Abstract
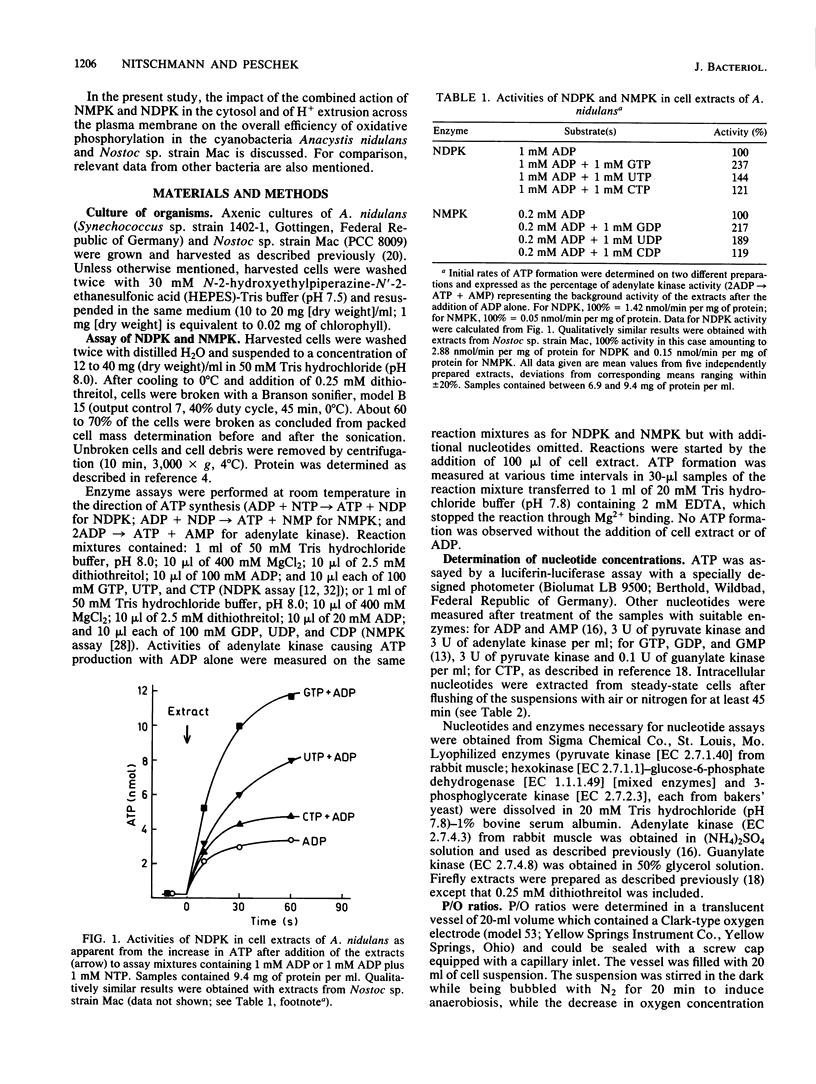
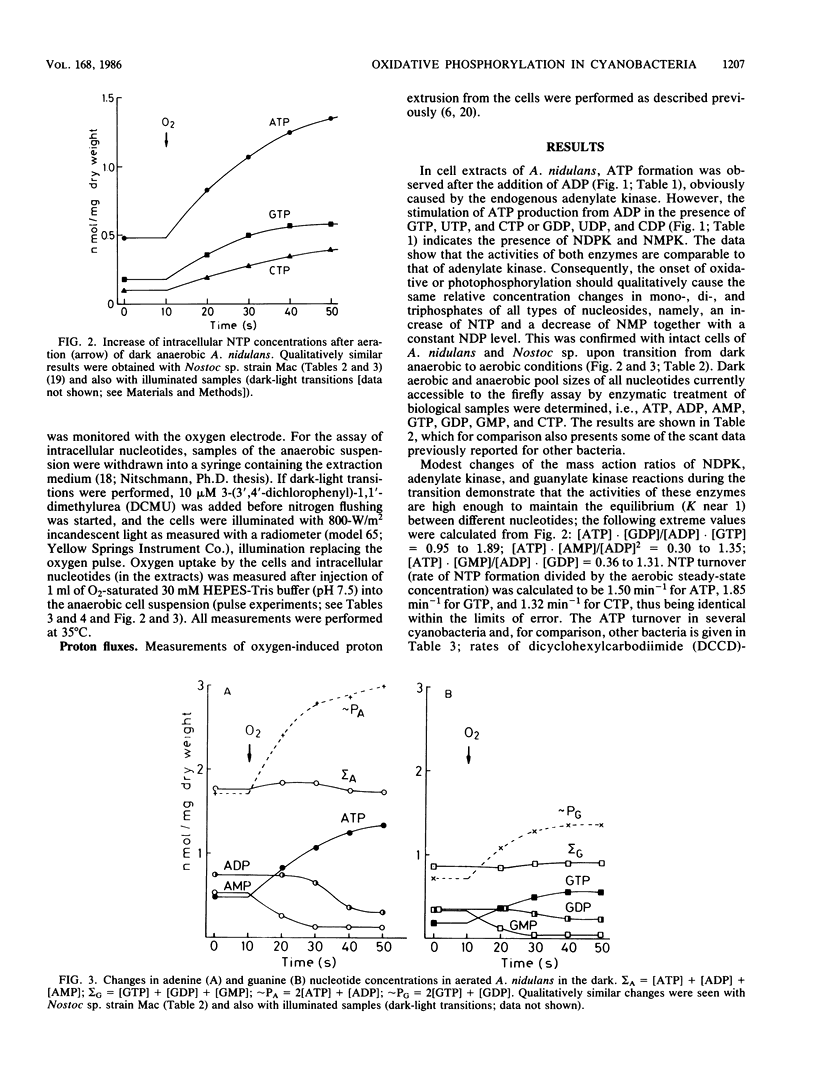
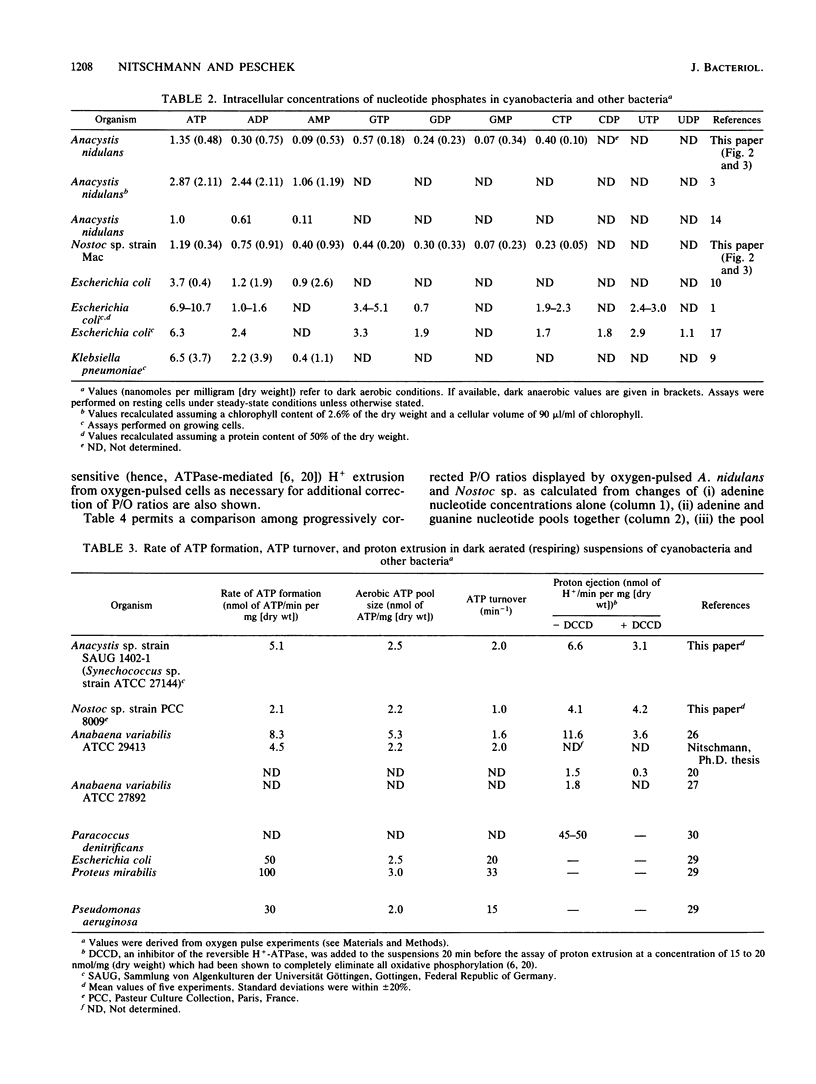
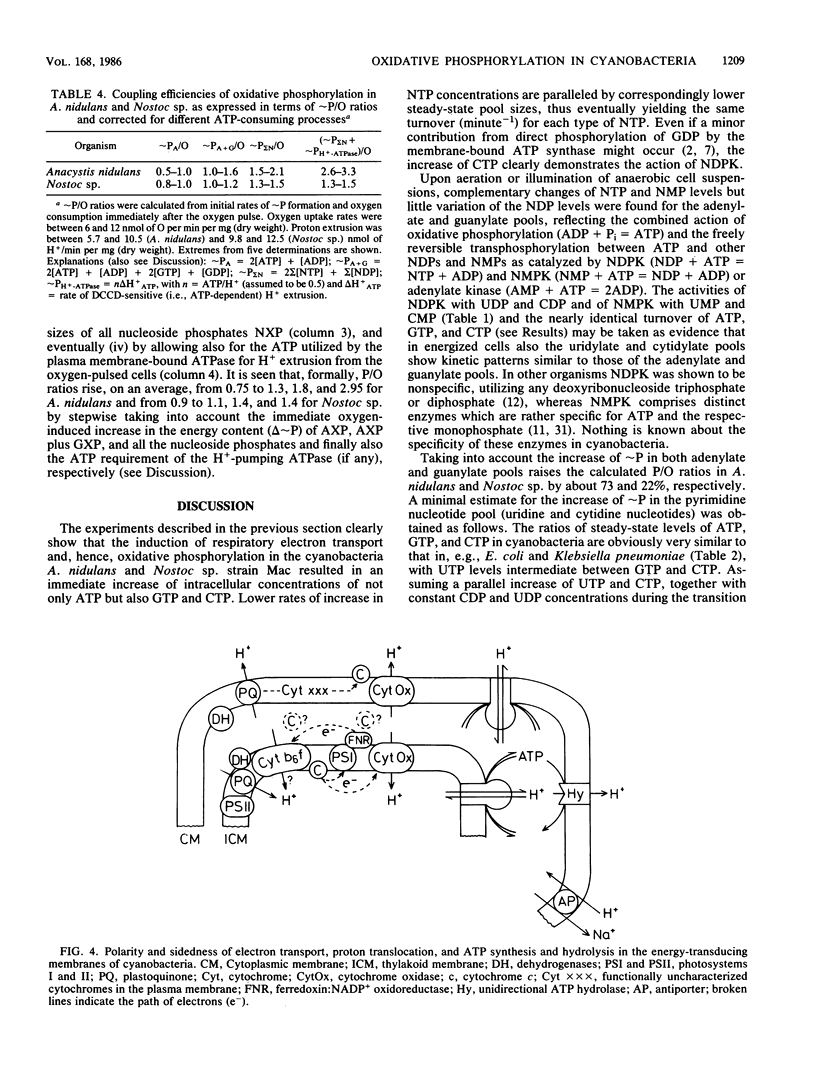
The onset of respiration in the cyanobacteria Anacystis nidulans and Nostoc sp. strain Mac upon a shift from dark anaerobic to aerobic conditions was accompanied by rapid energization of the adenylate pool (owing to the combined action of ATP synthase and adenylate kinase) and also the guanylate, uridylate, and cytidylate pools (owing to nucleoside diphosphate and nucleoside monophosphate kinases). Rates of the various transphosphorylation reactions were comparable to the rate of oxidative phosphorylation, thus explaining, in part, low approximately P/O ratios which incorporate adenylates only. The increase of ATP, GTP, UTP, and CTP levels (nanomoles per minute per milligram [dry weight]) in oxygen-pulsed cells of A. nidulans and Nostoc species was calculated to be, on average, 2.3, 1.05, 0.8, and 0.57, respectively. Together with aerobic steady-state pool sizes of 1.35, 0.57, 0.5, and 0.4 nmol/mg (dry weight) for these nucleotides, a fairly uniform turnover of 1.3 to 1.5 min-1 was derived. All types of nucleotides, therefore, may be conceived of as being in equilibrium with each other, reflecting the energetic homeostasis or energy buffering of the (respiring) cyanobacterial cell. For the calculation of net efficiencies of oxidative phosphorylation in terms of approximately P/O ratios, this energy buffering was taken into account. Moreover, in