Abstract
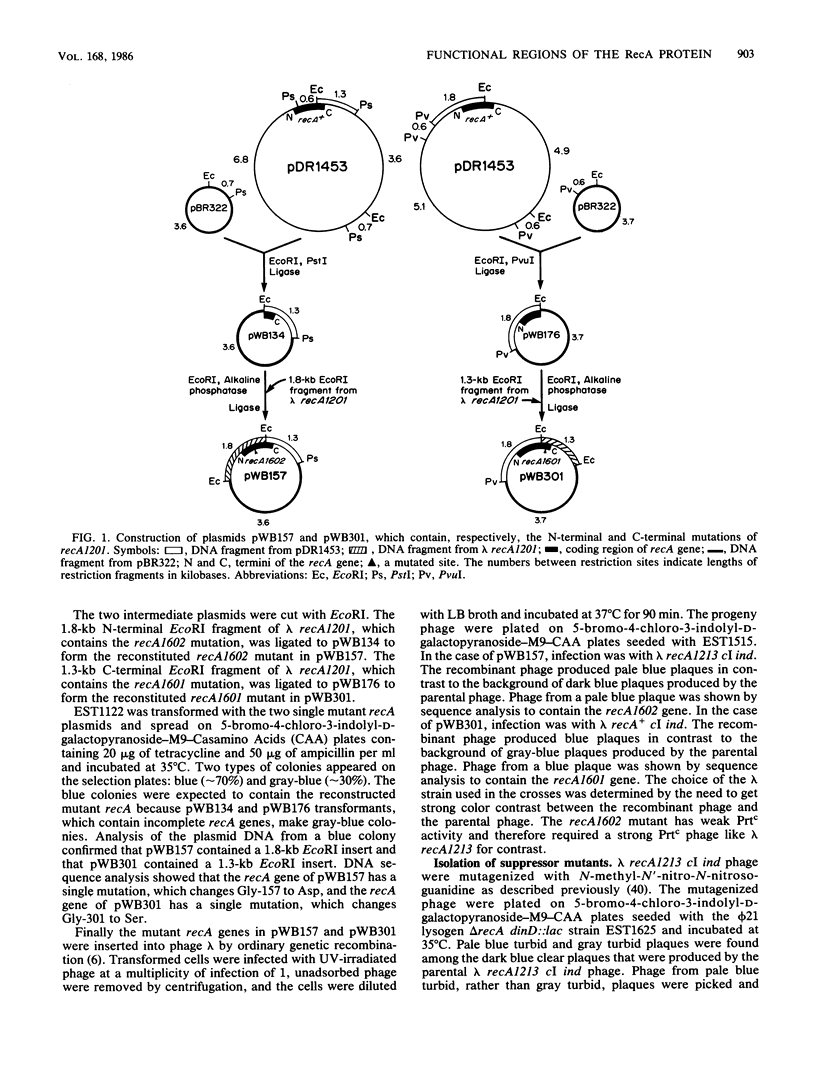
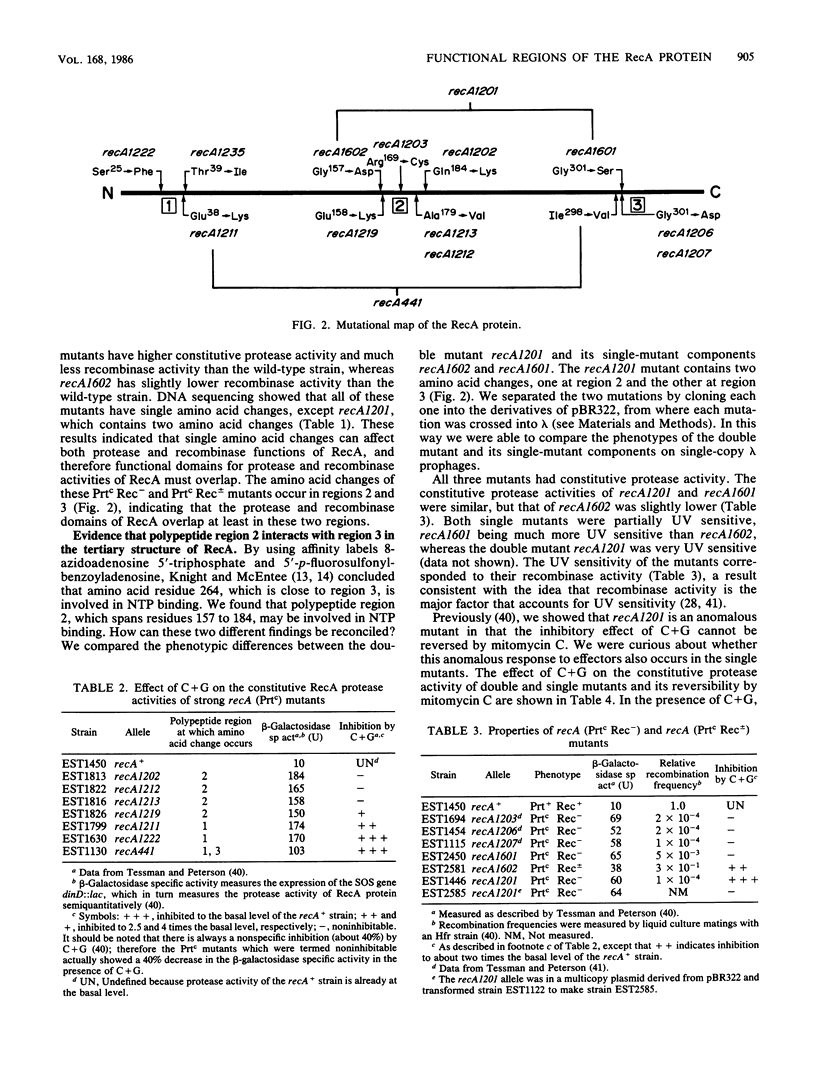
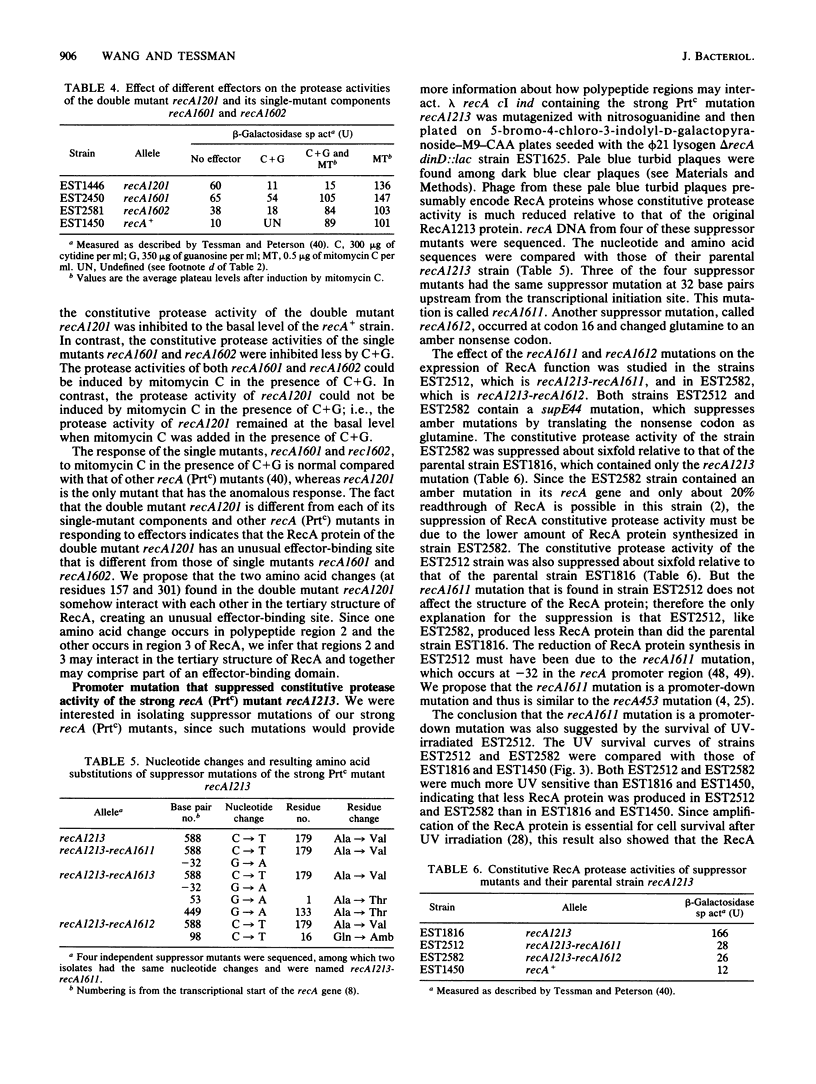
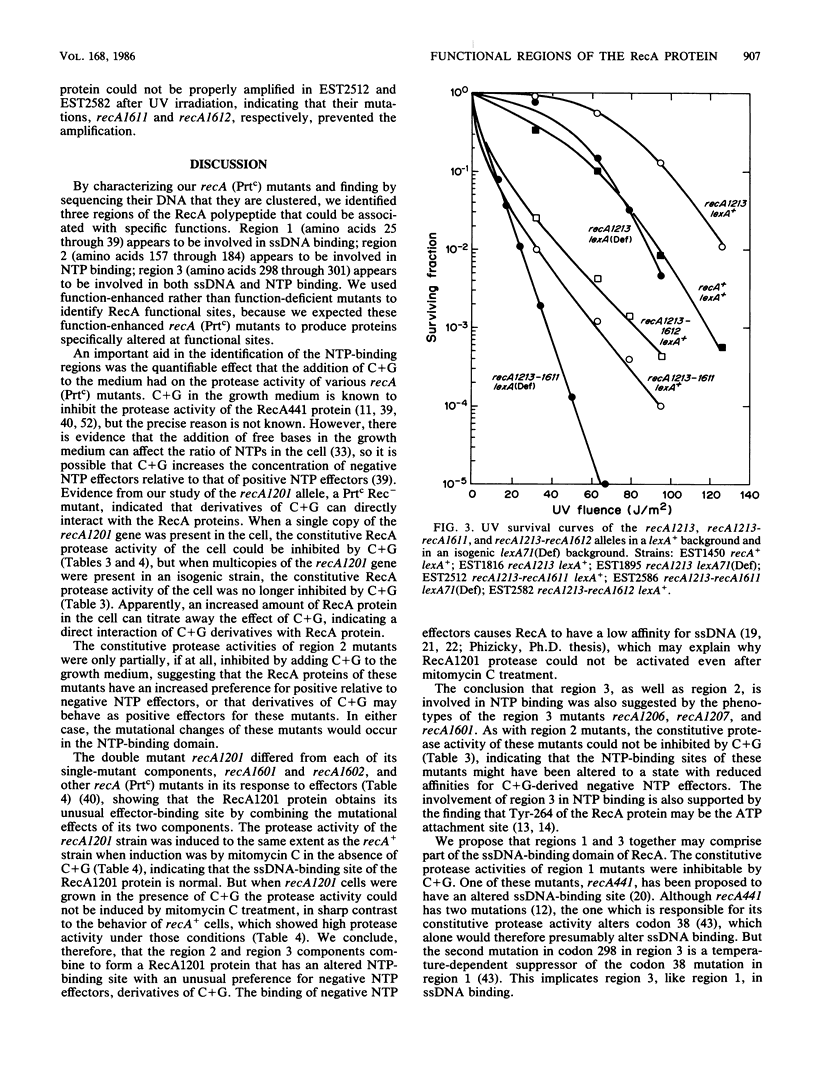
In previous work (E. S. Tessman and P. K. Peterson, J. Bacteriol. 163:677-687 and 688-695, 1985), we isolated many novel protease-constitutive (Prtc) recA mutants, i.e., mutants in which the RecA protein was always in the protease state without the usual need for DNA damage to activate it. Most Prtc mutants were recombinase positive and were designated Prtc Rec+; only a few Prtc mutants were recombinase negative, and those were designated Prtc Rec-. We report changes in DNA sequence of the recA gene for several of these mutants. The mutational changes clustered at three regions on the linear RecA polypeptide. Region 1 includes amino acid residues 25 through 39, region 2 includes amino acid residues 157 through 184, and region 3 includes amino acid residues 298 through 301. The in vivo response of these Prtc mutants to different effectors suggests that the RecA effector-binding sites have been altered. In particular we propose that the mutations may define single-stranded DNA- and nucleoside triphosphate-binding domains of RecA, that polypeptide regions 1 and 3 comprise part of the single-stranded DNA-binding domain, and that polypeptide regions 2 and 3 comprise part of the nucleoside triphosphate-binding domain. The overlapping of single-stranded DNA- and nucleoside triphosphate-binding domains in region 3 can explain previously known complex allosteric effects. Each of four Prtc Rec- mutants sequenced was found to contain a single amino acid change, showing that the change of just one amino acid can affect both the protease and recombinase activities and indicating that the functional domains for these two activities of RecA overlap. A recA promoter-down mutation was isolated by its ability to suppress the RecA protease activity of one of our strong Prtc mutants.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanar M. A., Kneller D., Clark A. J., Karu A. E., Cohen F. E., Langridge R., Kuntz I. D. A model for the core structure of the Escherichia coli RecA protein. Cold Spring Harb Symp Quant Biol. 1984;49:507–511. doi: 10.1101/sqb.1984.049.01.057. [DOI] [PubMed] [Google Scholar]
