Abstract
Recent studies demonstrated a number of links between chromatin structure, gene expression, extracellular signaling and cellular differentiation during lens development. Lens progenitor cells originate from a pool of common progenitor cells, the pre-placodal region (PPR) which is formed due to a complex exchange of extracellular signals between the neural plate, naïve ectoderm and mesendoderm. A specific commitment to the lens program over alternate choices such as the formation of olfactory epithelium or the anterior pituitary is manifested by the formation of a thickened surface ectoderm, the lens placode. Mouse lens progenitor cells are characterized by the expression of a complement of lens lineage-specific transcription factors including Pax6, Six3 and Sox2, controlled by FGF and BMP signaling, followed later by c-Maf, Mab21like1, Prox1 and FoxE3. Proliferation of lens progenitors together with their morphogenetic movements results in the formation of the lens vesicle. This transient structure, comprised of lens precursor cells, is polarized with its anterior cells retaining their epithelial morphology and proliferative capacity, whereas the posterior lens precursor cells initiate terminal differentiation forming the primary lens fibers. Lens differentiation is marked by expression and accumulation of crystallins and other structural proteins. The transcriptional control of crystallin genes is characterized by the reiterative use of transcription factors required for the establishment of lens precursors in combination with more ubiquitously expressed factors (e.g. AP-1, AP-2α, CREB and USF) and recruitment of histone acetyltransferases (HATs) CBP and p300, and chromatin remodeling complexes SWI/SNF and ISWI. These studies have poised the study of lens development at the forefront of efforts to understand the connections between development, cell signaling, gene transcription and chromatin remodeling.
Keywords: development, lens differentiation; histone acetylation and methylation; Pax6; c-Maf; chromatin remodeling; transcriptional regulation
1. Introduction
Multicellular organisms achieve cellular differentiation through precisely regulated gene expression. Humans, for example, use at least 22,500 different genes (the genetic information) to generate over 260 different, specialized cell types from the fertilized egg. However, the informational content of a fertilized mammalian egg at fertilization is not only limited to its primary DNA sequence, but also includes DNA methylation, histone modifications, the presence of specific macromolecules including RNAs and proteins, and their distribution throughout the plasma membrane and cytoplasm. These additional sources of inherited information are termed epigenetic regulatory mechanisms. While the genetic information generally remains constant during development, epigenetic information is reprogrammed in response to a myriad of cues including cell-to-cell interactions and extracellular signaling. The resulting activation and repression of specific genes directs the formation of individual progenitor cells that develop into whole tissues and organs. The ocular lens provides an excellent system to study this process at the molecular level as lens lineage-specific transcription factors and their hierarchies are among the best understood in mammalian systems.
This review describes how epigenetic regulatory mechanisms such as alterations in chromatin structure can control the expression of lens differentiation markers. First, in chapter two, we describe what is known about chromatin biology in other systems as this theme has not been covered in earlier reviews of gene expression in the lens (see Chow and Lang, 2001; Cvekl and Piatigorsky, 1996; Donner et al., 2006b; Duncan et al., 2004a; Kondoh, 1999; Medina-Martinez and Jamrich, 2007). In chapter three, we describe how alterations in gene transcription during embryonic development lead to the establishment of lens progenitor cells. In chapter 4, we present up-to-date analysis of the transcriptional control of both regulatory and structural gene expression in the lens. Finally, in chapter 5, we summarize the current gaps in our knowledge of how epigenetic and genetic networks regulate lens development.
2. Chromatin and gene regulation during development
2.1. Genetic and epigenetic regulatory mechanisms
Although somatic cells within an organism generally carry an identical genetic codebook, different cell types uses different portions of this code to control their differentiation state. All cells of mammalian embryos prior to the eight cell stage have the potential to form all somatic and germ cells of the embryo proper as well as all extraembryonic tissues (totipotency), while this potential is progressively restricted during subsequent development by epigenetic reprogramming of the cell nucleus in response to extracellular signaling pathways. This results in the restricted expression of subsets of genes from a pool of approximately 100 lineage-specifying transcription factors (“master” genes) in various progenitor cell lineages which are capable of further interactions with extracellular signaling pathways culminating in differentiation specific gene expression (see Margueron et al., 2005). Simultaneously, this global genome reprogramming results in the transcriptional repression of cohorts of genes responsible for the formation of alternate cell lineages (see Palacios and Puri, 2006).
The epigenetic program (epigenome) of a particular cell type (see Fig. 1A) is encoded in distinct patterns of nuclear organization, global chromatin structure, global and local covalent histone modifications, DNA methylation, as well as specific patterns of gene expression and repression. This information must remain stable during cell division so that individual cells can remember who they are. However, development also requires reprogramming of specific epigenetic marks and this process is often accomplished during cell division by a large group of enzymes that regulate chromatin structure in concert with the subnuclear distribution of individual chromosomal regions (see Sarma and Reinberg, 2005). This results in the coordinated expression of many distinct transcripts (a transcriptome) that dictates a particular cellular function such as lineage commitment and differentiation (see Kosak and Groudine, 2004). Thus, the dynamic control of epigenetic information is central to development (see Jenuwein and Allis, 2001).
Figure 1. Epigenetic regulation of gene expression and post-translational modifications of histones.

(A) Histone modifications (shown in box) influence other epigenetic regulatory processes listed outside of the box. (B) A summary of post-translational modifications of histones H4, H3, H2A, H2B and H1b (based on Margueron et al., 2005).