A. nidulans an additional 30% of the energy initially conserved in ATP by oxidative phosphorylation was immediately used up by a plasma membrane-bound reversible H+-ATPase for H+ extrusion. Consequently, by allowing for energy buffering and ATPase-linked H+ extrusion, maximum P/O ratios of 2.6 to 3.3 were calculated. By contrast, in Nostoc sp. all the H+ extrusion, appeared to be linked to a plasma membrane-bound respiratory chain, thus bypassing any ATP formation and leading to P/O ratios of only 1.3 to 1.5 despite the correction for energy buffering.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnara A. S., Finch L. R. The effects of bases and nucleosides on the intracellular contents of nucleotides and 5-phosphoribosyl 1-pyrophosphate in Escherichia coli. Eur J Biochem. 1974 Feb 1;41(3):421–430. doi: 10.1111/j.1432-1033.1974.tb03283.x. [DOI] [PubMed] [Google Scholar]
- Bennun A., Avron M. The relation of the light-dependent and light-triggered adenosine triphosphatases to photophosphorylation. Biochim Biophys Acta. 1965 Sep 27;109(1):117–127. doi: 10.1016/0926-6585(65)90096-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F. The cyanobacterial genome, its expression, and the control of that expression. Adv Microb Physiol. 1979;20:1–102. doi: 10.1016/s0065-2911(08)60206-4. [DOI] [PubMed] [Google Scholar]
- Erber W. W., Nitschmann W. H., Muchl R., Peschek G. A. Endogenous energy supply to the plasma membrane of dark aerobic cyanobacterium Anacystis nidulans: ATPase-independent efflux of H+ and Na+ from respiring cells. Arch Biochem Biophys. 1986 May 15;247(1):28–39. doi: 10.1016/0003-9861(86)90529-1. [DOI] [PubMed] [Google Scholar]