- Bossi L. Context effects: translation of UAG codon by suppressor tRNA is affected by the sequence following UAG in the message. J Mol Biol. 1983 Feb 15;164(1):73–87. doi: 10.1016/0022-2836(83)90088-8. [DOI] [PubMed] [Google Scholar]
- Bryant F. R., Taylor A. R., Lehman I. R. Interaction of the recA protein of Escherichia coli with single-stranded DNA. J Biol Chem. 1985 Jan 25;260(2):1196–1202. [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. Function of nucleoside triphosphate and polynucleotide in Escherichia coli recA protein-directed cleavage of phage lambda repressor. J Biol Chem. 1981 Aug 10;256(15):8039–8044. [PubMed] [Google Scholar]
- GOLDTHWAIT D., JACOB F. SUR LE M'ECANISME DE L'INDUCTION DU D'EVELOPPEMENT DU PROPHAGE CHEZ LES BACT'ERIES LYSOG'ENES. C R Hebd Seances Acad Sci. 1964 Jul 20;259:661–664. [PubMed] [Google Scholar]
- Horii T., Ogawa T., Ogawa H. Organization of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):313–317. doi: 10.1073/pnas.77.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., West S. C., Rusche J. R., Egelman E. H. Molecular mechanisms of general genetic recombination: the DNA-binding sites of RecA protein. Cold Spring Harb Symp Quant Biol. 1984;49:571–580. doi: 10.1101/sqb.1984.049.01.064. [DOI] [PubMed] [Google Scholar]
- Kawashima H., Horii T., Ogawa T., Ogawa H. Functional domains of Escherichia coli recA protein deduced from the mutational sites in the gene. Mol Gen Genet. 1984;193(2):288–292. doi: 10.1007/BF00330682. [DOI] [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K. L., Aoki K. H., Ujita E. L., McEntee K. Identification of the amino acid substitutions in two mutant forms of the recA protein from Escherichia coli: recA441 and recA629. J Biol Chem. 1984 Sep 25;259(18):11279–11283. [PubMed] [Google Scholar]
- Knight K. L., McEntee K. Affinity labeling of a tyrosine residue in the ATP binding site of the recA protein from Escherichia coli with 5'-p-fluorosulfonylbenzoyladenosine. J Biol Chem. 1985 Aug 25;260(18):10177–10184. [PubMed] [Google Scholar]
