Abstract
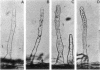
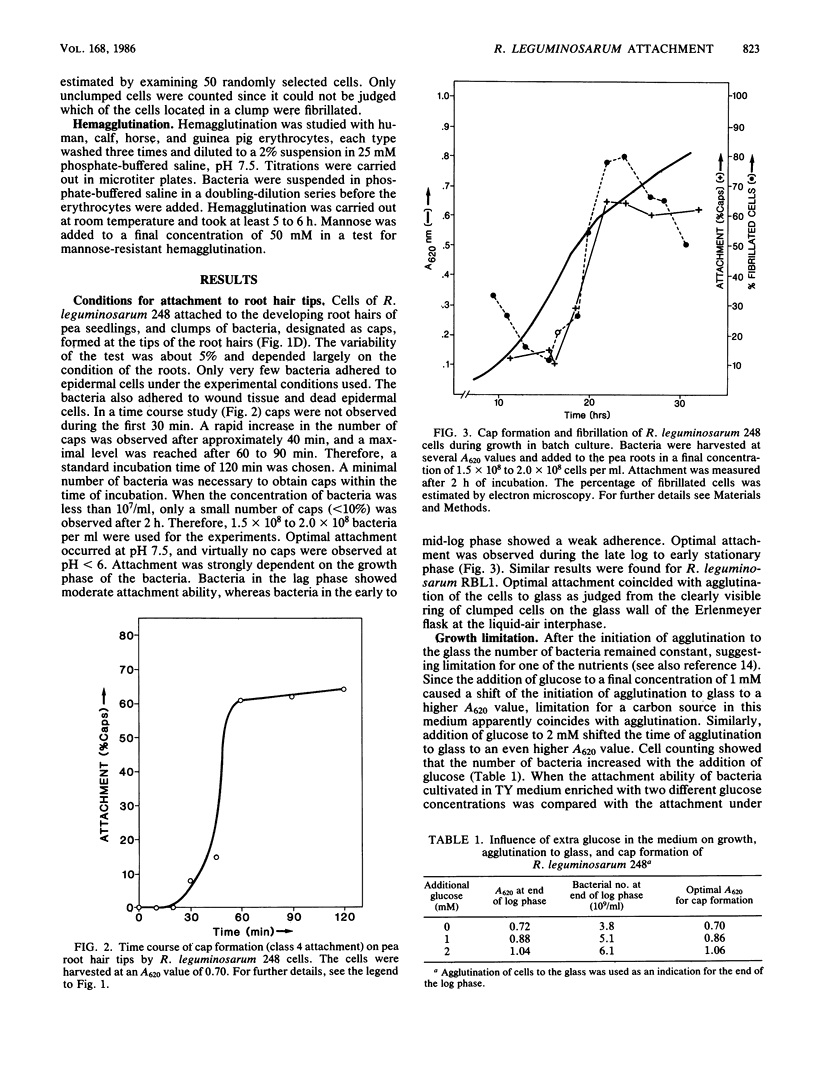
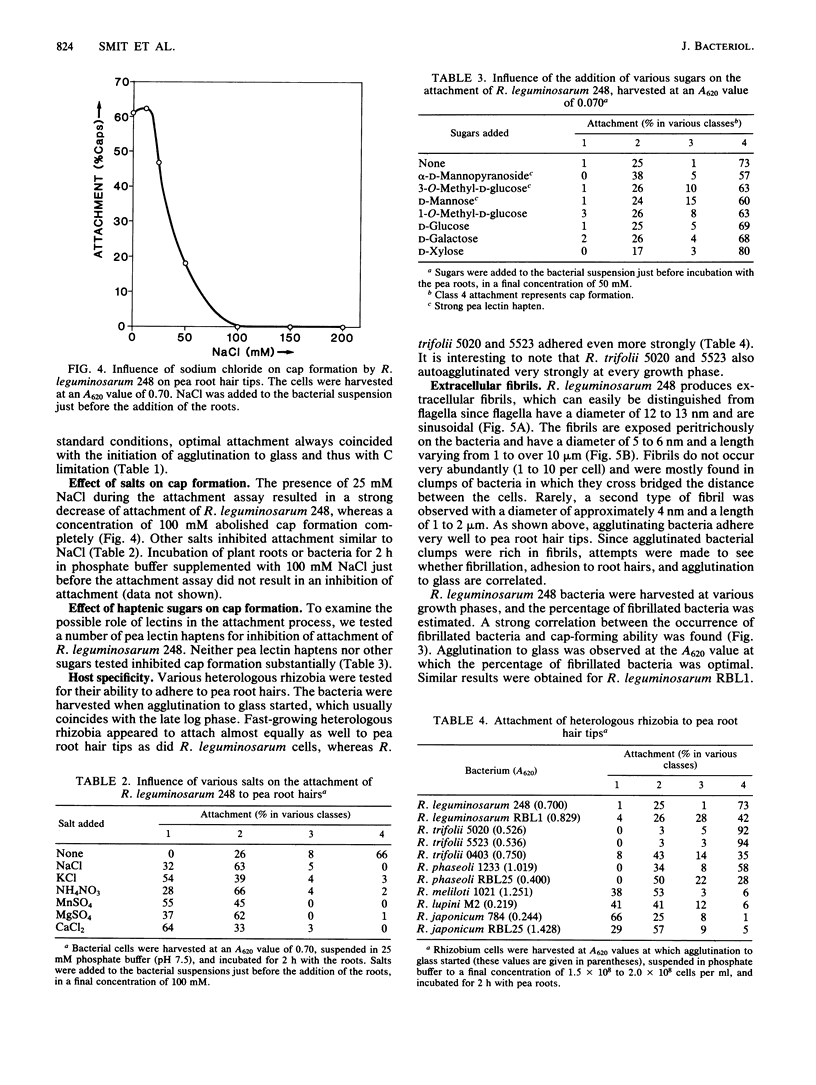
As part of a project meant to characterize molecules involved in nodulation, a semiquantitative microscopic assay was developed for measuring attachment of Rhizobium leguminosarum cells to pea root hair tips, i.e., the site at which R. leguminosarum initiates nodulation. This form of attachment, designated as cap formation, was dependent on the incubation pH and growth phase, with optimal attachment at pH 7.5 and with bacteria in the early stationary phase of growth. Addition of glucose to the growth medium delayed the initiation of the stationary phase and cap formation, suggesting a correlation between cap formation and carbon limitation. Attachment of R. leguminosarum was not inhibited by pea lectin haptens which makes it unlikely that lectins are involved under the tested conditions. Moreover, heterologous fast-growing rhizobia adhered equally well to pea root hair tips. Since the attachment characteristics of a Sym plasmid-cured derivative were indistinguishable from those of the wild-type strain, the Sym plasmidborne nodulation genes are not necessary for attachment. Sodium chloride and various other salts abolished attachment when present during the attachment assay in final concentrations of 100 mM. R. leguminosarum produced extracellular fibrils. A positive correlation between the percentage of fibrillated cells and the ability of the bacteria to form caps and to adhere to glass and erythrocytes was observed under various conditions, suggesting that these fibrils play a role in attachment of the bacteria to pea root hair tips, to glass, and to erythrocytes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badenoch-Jones J., Flanders D. J., Rolfe B. G. Association of Rhizobium Strains with Roots of Trifolium repens. Appl Environ Microbiol. 1985 Jun;49(6):1511–1520. doi: 10.1128/aem.49.6.1511-1520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Dazzo F. B., Napoli C. A., Hubbell D. H. Adsorption of bacteria to roots as related to host specificity in the Rhizobium-clover symbiosis. Appl Environ Microbiol. 