- Guffanti A. A., Bornstein R. F., Krulwich T. A. Oxidative phosphorylation by membrane vesicles from Bacillus alcalophilus. Biochim Biophys Acta. 1981 May 13;635(3):619–630. doi: 10.1016/0005-2728(81)90118-3. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Maitra P. K. Control of respiration and metabolism in growing Klebsiella aerogenes. The role of adenine nucleotides. Biochem J. 1969 May;112(5):647–656. doi: 10.1042/bj1120647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempfling W. P. Studies of the efficiency of oxidative phosphorylation in intact Escherichia coli B. Biochim Biophys Acta. 1970;205(2):169–182. doi: 10.1016/0005-2728(70)90247-1. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Singer M. F. Purification and characterization of adenylate kinase as an apparent adenosine triphosphate-dependent inhibitor of ribonuclease II in Escherichia coli. J Biol Chem. 1973 Mar 25;248(6):2014–2021. [PubMed] [Google Scholar]
- Ingraham J. L., Ginther C. L. Nucleoside diphosphokinase from Salmonella typhimurium. Methods Enzymol. 1978;51:371–375. doi: 10.1016/s0076-6879(78)51050-1. [DOI] [PubMed] [Google Scholar]
- Karl D. M. A rapid sensitive method for the measurement of guanine ribonucleotides in bacterial and environmental extracts. Anal Biochem. 1978 Sep;89(2):581–595. doi: 10.1016/0003-2697(78)90387-1. [DOI] [PubMed] [Google Scholar]