2.2. Spatial organization of gene activity within the nucleus
Human genomic DNA resides in the nucleus as 23 pairs of chromosomes containing 6×109 base pairs of DNA organized around 30 million core histone octamers and interacting with 1,900 specific DNA-binding transcription factors (Messina et al., 2004; Urnov and Wolffe, 2001). Experimental evidence suggests that each chromosome occupies a spatially confined territory within the nucleus, the chromosome territory. The space between chromosomal territories, the interchromatin compartment, is occupied by protruding chromatin loops and enriched in nuclear bodies involved in transcription and splicing (Cremer et al., 1993). In addition, this space permits high mobility of transcription factors (Hager et al., 2006) and their preferred recognition of binding sites on the outer surface of the chromosomal territories (Kosak and Groudine, 2004). Thus, the spatial organization of genes within the nucleus and linearly in the genome is thought to play a significant role in the orchestration of gene expression during cellular differentiation (see Kosak and Groudine, 2004).
The linear arrangement of genes represents genetic information and its spatial organization within the nucleus is an example of an epigenetic regulatory mechanism. What is currently known about the linear order of genes along chromosomes to facilitate the coordinated regulation of the transcriptome? The accumulating data support the hypothesis that an underlying linear order of genes along chromosomes exists to aid the coordinated regulation of all expressed genes (see Kosak and Groudine, 2004). Evidence exists that the human genome is organized into regions of high and low levels of gene activity (Caron et al., 2001). The regions of increased gene expression (RIDGEs), coincide with gene-dense chromosomal regions, and are separated by large gene poor regions of low activity, the valleys. Many gene-dense regions contain highly expressed housekeeping genes and a significant portion of tissue-specific genes are clustered in the genome (Divina et al., 2005; Lercher et al., 2002)
A rational explanation for a nonrandom order of genes along chromosomes originates from the mathematical concepts of graph (or network) theory and self-organization to form subcompartments such as the nucleolus and other types of nuclear bodies (Misteli, 2001). A special feature of biological networks is the formation of organized centers, or hubs, of highly connected nodes representing co-regulated genes (see Kosak and Groudine, 2004; Ragoczy et al., 2006). Thus, by spatially restricting the position of genes within the nucleus, their regulation can be efficiently controlled through sharing common regulatory proteins, distinct RNA polymerase holoenzymes or by spreading of chromatin modifications and activation mediated by distal regulatory elements (see Kosak and Groudine, 2004).
2.3. Nucleosome and chromatin domains
2.3.1. Structure of the nucleosome and its positioning
Histones are evolutionary conserved, small basic proteins composed of a central globular domain flanked by N- (in histones H2A, H2B, H3 and H4) and C-terminal (in histones H2A and H2B) peptides (histone “tails”). The basic structural unit of chromatin is the nucleosome core particle. Each nucleosome consists of a cylindrical core histone octamer composed of a central heterotetramer of histones H3 and H4 sandwiched between a pair of histones H2A-H2B heterodimers. The heterodimerization of the core histones, H4 and H3, and H2A and H2B, and their assembly into the histone octamer is mediated through the histone fold interaction motif (Gangloff et al., 2001), see Table 1. Each histone octamer has 147 base pairs (1.75 turns) of supercoiled DNA wrapped around its outer surface and this structure is stabilized by electrostatic interactions between DNA and exposed arginines of the histone fold domain while the histone tails protrude from the nucleosome. Each nucleosome is separated by variable species-specific DNA linkers of 28-43 base pairs in most vertebrates (see Woodcock et al., 2006) resembling “beads-on-a-string” to form a chromatin-fiber of 11 nm in diameter (see Margueron et al., 2005). The precise translational and rotational position that double helical DNA adopts with the respect to the histone core is termed “nucleosome positioning” (Wolffe, 1998). The translational position is the precise 146 bp region where the histone octamer begins and completes its association with DNA. The rotational position refers to which face of DNA is in contact with, or faces away from the histone octamer (Wolffe, 1998). Nucleosome positioning strongly influences accessibility of specific DNA sequences for trans-acting factors such as sequence specific DNA-binding transcription factors. Nucleosome repositioning and ejection are two important examples of dynamic properties of nucleosomes during transcriptional regulation (see 2.4.3.).
Table 1.
Structural and functional domains found in important chromatin-associated proteins
| Structure and Function | Examples of proteins | |
|---|---|---|
| Chromatin binding | ||
| Bromodomain | ~110 amino acids left-handed-up-and-down four α-helix bundle, present in single or duplicate copies, recognizes acetylated lysines in histones | ACF1, BAF180/polybromo, Brg1, Brwd1, Ring3, TAFII250, CBP, p300, PCAF, BPTF, Brd4/7, NoRC, TIF1γ |
| Chromodomain | ~60 amino acids (three β-strands and α-helix), single or multiple copies, binds to methylated histones | HP-1, CHD1, CHD3/4, Polycomb (Pc), Cbx2/4/6/7/8, Eaf3, RBP1, Su(var)3-9 |
| PHD finger | Plant homeodomain (PHD) zinc finger (C4HC3), binds H3 K4me3 | ATRX, BPTF, CHD3/4, CBP/p300, hPygo1/2, JARID1, JMJD2, MLL3, NURF, Pho23, Rbp2, TAFII140, Yng1/2 |
| SANT domain | ~130 amino acid histone-tail binding domain (SWI3/ADA2/NcoR/TFIIIB) similar to Myb DNA-binding domains | Snf2h, Snf2l, NCoR, hMI-ER1, SMRT1/2, coREST, polycomb protein E(z), MTA1/2/3 |
| SLIDE domain | Similar to SLIDE and Myb DNA-binding domains | Snf2h |
| Tudor domain | ~50-70 amino acids adopting a barrel-like fold, single or multiple copies. Recognizes H3 K4me | JMJD2a/b/c, h53BP1, RBP1, RBP1L1 |
| WD40 repeat | A variable region of 6-30 residues and a 28-residues core which usually starts with a Gly-His pair and ends with Trp-Asp (WD) pair. Up to eight repeats per protein. Binds H3 K4me. | WDR5 (subunit of Trithorax complex), ram/WDR9, L2DTL, BRWD1, Set1 |