1976 Jul;32(1):166–171. doi: 10.1128/aem.32.1.166-171.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Truchet G. L., Sherwood J. E., Hrabak E. M., Abe M., Pankratz S. H. Specific phases of root hair attachment in the Rhizobium trifolii-clover symbiosis. Appl Environ Microbiol. 1984 Dec;48(6):1140–1150. doi: 10.1128/aem.48.6.1140-1150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinema M. H., Zevenhuizen L. P. Formation of cellulose fibrils by gram-negative bacteria and their role in bacterial flocculation. Arch Mikrobiol. 1971;78(1):42–51. doi: 10.1007/BF00409087. [DOI] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahtela K., Tarkka E., Korhonen T. K. Type 1 fimbria-mediated adhesion of enteric bacteria to grass roots. Appl Environ Microbiol. 1985 May;49(5):1182–1185. doi: 10.1128/aem.49.5.1182-1185.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper P., Bias W. B., Hutchinson J. R., McKusick V. A. ABH secretor status of the fetus: a genetic marker identifiable by amniocentesis. J Med Genet. 1971 Dec;8(4):438–440. doi: 10.1136/jmg.8.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann W. Conjugation in starforming Rhizobium lupini. Mol Gen Genet. 1968;102(2):132–144. doi: 10.1007/BF01789140. [DOI] [PubMed] [Google Scholar]
- Hrabak E. M., Urbano M. R., Dazzo F. B. Growth-phase-dependent immunodeterminants of Rhizobium trifolii lipopolysaccharide which bind trifoliin A, a white clover lectin. J Bacteriol. 1981 Nov;148(2):697–711. doi: 10.1128/jb.148.2.697-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Matthysse A. G., Holmes K. V., Gurlitz R. H. Elaboration of cellulose fibrils by Agrobacterium tumefaciens during attachment to carrot cells. J Bacteriol. 1981 Jan;145(1):583–595. doi: 10.1128/jb.145.1.583-595.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Dazzo F., Hubbell D. Production of cellulose microfibrils by Rhizobium. Appl Microbiol. 1975 Jul;30(1):123–131. doi: 10.1128/am.30.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K., Pelcher L. E., Schaefer A. In Vitro Binding of Agrobacterium tumefaciens to Plant Cells from Suspension Culture. Plant Physiol. 1979 Feb;63(2):382–387. doi: 10.1104/pp.63.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planqué K., Kijne J. W. Binding of pea lectins to a glycan type polysaccharide in the cell walls of Rhizobium leguminosarum. FEBS Lett. 1977 Jan 15;73(1):64–66. doi: 10.1016/0014-5793(77)80016-1. [DOI] [PubMed] [Google Scholar]