- Lawson J. W., Veech R. L. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979 Jul 25;254(14):6528–6537. [PubMed] [Google Scholar]
- Lundin A., Thore A. Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl Microbiol. 1975 Nov;30(5):713–721. doi: 10.1128/am.30.5.713-721.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazar R. N., Wong J. T. Nucleotide changes and the regulation of ribonucleic acid accumulation during growth rate shifts in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):790–797. [PubMed] [Google Scholar]
- Nitschmann W. H. A firefly luciferase assay for determination of cytidine 5'-triphosphate in biological samples. Anal Biochem. 1985 May 15;147(1):186–193. doi: 10.1016/0003-2697(85)90026-0. [DOI] [PubMed] [Google Scholar]
- Pelroy R. A., Bassham J. A. Efficiency of energy conversion by aerobic glucose metabolism in Aphancapsa 6714. J Bacteriol. 1973 Sep;115(3):937–942. doi: 10.1128/jb.115.3.937-942.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschek G. A., Hinterstoisser B., Riedler M., Muchl R., Nitschmann W. H. Exogenous energy supply to the plasma membrane of dark anaerobic cyanobacterium Anacystis nidulans: thermodynamic and kinetic characterization of the ATP synthesis effected by an artificial proton motive force. Arch Biochem Biophys. 1986 May 15;247(1):40–48. doi: 10.1016/0003-9861(86)90530-8. [DOI] [PubMed] [Google Scholar]