- Knight K. L., McEntee K. Tyrosine 264 in the recA protein from Escherichia coli is the site of modification by the photoaffinity label 8-azidoadenosine 5'-triphosphate. J Biol Chem. 1985 Aug 25;260(18):10185–10191. [PubMed] [Google Scholar]
- Little J. W. Autodigestion of lexA and phage lambda repressors. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M., Lehman I. R. Binding of the recA protein of Escherichia coli to single- and double-stranded DNA. J Biol Chem. 1981 Aug 25;256(16):8835–8844. [PubMed] [Google Scholar]
- McEntee K., Weinstock G. M. tif-1 mutation alters polynucleotide recognition by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6061–6065. doi: 10.1073/pnas.78.10.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetski J. P., Kowalczykowski S. C. Interaction of recA protein with single-stranded DNA. Quantitative aspects of binding affinity modulation by nucleotide cofactors. J Mol Biol. 1985 Jan 20;181(2):281–295. doi: 10.1016/0022-2836(85)90092-0. [DOI] [PubMed] [Google Scholar]
- Miller J. H. Mutational specificity in bacteria. Annu Rev Genet. 1983;17:215–238. doi: 10.1146/annurev.ge.17.120183.001243. [DOI] [PubMed] [Google Scholar]
- Moreau P. L., Roberts J. W. RecA protein--promoted lambda repressor cleavage: complementation between RecA441 and RecA430 proteins in vitro. Mol Gen Genet. 1984;198(2):25–34. doi: 10.1007/BF00328696. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Wabiko H., Tsurimoto T., Horii T., Masukata H., Ogawa H. Characteristics of purified recA protein and the regulation of its synthesis in vivo. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):909–915. doi: 10.1101/sqb.1979.043.01.099. [DOI] [PubMed] [Google Scholar]
- Phizicky E. M., Roberts J. W. Induction of SOS functions: regulation of proteolytic activity of E. coli RecA protein by interaction with DNA and nucleoside triphosphate. Cell. 1981 Jul;25(1):259–267. doi: 10.1016/0092-8674(81)90251-8. [DOI] [PubMed] [Google Scholar]
