Abstract
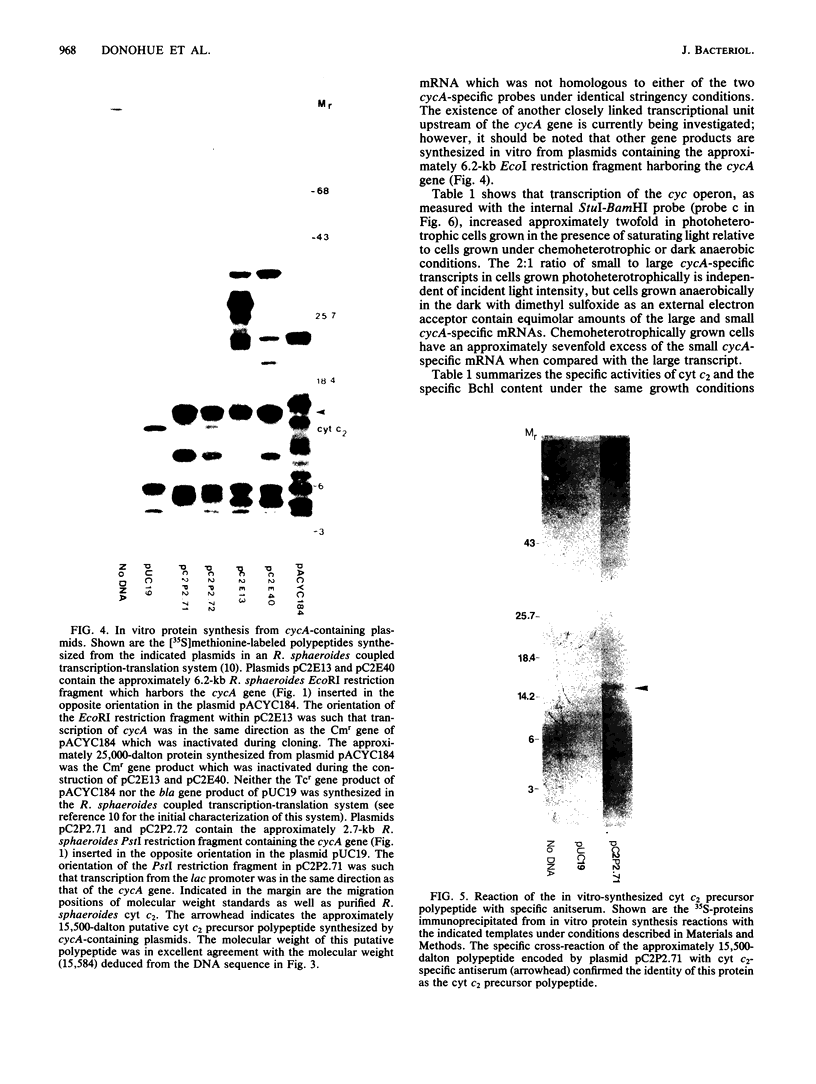
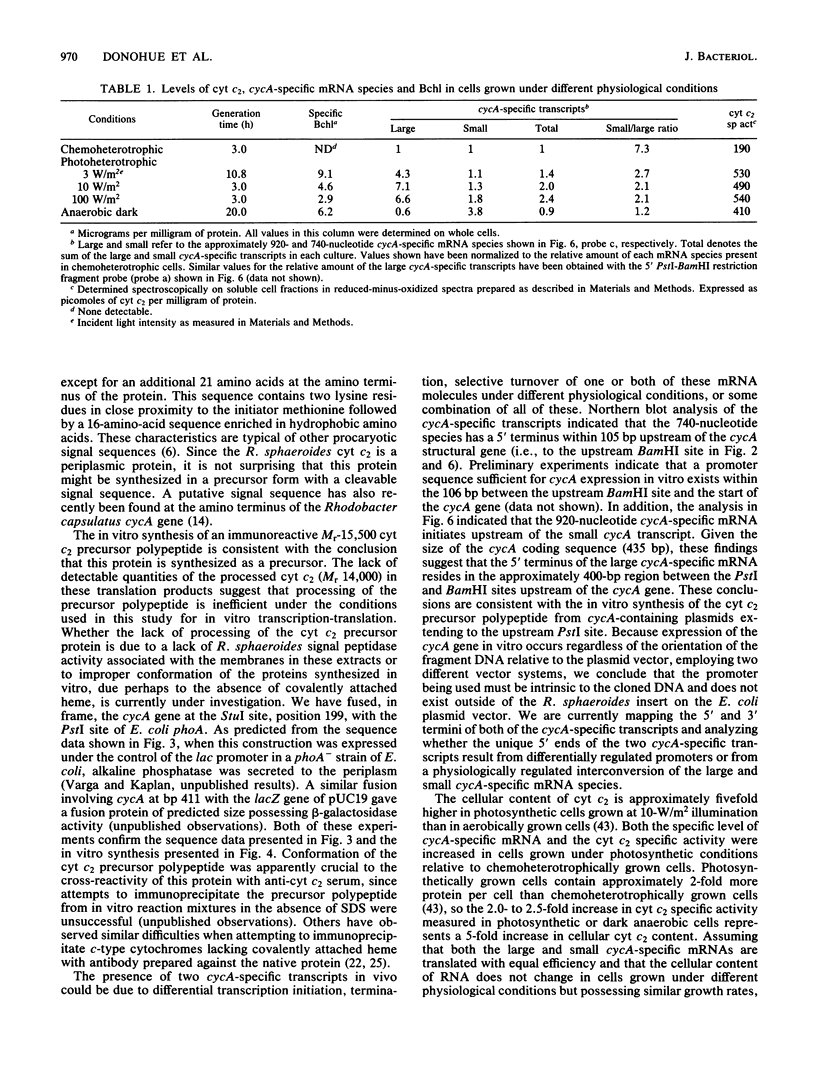
The Rhodobacter sphaeroides cytochrome c2 functions as a mobile electron carrier in both aerobic and photosynthetic electron transport chains. Synthetic deoxyoligonucleotide probes, based on the known amino acid sequence of this protein (Mr 14,000), were used to identify and clone the cytochrome c2 structural gene (cycA). DNA sequence analysis of the cycA gene indicated the presence of a typical procaryotic 21-residue signal sequence, suggesting that this periplasmic protein is synthesized in vivo as a precursor. Synthesis of an immunoreactive cytochrome c2 precursor protein (Mr 15,500) was observed in vitro when plasmids containing the cycA gene were used as templates in an R. sphaeroides coupled transcription-translation system. Approximately 500 base pairs of DNA upstream of the cycA gene was sufficient to allow expression of this gene product in vitro. Northern blot analysis with an internal cycA-specific probe identified at least two possibly monocistronic transcripts present in both different cellular levels and relative stoichiometries in steady-state cells grown under different physiological conditions. The ratio of the small (740-nucleotide) and large (920-nucleotide) cycA-specific mRNA species was dependent on cultural conditions but was not affected by light intensity under photosynthetic conditions. Our results suggest that the increase in the cellular level of the cytochrome c2 protein found in photosynthetic cells was due, in part, to increased transcription of the single-copy cyc operon.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage J. P., Ingham C., Evans M. C. Role of proton motive force in phototactic and aerotactic responses of Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Mar;161(3):967–972. doi: 10.1128/jb.161.3.967-972.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes W. M., Bevan M., Son P. H. Kilo-sequencing: creation of an ordered nest of asymmetric deletions across a large target sequence carried on phage M13. Methods Enzymol. 1983;101:98–122. doi: 10.1016/0076-6879(83)01008-3. [DOI] [PubMed] [Google Scholar]
