeh S. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J Bacteriol. 1977 Jan;129(1):378–387. doi: 10.1128/jb.129.1.378-387.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M., Graffe M., Goursot R., Grunberg-Manago M. Physical localisation and cloning of the structural gene for E. coli initiation factor IF3 from a group of genes concerned with translation. Gene. 1980 Oct;11(1-2):33–42. doi: 10.1016/0378-1119(80)90084-0. [DOI] [PubMed] [Google Scholar]
- Pramanik A., Schwartz I. The gene encoding translation initiation factor 3 is highly conserved in gram-negative bacteria. Arch Biochem Biophys. 1984 Nov 15;235(1):276–282. doi: 10.1016/0003-9861(84)90276-5. [DOI] [PubMed] [Google Scholar]
- Sacerdot C., Fayat G., Dessen P., Springer M., Plumbridge J. A., Grunberg-Manago M., Blanquet S. Sequence of a 1.26-kb DNA fragment containing the structural gene for E.coli initiation factor IF3: presence of an AUU initiator codon. EMBO J. 1982;1(3):311–315. doi: 10.1002/j.1460-2075.1982.tb01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W., Gesteland R. F., Bolle A. In vitro synthesis of bacteriophage lysozyme. Nature. 1967 Aug 5;215(5101):588–591. doi: 10.1038/215588a0. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz I., Klotsky R. A., Elseviers D., Gallagher P. J., Krauskopf M., Siddiqui M. A., Wong J. F., Roe B. A. Molecular cloning and sequencing of pheU, a gene for Escherichia coli tRNAPhe. Nucleic Acids Res. 1983 Jul 11;11(13):4379–4389. doi: 10.1093/nar/11.13.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz I., Vincent M., Strycharz W. A., Kahan L. Photochemical cross-linking of translation initiation factor 3 to Escherichia coli 50S ribosomal subunits. Biochemistry. 1983 Mar 15;22(6):1483–1489. doi: 10.1021/bi00275a024. [DOI] [PubMed] [Google Scholar]
- Springer M., Plumbridge J. A., Butler J. S., Graffe M., Dondon J., Mayaux J. F., Fayat G., Lestienne P., Blanquet S., Grunberg-Manago M. Autogenous control of Escherichia coli threonyl-tRNA synthetase expression in vivo. J Mol Biol. 1985 Sep 5;185(1):93–104. doi: 10.1016/0022-2836(85)90185-8. [DOI] [PubMed] [Google Scholar]
- Springer M., Plumbridge J. A., Trudel M., Graffe M., Grunberg-Manago M. Transcription units around the gene for E. coli translation initiation factor IF3 (infC). Mol Gen Genet. 1982;186(2):247–252. doi: 10.1007/BF00331857. [DOI] [PubMed] [Google Scholar]
- Wu T. H., Wood D. L., Stein P. L., Comer M. M. Transcription of a gene cluster coding for two aminoacyl-tRNA synthetases and an initiation factor in Escherichia coli. J Mol Biol. 1984 Feb 25;173(2):177–209. doi: 10.1016/0022-2836(84)90189-x. [DOI] [PubMed] [Google Scholar]