| DNA-binding | ||
| ARID/BRIGHT | AT-rich interactive domain (~94 amino acids) | JARID1a/RPB2, JARID1b/PLU-1, JARID1c/SMOX, JARID1d/SMCY, BAF250/OSA1, RBP1/2, Bright/DRIL1 |
| AT-hook | Binds to AT-rich DNA sequences | Brg1, Cbx2, PSIP1/LEDGF/p75, MLL1 |
| HMG-box | Comprised of multiple AT-hooks | HMGA1 and 2, HMGB1, HMGN1, NSBP1, Sox1, Sox2 |
| Enzyme function | ||
| Deacetylase type I | ~ 390 amino acid deacetylase core consisting of a tubular active site pocket containing the binding site for trichostatin A and a bound Zn2+ at the base | HDAC1/2/3/8, HDLP |
| Deacetylase type III (sirtuins) | ~ 270 amino acid deacetylase core containing a large Rossmann fold domain that binds NAD+/NADH cofactor and a small domain with structural Zn2+ | SIRT1 to SIRT7, Sir2 |
| DEAD/H-box helicase domain | ~170 amino acid ATP-binding helicase | Brg1, Brm, CHD3/4, Snf2h, Snf2l |
| E4 ligase | Ubiquitin ligase specific to histone H1 (TAFII250) and p53 (p300) | TAFII250, p300 |
| Histone acetyltransferase (HAT) | A variable ~410-740 amino acid domain, the central core subdomain binds acetyl-coenzyme A cofactor, the flanking subdomains bind to histones | ATF2, CBP/p300, ESA1, P/CAF, SRC-1, TAFII250 |
| JmjC demethylase | ~ 120 amino acid histone demethylase signature domain with Fe2+ in the catalytical core | JARID1a/RPB2, JARID1b/PLU-1, JARID1c/SMOX, JARID1d/SMCY |
| SET | ~130-140 amino acid protein L-shaped methylase domain (Su(var)3-9, Enhancer-of-zeste and Trithorax) | SUV39, SET1/2/7/8/9, polycomb protein E(z), RIZ1, SMYD, SUV4-20 |
| Protein-protein interactions | ||
| Histone fold | ~65 amino acid domain (three tandem α-helices connected by two short β-strand regions) | H2A, H2B, H3 and H4 histones, CHRAC15/17, DR1, DRAP1, TAFII15, TAFII80, |
| LXXLL motif | One or more LXXLL motif serve as binding sites for liganded nuclear hormone receptors or Sirtui-type HDacs | ASC-2, BAF250/OSA1, CBP, FoxO1, JARID1a/RPB2, JARID1b/PLU-1, JARID1c/SMOX, JARID1d/SMCY, p300, PPAR- binding protein (PBP), Prox1, Stat6 |
Nucleosome positioning is regulated by the posttranslational processing of histone tails by a range of reversible modifications including acetylation of lysine residues, methylation of arginine and lysine residues, phosphorylation of serine and threonine residues, and sumoylation and ubiquitination of lysine residues (see section 2.4.2.). Recruitment of histone acetyltransferases (HATs), histone deacetyltransferases (HDACs), histone methyltransferases (HMTs) and histone demethylases by DNA-binding transcription factors control the majority of histone covalent modifications. Notably, these core histone modifications do not alter chromatin dynamics by themselves but rather through their recruitment of nonhistone proteins to the chromatin fiber. For example, acetylated lysines are recognized by a special interacting domain, the bromodomain (Dhalluin et al., 1999; R. H. Jacobson et al., 2000) that is found in ATP-dependent chromatin remodeling enzymes (see section 2.4.3.) as well as in other chromatin-associated proteins (see Table 1).
The 11 nm fiber can be folded like a solenoid with the help of linker histones (histone H1 family) into more condensed, 30 nm thick, chromatin fibers. The 30 nm fibers are further compacted to form 100-400 nm thick interphase fibers (Wolffe, 1998). In this conformation, the accessibility of DNA to non-histone proteins and enzymes involved in DNA replication, transcription and repair is greatly obstructed. Thus, local chromatin folding-unfolding transitions are important epigenetic mechanisms which generate differential accessibility of the genetic information in different cell types.
2.3.2. Euchromatin, heterochromatin and barrier insulators
Chromatin in the interphase nucleus is organized into transcriptionally inactive heterochromatin and transcriptionally active euchromatin. The transcriptional competence of euchromatin results from its relatively low compactness which can be measured by as a greater sensitivity to nuclease digestion. Some regions within euchromatin exhibit “nuclease-hypersensitivity”, which is an indirect result of the presence of DNA-binding transcription factors and irregular spacing of the nucleosomes. Heterochromatin can be recognized by its more condensed structure leading to a reduced sensitivity to nuclease digestion (see Gaszner and Felsenfeld, 2006). Some gene poor regions of DNA (constitutive heterochromatin) are always highly methylated at CpG dinucleotides and highly condensed in all cell types, while facultative heterochromatin is cell lineage-dependent and actively formed in chromosomal regions harboring genes whose suppression is necessary for that cell type (see Kosak and Groudine, 2004).
Heterochromatic regions are marked by methylation of residues K9 and K27 of histone H3 combined with the lack of H3 acetylation (see 2.4.2.). Its maintenance requires large amounts of heterochromatin protein 1 (HP1) that directly mediates its highly condensed structure and regular nucleosomal spacing (Kosak and Groudine, 2004). The growth of heterochromatin regions is catalyzed by a self-perpetuating cycle of reactions: methylation of H3 K9 leads to the recruitment of HP1 to the region (see 2.4.2. and 2.4.4.) while HP1 recruits additional protein lysine methyltransferase activity (see Gaszner and Felsenfeld, 2006). The extent of heterochromatin formation is constrained by the presence of barrier insulators which result in the gradual transition between euchromatin and heterochromatin, an effect termed “position-effect variegation” (PEV). Barrier insulators are thought to function by changing the local balance of euchromatin-promoting enzymes such as histone acetyltransferases, H3 K4 and H4 R3 histone methyltransferases (see 2.4.2) and ATP-dependent chromatin remodeling enzymes (see 2.4.3). Another possibility for their action is through tethering the chromatin fiber to an interchromosomal compartment in which the protein composition is unfavorable to heterochromatin formation (see Gaszner and Felsenfeld, 2006). Some barrier insulators can also act as enhancer-blocking insulators (see 2.4.1). Barrier-insulator activity can also reside within locus control regions (LCRs), complex enhancers that confer strong position-independent expression on transgenes as described in detail in 2.4.1.