- Barrett J., Jones O. T. Localization of ferrochelatase and of newly synthesized haem in membrane fractions from Rhodopseudomonas spheroides. Biochem J. 1978 Jul 15;174(1):277–281. doi: 10.1042/bj1740277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. E., Hesse S. D., Waechter-Brulla D. A., Lynn S. P., Gumport R. I., Gardner J. F. A genetic enrichment for mutations constructed by oligodeoxynucleotide-directed mutagenesis. Gene. 1985;37(1-3):73–81. doi: 10.1016/0378-1119(85)90259-8. [DOI] [PubMed] [Google Scholar]
- Benson S. A., Hall M. N., Silhavy T. J. Genetic analysis of protein export in Escherichia coli K12. Annu Rev Biochem. 1985;54:101–134. doi: 10.1146/annurev.bi.54.070185.000533. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Rhoads D., Tai P. C. Alkaline phosphatase and OmpA protein can be translocated posttranslationally into membrane vesicles of Escherichia coli. J Bacteriol. 1985 Mar;161(3):973–980. doi: 10.1128/jb.161.3.973-980.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J., Donohue T. J., Varga A. R., Staehelin L. A., Kaplan S. Induction of the photosynthetic membranes of Rhodopseudomonas sphaeroides: biochemical and morphological studies. J Bacteriol. 1984 Aug;159(2):540–554. doi: 10.1128/jb.159.2.540-554.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J., Kaplan S. The in vitro transcription-translation of DNA and RNA templates by extracts of Rhodopseudomonas sphaeroides. Optimization and comparison of template specificity with Escherichia coli extracts and in vivo synthesis. J Biol Chem. 1982 Dec 25;257(24):15110–15121. [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E., Harbin B. M. Ferrochelatase from Rhodopseudomonas sphaeroides: substrate specificity and role of sulfhydryl and arginyl residues. J Bacteriol. 1986 Jan;165(1):1–5. doi: 10.1128/jb.165.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldal F., Cheng S., Applebaum J., Davidson E., Prince R. C. Cytochrome c(2) is not essential for photosynthetic growth of Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2012–2016. doi: 10.1073/pnas.83.7.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartini M., Inouye M. Interaction between two major outer membrane proteins of Escherichia coli: the matrix protein and the lipoprotein. J Bacteriol. 1978 Jan;133(1):329–335. doi: 10.1128/jb.133.1.329-335.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E., Timkovich R., Almassy R. J. The cytochrome fold and the evolution of bacterial energy metabolism. J Mol Biol. 1976 Feb 5;100(4):473–491. doi: 10.1016/s0022-2836(76)80041-1. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Gabellini N., Bowyer J. R., Hurt E., Melandri B. A., Hauska G. A cytochrome b/c1 complex with ubiquinol--cytochrome c2 oxidoreductase activity from Rhodopseudomonas sphaeroides GA. Eur J Biochem. 1982 Aug;126(1):105–111. doi: 10.1111/j.1432-1033.1982.tb06753.x. [DOI] [PubMed] [Google Scholar]