- Pull S. P., Pueppke S. G., Hymowitz T., Orf J. H. Soybean lines lacking the 120,000-dalton seed lectin. Science. 1978 Jun 16;200(4347):1277–1279. doi: 10.1126/science.200.4347.1277. [DOI] [PubMed] [Google Scholar]
- Stacey G., Paau A. S., Brill W. J. Host recognition in the Rhizobium-soybean symbiosis. Plant Physiol. 1980 Oct;66(4):609–614. doi: 10.1104/pp.66.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brussel A. A., Zaat S. A., Cremers H. C., Wijffelman C. A., Pees E., Tak T., Lugtenberg B. J. Role of plant root exudate and Sym plasmid-localized nodulation genes in the synthesis by Rhizobium leguminosarum of Tsr factor, which causes thick and short roots on common vetch. J Bacteriol. 1986 Feb;165(2):517–522. doi: 10.1128/jb.165.2.517-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert J. S., Albersheim P. Host-symbiont interactions. I. The lectins of legumes interact with the o-antigen-containing lipopolysaccharides of their symbiont Rhizobia. Biochem Biophys Res Commun. 1976 Jun 7;70(3):729–737. doi: 10.1016/0006-291x(76)90653-7. [DOI] [PubMed] [Google Scholar]
- Zurkowski W. Specific adsorption of bacteria to clover root hairs, related to the presence of the plasmid pWZ2 in cells of Rhizobium trifolii. Microbios. 1980;27(107):27–32. [PubMed] [Google Scholar]
- van Die I., van Megen I., Hoekstra W., Bergmans H. Molecular organisation of the genes involved in the production of F7(2) fimbriae, causing mannose-resistant haemagglutination, of a uropathogenic Escherichia coli 06:K2:H1:F7 strain. Mol Gen Genet. 1984;194(3):528–533. doi: 10.1007/BF00425570. [DOI] [PubMed] [Google Scholar]
- van der Schaal I. A., Logman T. J., Díaz C. L., Kijne J. W. An enzyme-linked lectin binding assay for quantitative determination of lectin receptors. Anal Biochem. 1984 Jul;140(1):48–55. doi: 10.1016/0003-2697(84)90131-3. [DOI] [PubMed] [Google Scholar]