2.3.3. Chromatin architectural proteins
Linker histones, histone variants and high mobility group (HMG) proteins are diverse chromatin architectural proteins participating differently in the regulation of gene expression. H1 is the linker histone associated with compaction of the chromatin fiber and the general repression of transcription. In mice, there are six somatic linker histone subtypes (H1a, H1b, H1c, H1d, H1e and H10) as well as testis- and oocyte-specific subtypes, which differ in primary sequence and relative abundance in different cell types. For example, H10 is most abundant in erythrocytes derived from yolk sac and lens fiber cells (Gjerset et al., 1982). The function of many linker histone H1 variants was determined using their gene inactivation in mouse: deletion of one or two H1 subtypes from the mouse genome was followed by a compensatory increase of the remaining subtypes, resulting in the normal H1 to nucleosome ratio (Fan et al., 2003; Sirotkin et al., 1995). Deletion of lens-preferred H10 does not impair lens fiber cell differentiation (Sirotkin et al., 1995). In contrast, inactivation of three H1 subtypes (H1c, H1d and H1e) results in embryonic lethality prior to E11.5 (Fan et al., 2005). Analysis of gene expression profiles in triple mutant ES cells derived from earlier blastocysts revealed that a significant proportion of genes affected by the triple mutation were regulated at the level of DNA methylation. Thus, linker H1 histones are involved at a number of different processes that control gene expression.
Histone variants (“replacement” histones) are of highly similar sequence to the canonical histones although they are mostly encoded by intron-containing single genes located outside of the histone gene clusters. While there are three known variants of histone H3 (H3.1, H3.2 and H3.3; see (Kamakaka and Biggins, 2005; Sarma and Reinberg, 2005) and five variants of histone H2A (H2A.Z, H2A.X, MacroH2A1 and 2 and H2A.Bbd) only four of these, H3.3, H2A.Z, MacroH2A1 and 2, have been shown to play roles in transcriptional regulation. The synthesis of murine H3.3 increases during terminal differentiation and the histone chaperone, HIRA, deposits H3.3 in a transcription dependent, replication-independent manner into nucleosomes along the transcribed region (Fig. 3C) (see Sarma and Reinberg, 2005). H2A.Z is essential for early mouse development and appears to be involved in both transcriptional activation and repression. It differs from the canonical H2A by the presence of an acidic patch motif between the last two α-helices of the core structure (Suto et al., 2000) which seems to provide a stronger docking domain for the H4 tail of an adjacent nucleosome. MacroH2A1 and 2 are histone H2A variants which contain an additional 25 kDa “macrodomain” consisting of a basic region and a putative leucine zipper, a structure that can be used for homo- and heterodimerization of proteins. MacroH2A represses transcription as it interferes with binding of NF-κB and chromatin remodeling by the SWI/SNF complex (Angelov et al., 2003). Collectively, incorporation of histone variants enriches the repertoire of mechanisms that perturb regular chromatin structure and participate in the control of gene expression.
Figure 3. Four examples of many possible combination of the “histone code”.

(A) Transcriptional activation via histone methylation (H3 K4me) that supports a broad histone acetylation (H3 K9ac, 14ac, 17ac and 23ac and H4 K5ac, K8ac, K12ac and K16ac) catalyzed by one or more HATs. (B) Transcriptional repression elicited by a specific histone methylation, H3 K9me, that inhibits acetylation of H3 and H4. (C) Transcriptional activation through phosphorylation of H3 S10ph followed by acetylation of H3K9ac and K14ac. (D) Transcriptional repression elicited by H3 K9me that inhibits acetylation of H3 K9 and K14. See text in 2.4.4.
Three families of high mobility group proteins, HMGA, HMGB and HMGN (see Bustin, 2001; Catez et al., 2003) are comprised of proteins with a molecular weight around 10 kD that are thought to act as chromatin architectural regulators. HMGN proteins are the only nuclear proteins that bind specifically to the core nucleosome particle, contacting both histones and DNA, although the DNA interactions are non-specific. The binding of HMGN proteins to nucleosomes disrupts the 30 nm fiber and promotes access to the nucleosome by competing with linker histone H1 (Catez et al., 2002). Further HMGN protein interaction with nucleosomes results in alterations in histone acetylation and phosphorylation allowing for the further modulation of chromatin structure. HMGN proteins are found clustered in domains containing six contiguous nucleosome-HMGN complexes (Postnikov et al., 1997) which appear to contribute to the general structural and compositional heterogeneity of chromatin fiber. HMGN proteins share three functional domains: a bipartite nuclear localization motif, a nucleosome-binding domain and a chromatin-unfolding domain. The nucleosome-binding domain is positively charged and contacts multiple core histones. In contrast, the negatively charged chromatin-unfolding domains interact with the N-terminus of histone H3 (Trieschmann et al., 1998). HMGN1 and HMGN2 (previous names HMG-14 and HMG-17, respectively) are ubiquitously expressed and form functional homodimers but not heterodimers. The more recently added HMGN3 (Trip7), HMGN4 and NSBP1 are expressed in a limited number of tissues (West et al., 2004) with HMGN3 highly expressed in mouse lens fiber cells (Lucey et al., 2005).