- Gennis R. B., Casey R. P., Azzi A., Ludwig B. Purification and characterization of the cytochrome c oxidase from Rhodopseudomonas sphaeroides. Eur J Biochem. 1982 Jun 15;125(1):189–195. doi: 10.1111/j.1432-1033.1982.tb06667.x. [DOI] [PubMed] [Google Scholar]
- Hendry G. A., Houghton J. D., Jones O. T. The cytochromes in microsomal fractions of germinating mung beans. Biochem J. 1981 Mar 15;194(3):743–751. doi: 10.1042/bj1940743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B., Neupert W. Assembly of cytochrome c. Apocytochrome c is bound to specific sites on mitochondria before its conversion to holocytochrome c. Eur J Biochem. 1981 Dec;121(1):203–212. doi: 10.1111/j.1432-1033.1981.tb06450.x. [DOI] [PubMed] [Google Scholar]
- Korb H., Neupert W. Biogenesis of cytochrome c in Neurospora crassa. Synthesis of apocytochrome c, transfer to mitochondria and conversion to Holocytochrome c. Eur J Biochem. 1978 Nov 15;91(2):609–620. doi: 10.1111/j.1432-1033.1978.tb12714.x. [DOI] [PubMed] [Google Scholar]
- Lopatin D. E., Voss E. W., Jr Anti-lysergyl antibody: measurement of binding parameters in IgG fractions. Immunochemistry. 1974 Jun;11(6):285–293. doi: 10.1016/0019-2791(74)90364-4. [DOI] [PubMed] [Google Scholar]
- Lueking D. R., Fraley R. T., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Fate of "old" and "new" membrane. J Biol Chem. 1978 Jan 25;253(2):451–457. [PubMed] [Google Scholar]
- Maquat L. E., Reznikoff W. S. In vitro analysis of the Escherichia coli RNA polymerase interaction with wild-type and mutant lactose promoters. J Mol Biol. 1978 Nov 15;125(4):467–490. doi: 10.1016/0022-2836(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Maquat L. E., Thornton K., Reznikoff W. S. lac Promoter mutations located downstream from the transcription start site. J Mol Biol. 1980 May 25;139(3):537–549. doi: 10.1016/0022-2836(80)90145-x. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McEwan A. G., Ferguson S. J., Jackson J. B. Electron flow to dimethylsulphoxide or trimethylamine-N-oxide generates a membrane potential in Rhodopseudomonas capsulata. Arch Microbiol. 