- Quillardet P., Moreau P. L., Ginsburg H., Mount D. W., Devoret R. Cell survival, UV-reactivation and induction of prophage lambda in Escherichia coli K12 overproducing RecA protein. Mol Gen Genet. 1982;188(1):37–43. doi: 10.1007/BF00332993. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Phizicky E. M., Burbee D. G., Roberts C. W., Moreau P. L. A brief consideration of the SOS inducing signal. Biochimie. 1982 Aug-Sep;64(8-9):805–807. doi: 10.1016/s0300-9084(82)80133-8. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L., Phizicky E. M. Activity of the Escherichia coli recA-gene product. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):917–920. doi: 10.1101/sqb.1979.043.01.100. [DOI] [PubMed] [Google Scholar]
- Ruff W., Kirby E. P., Goldthwait D. A. Cell division and prophage induction in Escherichia coli: studies of nucleotide levels. J Bacteriol. 1971 Jun;106(3):994–1004. doi: 10.1128/jb.106.3.994-1004.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche J. R., Konigsberg W., Howard-Flanders P. Isolation of altered recA polypeptides and interaction with ATP and DNA. J Biol Chem. 1985 Jan 25;260(2):949–955. [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. Physical map of the recA gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3144–3148. doi: 10.1073/pnas.76.7.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., DasGupta C., Cunningham R. P., Williams J. G., Osber L., Radding C. M. Homologous pairing in genetic recombination. The pairing reaction catalyzed by Escherichia coli recA protein. J Biol Chem. 1981 Jul 25;256(14):7565–7572. [PubMed] [Google Scholar]
- Tessman E. S., Peterson P. K. Isolation of protease-proficient, recombinase-deficient recA mutants of Escherichia coli K-12. J Bacteriol. 1985 Aug;163(2):688–695. doi: 10.1128/jb.163.2.688-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman E. S., Peterson P. K. tif-dependent induction of colicin E1, prophage lambda, and filamentation in Escherichia coli K-12. J Bacteriol. 1980 Sep;143(3):1307–1317. doi: 10.1128/jb.143.3.1307-1317.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman E. S., Peterson P. Plaque color method for rapid isolation of novel recA mutants of Escherichia coli K-12: new classes of protease-constitutive recA mutants. J Bacteriol. 1985 Aug;163(2):677–687. doi: 10.1128/jb.163.2.677-687.