Members of the HMGB and HMGA families are characterized by two structural motifs, the HMG-box (also found in Sox proteins which are important for lens development) and the AT-hook (see Table 1). HMGB1 (formerly HMG-1) interacts with minor groove of DNA resulting in DNA bending. This, in turn, enhances binding of various transcription factors to DNA (Jayaraman et al., 1998; Mitsouras et al., 2002). In HMGA, three copies of the AT-hook preferentially bind to the minor groove of AT-sequences and interact with a large number of specific DNA-binding transcription factors including NF-κB, Oct-6 and SRF (see Reeves, 2001). In vivo function of these proteins awaits confirmations using tissue-specific knockouts of each HMG-containing gene.
2.4. Chromatin and transcription
2.4.1. Promoters, enhancers, locus control regions, silencers and enhancer-blocking insulators
The genomic DNA regulatory sequences for transcription include promoters, enhancers, locus control regions (LCRs), silencers and enhancer-blocking insulators. The common denominator between these elements is the presence of an array of cis-acting elements recognized by DNA sequence-specific transcription factors and the abundant presence of a variety of proteins/enzymes that remodel nucleosomes. These activities combine in various ingenious ways to dictate both the quality and quantity of diverse local chromatin structures that regulate transcriptional initiation and elongation (see Gaszner and Felsenfeld, 2006).
A promoter is a region of DNA that contains the start site of transcription surrounded by a few “basal” regulatory elements. A significant number of promoters, especially those linked to tissue-specific genes such as lens crystallins, contain a sequence TATA(T/A)A (the TATA-box) approximately 25-30 base pairs upstream from the start site of transcription. The TATA-box organizes transcriptional initiation by serving as the binding site for the TATA-box binding protein, TBP, a component of the TFIID transcriptional complex comprised of at least 11 other subunits, the TAFIIs. The largest subunit of the TFIID complex, TAFII250, is a multifunctional enzyme (see Table 1) with N-terminal kinase, internal histone acetyltransferase (HAT), and ubiquitin-activating/conjugating and C-terminal kinase domains (see Wassarman and Sauer, 2001). In addition, two bromodomains and other surfaces are used for interactions with acetylated lysine residues of core histones (Jacobson et al., 2000) and stabilization of the TFIID complex, respectively. Crystal structure of TAFII250 double bromodomain module predicts recognition of diacetylated histone H4 tails (Jacobson et al., 2000). TAFII250 as well as the majority of other TAFs contain the histone fold domain (see Table 1). However the key arginine residues in the core histones are not conserved in TAFIIs, it is unlikely that individual TAFIIs can bind DNA like histones. The second important sequence-specific DNA-binding region in many eukaryotic promoters is the initiator (Inr) with consensus sequence CTCANTCT, where A represents the start site of transcription. The Inr is recognized by TFII I (see Roy, 2001) and TAFII250 (see Wassarman and Sauer, 2001).
Nucleosomal positioning obviously controls both the TATA-box and Inr accessibility (see Segal et al., 2006). When the TATA-box is located outside of a nucleosome, binding of TFIID complex seems to occur without any obstruction. If the TATA-sequence is rotationally positioned on a nucleosome to allow for TFIID recognition, the transcription machinery can assemble at a promoter without loss of nucleosomes (Ioshikhes et al., 2006). However, a small shift in rotational positioning can move the TATA-box towards the histone surface, rendering it poorly accessible (Martinez-Campa et al., 2004). Genome-wide studies in yeast indicate that the majority of transcriptional start sites are surrounded by nucleosome-destabilizing (“antipositioning”) sequences (Ioshikhes et al., 2006). Thus, differences in nucleosomal positioning in and around a promoter may require different mechanistic solutions to recruit transcriptional machinery to the start site of transcription (see 2.5.3 and 4.2.1).
Enhancers are a class of regulatory sequences that, like promoters, possess arrays of cis-acting elements, however, they function at a distance from their target promoters to increase transcriptional activity of these promoters (see Dean, 2006). Enhancers range in length between 200 to 800 base pairs and can accommodate a string of nucleosomes. It is hypothesized that a multiprotein-enhancer complex is formed using a series of cooperative interactions between the individual DNA-binding proteins that promote recruitment of transcriptional co-activators and co-repressors possessing the chromatin remodeling activities (see below) required for transcription. The promoter- and enhancer-bound proteins can establish physical contacts through local DNA-looping as shown by electron microscopy (Stenger et al., 1994) and chromatin conformation capture (3C) studies (Splinter et al., 2004). In contrast to enhancers, silencers preferentially recruit transcriptional co-repressors and proteins stimulating chromatin condensation. Enhancer-blocking insulators interfere with enhancer-promoter communication in a position-dependent manner, i.e. when placed between the two, but not otherwise (see Gaszner and Felsenfeld, 2006). The leading model to explain their function is based on the ability of the zinc finger-containing protein CTCF to block the movement of RNA polymerase II and the spread of histone acetylation (Zhao and Dean, 2004) when multiple copies are bound to an insulator. It is also possible that CTCF proteins can aid the formation of chromatin loop domains in which the enhancer and promoter are spatially separated (see Gaszner and Felsenfeld, 2006).
LCRs are complex transcriptional enhancers that provide exquisite control over gene regulation. LCRs are distinguished from enhancers by their ability to overcome chromosomal positional effects in transgenic experiments (see Dean, 2006). As the number of well-characterized LCRs is limited (β-globin cluster, α-globin, rhodopsin X-chromosome linked locus, lysozyme and thymosin), their function is best illustrated with respect to transcriptional control of mammalian erythrocyte-specific genes. The current concept of LCRs’ activity operates with three modes. First, chromatin loops are tethered through active transcription units to a “hub” or “factory” (see Bartlett et al., 2006; Dillon, 2006; Ragoczy et al., 2006). For example, the LCR of the β-globin gene cluster is in close physical contact with the Eraf gene (encodes a α-globin stabilizing protein) in erythroid nuclei though they are 25 Mbp away from each other on the chromosome (Ragoczy et al., 2006). In contrast, this complex was not detected in brain nuclei not expressing these genes. Second, transcription factories are characterized by a local concentration of RNA polymerase that allows efficient use of the enzyme. In fact, some LCRs represent non-coding transcriptional units (Ho et al., 2006). Third, highly expressed genes such as mouse β-globin move from the nuclear periphery to the nuclear interior as erythrocyte differentiation progresses and this relocalization depends on the LCR (Ragoczy et al., 2006).