1983 Dec;136(4):300–305. doi: 10.1007/BF00425221. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Cusanovich M. A. Soluble cytochrome composition of the purple phototrophic bacterium, Rhodopseudomonas sphaeroides ATCC 17023. Biochim Biophys Acta. 1985 May 31;807(3):308–319. doi: 10.1016/0005-2728(85)90263-4. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Kamen M. D. New perspectives on c-type cytochromes. Adv Protein Chem. 1982;35:105–212. doi: 10.1016/s0065-3233(08)60469-6. [DOI] [PubMed] [Google Scholar]
- Nano F. E., Kaplan S. Expression of the transposable lac operon Tn951 in Rhodopseudomonas sphaeroides. J Bacteriol. 1982 Nov;152(2):924–927. doi: 10.1128/jb.152.2.924-927.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nano F. E., Kaplan S. Plasmid rearrangements in the photosynthetic bacterium Rhodopseudomonas sphaeroides. J Bacteriol. 1984 Jun;158(3):1094–1103. doi: 10.1128/jb.158.3.1094-1103.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince R. C., Baccarini-Melandri A., Hauska G. A., Melandri B. A., Crofts A. R. Asymmetry of an energy transducing membrane the location of cytochrome c2 in Rhodopseudomonas spheroides and Rhodopseudomonas capsulata. Biochim Biophys Acta. 1975 May 15;387(2):212–227. doi: 10.1016/0005-2728(75)90104-8. [DOI] [PubMed] [Google Scholar]
- Reichlin M. Use of glutaraldehyde as a coupling agent for proteins and peptides. Methods Enzymol. 1980;70(A):159–165. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Tai S. P., Kaplan S. Intracellular localization of phospholipid transfer activity in Rhodopseudomonas sphaeroides and a possible role in membrane biogenesis. J Bacteriol. 1985 Oct;164(1):181–186. doi: 10.1128/jb.164.1.181-186.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybulewicz V. L., Falk G., Walker J. E. Rhodopseudomonas blastica atp operon. Nucleotide sequence and transcription. J Mol Biol. 1984 Oct 25;179(2):185–214. doi: 10.1016/0022-2836(84)90465-0. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Zhu Y. S., Kaplan S. Effects of light, oxygen, and substrates on steady-state levels of mRNA coding for ribulose-1,5-bisphosphate carboxylase and light-harvesting and reaction center polypeptides in Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jun;162(3):925–932. doi: 10.1128/jb.162.3.925-932.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]