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. B., Tessman E. S. Evidence that the recA441 (tif-1) mutant of Escherichia coli K-12 contains a thermosensitive intragenic suppressor of RecA constitutive protease activity. J Bacteriol. 1985 Jul;163(1):407–409. doi: 10.1128/jb.163.1.407-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. ATP-dependent renaturation of DNA catalyzed by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):126–130. doi: 10.1073/pnas.76.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. Hydrolysis of nucleoside triphosphates catalyzed by the recA protein of Escherichia coli. Steady state kinetic analysis of ATP hydrolysis. J Biol Chem. 1981 Aug 25;256(16):8845–8849. [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. Interaction of the recA protein of Escherichia coli with adenosine 5'-O-(3-thiotriphosphate). J Biol Chem. 1981 Aug 25;256(16):8850–8855. [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K. RecA protein-dependent proteolysis of bacteriophage lambda repressor Characterization of the reaction and stimulation by DNA-binding proteins. J Biol Chem. 1981 Nov 10;256(21):10883–10888. [PubMed] [Google Scholar]
- Weisemann J. M., Weinstock G. M. Direct selection of mutations reducing transcription or translation of the recA gene of Escherichia coli with a recA-lacZ protein fusion. J Bacteriol. 1985 Aug;163(2):748–755. doi: 10.1128/jb.163.2.748-755.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman K. F., Mount D. W. Nucleotide sequence binding specificity of the LexA repressor of Escherichia coli K-12. J Bacteriol. 1985 Jul;163(1):376–384. doi: 10.1128/jb.163.1.376-384.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Spengler S. J. Fibers of RecA protein and complexes of RecA protein and single-stranded phi X174 DNA as visualized by negative-stain electron microscopy. J Mol Biol. 1986 Jan 5;187(1):109–118. doi: 10.1016/0022-2836(86)90410-9. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarranton G. T., Sedgwick S. G. Cloned truncated recA genes in E. coli II. Effects of truncated gene products on in vivo recA+ protein activity. Mol Gen Genet. 1982;185(1):99–104. doi: 10.1007/BF00333797. [DOI] [PubMed] [Google Scholar]