2.4.2. Histone modifications and histone-modifying enzymes
Postranslational modifications of histones serve as a major source of epigenetic information which controls the regulation of gene expression (see Fig. 1B). Covalent modifications of histone tails result in structural and conformational changes of these regions that enable histone tails to engage in diverse sets of novel physical interactions with non-histone proteins involved in transcription. The “bulk” histones (H2A, H2B, H3 and H4) are encoded by multi-copy intron-less genes transcribed by RNA polymerase II into nonpolyadenylated mRNAs. The N-terminal tail of histone H3 comprised of 81 amino acid residues contains at least 14 residues with known modifications (see Fig. 1B). Multiple modifications are allowed to occur simultaneously within the same histone. Some residues, e.g. lysine 4, 9 and 27 of H3 can be either acetylated (ac) or methylated (me) and each lysine residue can be modified by one (me1), two (me2) or three (me3) methyl groups (Turner, 2005). In addition, an arginine residue can be either mono- or di-methylated. Posttranslational modifications of histones have both positive and negative impacts on transcription.
The biochemical and genetic studies of HATs, HDACs and HMTs have resulted in an enormous expansion of information about histone metabolism and gene regulation during the last decade (see Mellor, 2005; Mellor, 2006a; Mellor, 2006b; Peterson and Laniel, 2004; Workman, 2006). Over 20 mammalian HATs are classified in seven distinct families (see Marmorstein and Roth, 2001). The most extensive studies were performed with p300 and CREB-binding protein, CBP. Despite their high degree of similarity, p300 and CBP are not redundant and have unique roles in vivo (Kasper et al., 2006). Both p300 and CBP interact with lens-regulatory factors such as CREB (Chrivia et al., 1993), Pax6 (Hussain and Habener, 1999) and c-Maf (Q. Chen et al., 2002). Mutations in human CBP and less frequently in its homologue gene p300, cause Rubinstein-Taybi syndrome, which manifests with a variety of ocular defects including cataracts (van Genderen et al., 2000). Both p300 and CBP have been shown to catalyze acetylation of H2A (K5), H2B (K12 and 15), and H3 (K18 and 23) in vitro. In addition, p300 has been shown to acetylate H2B (K20), H3 (K14) and H4 (K5 and 8). The most widely-studied acetylations of H3 (K9 and 27) are catalyzed by PCAF. Acetylation of H3 K14 is also catalyzed by PCAF, Esa1 and SRC1 (see Marmorstein and Roth, 2001). Chromatin immunoprecipitations (ChIPs) using antibodies recognizing acetylated histone tails (e.g. H3 K9, H2A K4 and H2B K5, see Fig. 1B) have shown that increased acetylation in promoters of specific genes correlates with recruitment of HATs and transcriptional activity.
In contrast, hypoacetylation of specific promoters has similarly been correlated with recruitment of HDACs and reduced transcription originating from hypoacetylated promoters (Zupkovitz et al., 2006). HDACs often interact with the co-repressor Sin3, a commonly used bridging protein used by transcriptional repressors such as pRb, Mxi1 and Dach1. Although histone deacetylation is generally associated with transcriptional repression, loss-of-function studies of HDAC1-deficient ES cells identified a small number of genes that require recruitment of HDAC1 for their transcriptional activation (Zupkovitz et al., 2006). In addition, a group of cytokine-inducible genes are also positively regulated by HDACs (Nusinzon and Horvath, 2005). Given the complexity of the primary sequences of core histones, the growing list of specific acetylation events and the lack of genetic data for individual tissues including the lens, a large body of work remains to be performed in this field.
Although the total number of methylated arginine and lysine residues in core histones is lower than the number of acetylated lysine residues (see Fig. 1B), the biology of methylated histones is more complex due to the increased stability (i.e. lower turnover) of this modification and the three potential stages of methylation. Histone methyltransferases (HMTs) are represented by two families of enzymes, the protein lysine methyltransferases (PKMTs) and protein arginine methyltransferases (PRMTs). Demethylation of di- and tri-methylated lysines is catalyzed by a family of jumonji (jmjC) domain enzymes (see Klose et al., 2006a; Klose and Zhang, 2007).
All PKMTs contain an evolutionary conserved 130 amino acid SET domain (See Table 1) and can be grouped into seven subfamilies – the SUV39, SET1, SET2, EZ, RIZ, SMYD and SUV4-20 – as well as a few orphan members such as SET7/9 and SET8 (see Dillon et al., 2005). Particular members of each subfamily differ by the presence of additional domains that include the chromodomain, bromodomain, PHD fingers, AT-hook and others (see Table 1). These domains are thought to provide specificity via interactions with other proteins and DNA. H3 K4 methylation is mostly associated with transcriptional activation. In contrast, H3 K9 and H3 K27 methylations function in transcriptional repression. Genetic studies of Suvar39h1 and ESET (SUV39 subfamily, H3 K9 methylation) showed reduced viability (Peters et al., 2001) or preimplantation lethality (Dodge et al., 2004). Inactivation of G9a (SUV39 subfamily, H3 K27 methylation is lethal at E8.5 (Feldman et al., 2006). Expression of MLL3 (SET1 subfamily) was shown in the developing eye, particularly in the optic vesicles (E9.0), in the lens vesicle and in primary lens fibers up to E14.5, and in many other tissues (Brun et al., 2006). This enzyme is a component of a large multisubunit complex ASCOM, a coactivator complex of nuclear receptors. The complex includes activating signal cointegrator 2 (ASC-2) with a pair of LXXLL motifs (Plevin et al., 2005) (see Table 1) that was earlier shown to participate in regulation of mouse lens development and crystallin gene expression (Kim et al., 2002).