- Peschek G. A. Proton pump coupled to cytochrome c oxidase in the cyanobacterium Anacystis nidulans. J Bacteriol. 1983 Jan;153(1):539–542. doi: 10.1128/jb.153.1.539-542.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschek G. A. Structure and function of respiratory membranes in cyanobacteria (blue-green algae). Subcell Biochem. 1984;10:85–191. doi: 10.1007/978-1-4613-2709-7_2. [DOI] [PubMed] [Google Scholar]
- Scherer S., Stürzl E., Böger P. Oxygen-dependent proton efflux in cyanobacteria (blue-green algae). J Bacteriol. 1984 May;158(2):609–614. doi: 10.1128/jb.158.2.609-614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes P., Mitchell P., Moyle J. The polarity of proton translocation in some photosynthetic microorganisms. Eur J Biochem. 1969 Apr;8(3):450–454. doi: 10.1111/j.1432-1033.1969.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Scott E. M., Wright R. C. Kinetics and equilibria of pyrimidine nucleoside monophosphate kinase from human erythrocytes. Biochim Biophys Acta. 1979 Nov 9;571(1):45–54. doi: 10.1016/0005-2744(79)90223-7. [DOI] [PubMed] [Google Scholar]
- Waleh N. S., Ingraham J. L. Pyrimidine ribonucleoside monophosphokinase and the mode of RNA turnover in Bacillus subtilis. Arch Microbiol. 1976 Oct 11;110(1):49–54. doi: 10.1007/BF00416968. [DOI] [PubMed] [Google Scholar]
- van Verseveld H. W., Krab K., Stouthamer A. H. Proton pump coupled to cytochrome c oxidase in Paracoccus denitrificans. Biochim Biophys Acta. 1981 May 13;635(3):525–534. doi: 10.1016/0005-2728(81)90111-0. [DOI] [PubMed] [Google Scholar]
- van der Beek E. G., Stouthamer A. H. Oxidative phosphorylation in intact bacteria. Arch Mikrobiol. 1973;89(4):327–339. doi: 10.1007/BF00408900. [DOI] [PubMed] [Google Scholar]