Histone arginine methylation is catalyzed by at least six PRMTs with PRMT1 and CARM1/PRMT4 studied at both biochemical and genetic levels. PRMT1 methylates Arg3 on histone H4 and is involved in nuclear-receptor-mediated transcriptional activation (Strahl and Allis, 2000). The glucocorticoid receptor-associated CARM1/PRMT4 methylates histone H3 at arginine residues 2, 17 and 26 (Strahl et al., 2001). Gene targeting in mice demonstrated that homozygous embryos were grossly normal at E12.5, however, they exhibit perinatal lethality (Yadav et al., 2003). Expression of these enzymes and their function in the mammalian eye remains to be studied.
The identification of histone lysine demethylases remained elusive until the discovery of LSD1 (lysine specific demethylase 1), a nuclear FAD-dependent amine oxidase specific for mono- and di-methylated H3 K4 residues (Shi et al., 2004). LSD1 is a component of various transcriptional co-repressors complexes that often contain HDAC1/2 enzymes and CoREST, providing the DNA-binding subunit to the complex. An alternate way to reverse histone monomethylation is a calcium-dependent conversion of the methylated arginine residues in core histones H3 and H4 by peptidyl arginine deaminase (PAD4) into citrulline (Wang et al., 2004c).
Demethylation of di- and tri-methylated lysine residues in histones is catalyzed by a family of JmjC domain-containing oxidases that require Fe2+, α-ketoglutarate and generate formaldehyde and succinate byproducts (see Klose et al., 2006a; Klose and Zhang, 2007). These enzymes are comprised of an N-terminal ARID domain (see Wilsker et al., 2002) followed by the JmjC demethylase domain, and one or more internal PHD domain (see Table 1). The JMJD2 group also contains a pair of C-terminal tudor domains recognizing methylated histones (see Table 1). JHDM1 is specific for H3 K36me2 histones and it can also demethylate K36me1 (Tsukada et al., 2006). The demethylation of H3 K9me3 and K36me3 residues is catalyzed by a JMJD2 group of four enzymes (Whetstine et al., 2006). Overexpression of JMJD2A/JHDM3A abrogates recruitment of HP1 to heterochromatin (see 2.3.2.). In euchromatin, this enzyme may function to remove histone methylation marks that are associated with active transcription (Klose et al., 2006b). JMJD2A has been recently shown to associate with pRb, HDACs and the co-repressor NcoR suggesting a number of possibilities by which JMJD2 can be recruited to the nucleosomes (Whetstine et al., 2006). The JHDM3/JMJD2 group can demethylate both di- and tri-methylated H3 K9 and K36 histone tails (see Klose and Zhang, 2007)). Four members of JARID1 family, JARID1a/RPB2, JARID1b/PLU-1, JARID1c/SMOX, JARID1d/SMCY, catalyze demethylation of H3 K4me3 and me2 (Christensen et al., 2007; Iwase et al., 2007; M. G. Lee et al., 2007). Thus, elaborate (Christensen et al., 2007; Iwase et al., 2007; M. G. Lee et al., 2007) enzymatic systems exist within the cell to catalyze reversible histone acetylations and methylations. The current view of the functional role of histone tail modifications is best described by the “histone code” hypothesis as described in 2.4.4.
2.4.3. ATP-dependent chromatin remodeling
Relocation of nucleosomes by ATP-dependent chromatin remodeling is catalyzed by at least five families of complexes: SWI/SNF, ISWI, INO80, SWR1 and NuRD (see Saha et al., 2006). Each complex contains a different ATPase, i.e. Brg1 or Brm (SWI/SNF), Snf2h or Snf2l (ISWI), or one of the nine variants of CHD3/4 (NuRD). All remodeller ATPase domains (see Table 1) belong to the superfamily II of evolutionary conserved DEAD/H-box helicases and translocases (see Saha et al., 2006).
The mammalian SWI/SNF complexes, SWI/SNF-A and SWI/SNF-B/PBAF, contain either Brg1 (Brahma-related gene 1, other gene names are Snf2β and SMARCA4) or Brm (Brahma/Snf2α is a Drosophila homologue of yeast SWI2/SNF2), as their ATPases. Brg1 is a large enzyme (205 kD) with a C-terminal bromodomain and an AT-hook domain. The SWI/SNF-A complex contains seven non-catalytic subunits (BAF250/OSA1, BAF170, BAF155/SRG3, BAF60a/b/c/d, Ini1, BAF50 and BAF47. The SWI/SNF-B complex also contains BAF180/polybromo subunit with six bromodomains (see Table 1). Both Brg1 and Brm are ubiquitously expressed although elevated expression of Brg1 was detected in mouse retinal ganglion cells and cerebellum (Randazzo et al., 1994). In lens, Brg1 is expressed both in lens epithelium and lens fibers (Chauhan et al., 2002). Expression of Brm in lens was not spatially evaluated, however, RT-PCR studies revealed its expression in the adult mouse lens (Chauhan et al., 2002). Expression of the regulatory subunit BAF250/OSA1was shown in cultured lens epithelial cells (Kozmik et al., 2001) and recent studies of cancer cells suggested its critical role for cell cycle arrest (Nagl et al., 2006). Genetic studies in mouse have shown that Brg1 is indispensable for early mouse development as embryos die during the preimplantation period (Bultman et al., 2000). The zebrafish mutant young (yng) harbors a Brg1 mutation and exhibits impaired retinal differentiation (Gregg et al., 2003). In contrast, inactivation of Brm does not compromise mouse embryonic development and leads to an increased rate of cellular proliferation in adult mice (Reyes et al., 1998). Inactivation of a single allele of BAF155/SRG3 in mice yielded defects in neuronal development (Kim et al., 2001).
Three types of ISWI complexes, RSF, ACF/WCRF and CHRAC who share the common ATPase subunit Snf2h (other name SMARCA5) were found in mammalian cells. Snf2h is a 135 kD protein with C-terminal SANT and SLIDE domains (Table 1) which are thought to bind methylated histones (Boyer et al., 2004). The RSF and ACF/WCRF complexes contain only one additional subunit, p325 or ACF/WCRF180, respectively. The CHRAC complex is similar to the ACF/WCRF complex as it contains two additional subunits, p15 and p17. As with Brg1 (Bultman et al., 2000), Snf2h (Stopka and Skoultchi, 2003) is essential for early embryonic development and is expressed in many tissues and cells including the lens and retina (Yang et al., 2006). Inhibition of ISWI activity in fertilized Xenopus eggs using morpholinos specific to Snf2h results in aberrant neural and eye development and cataract formation (Dirscherl et al., 2005). In contrast to Snf2h, Snf2l is expressed only in terminally differentiated neurons, ovaries and testes. Thus, Snf2l and variants of BAF60 are best-known examples of tissue-specific subunits of ATP-dependent chromatin remodeling complexes.
The mammalian NuRD (nucleosome remodeling and histone deacetylase) complex is composed of eight subunits: CHD3/4 (other name Mi-2) ATPase, HDAC1, HDAC2, MBD3, MTA1, MTA2, RbA (p48) and RbA (p46) (Bowen et al., 2004). Mi-2 is a 200 kD enzyme with an N-terminal PHD finger domain and internal chromodomain (Table 1). The NuRD complex was shown to act in both transcriptional repression (via HDACs) and activation via cooperation with HATs. However, to date there are no data suggesting roles of the NuRD complex in ocular development.
A series of studies in vitro and in cultured cells have addressed the mechanistic aspects of chromatin remodeling. Studies of recombinant Brg1, Snf2h and Mi-2 showed that these enzymes alone are capable of moving nucleosomes in vitro with specific activities within 15-fold of each other (Narlikar et al., 2002). The yeast SWI/SNF complex can slide a nucleosome from the end of a 2 kb long DNA fragment to several internal positions (Whitehouse et al., 1999). In addition, both yeast and human enzymes can catalyze trans-displacement of a nucleosome (Lorch et al., 1999), a mechanism that is likely to be important to remove nucleosomes from promoters and other regulatory regions. In vitro experiments also suggested that SWI/SNF complexes can alter conformation of the nucleosome by changing either the DNA contacts with the surface of histone octamer, the shape of the nucleosome, or both (Narlikar et al., 2002; Saha et al., 2006) as shown in Fig. 2. SNF/SWI and Mi-2 but not Snf2h-containing complexes can remodel nucleosomes assembled from histones lacking their N-terminal tails. It was shown that the histone H4 N-terminal tail is critical for stimulation of ISWI ATPase activity but not for binding of ISWI (Clapier et al., 2001). The current data on Snf2h catalyzed chromatin remodeling indicates that the most likely mechanism to carry out this process is sliding of the DNA (see Narlikar et al., 2002). In summary, a large number of distinct ATP-dependent chromatin remodeling complexes provide the cells a tool kit to execute a variety of specific differentiation programs.
Figure 2. Diagrammatic representation of three types of chromatin remodeling.

(A) ATP-dependent chromatin remodeling resulting in nucleosome eviction. (B) ATP-dependent conformational change of the nucleosome core. (C) Incorporation of core variant histones H3.3 into the RNA polymerase II transcribed region via histone chaperone complex, HIRA, is a replication-independent process to “mark” transcriptionally active regions.
2.4.4. “Histone code” hypothesis
In 2000, Allis and co-workers proposed that a particular combination of histone marks dictate the same biological function (Strahl and Allis, 2000). The rationale for this hypothesis was that histone modifications control the structure, and, hence, the function of chromatin fibers, with different modifications influencing transcription either positively, neutrally or negatively. The current data confirm that specific patterns of histone marks are indeed associated with transcriptional activation and repression. Four examples of specific histone code outputs are shown in Fig. 3 (see Jenuwein and Allis, 2001; Margueron et al., 2005; Nightingale et al., 2006). The formation of histone modifications is often referred to as a “writing” phase of the histone code (Wang et al., 2004b). In the “reading” stage, a class of reader proteins equipped with a repertoire of recognition domains such as the chromodomain and bromodomain (see Table 1) is recruited to DNA (see Mellor, 2006b). Three examples follows: Acetylation of lysine 8 in histone H4 is recognized by the chromatin remodeling complex SWI/SNF, via a bromodomain in the Brg1 subunit. Dimethylation of lysine 9 in histone H3 is recognized by heterochromatin protein 1 (HP1) via its chromodomain (Table 1) resulting in chromatin condensation. In contrast, dimethylation of lysine 27 of the same histone recruits the repressive Polycomb (PcG) complex via its EZH2 subunit and its chromodomain.
The definition of the epigenetic code is a complex problem as the number of possible combinations is enormous compared to the triplet codon code used for protein translation which is built from only four bases (see Turner, 2007). The link between embryonic development, transcriptional memory and epigenetic code resulted in the following definition: “The epigenetic code describes the way in which the potential for expression of genes in a particular cell type is specified by chromatin modifications put in place at an earlier stage of differentiation” (Turner, 2007). Thus, the histone code can be viewed as a specific type of epigenetic code that regulates development. To fully appreciate the extent of epigenetic and histone codes and to decrypt their set of rules in vivo will require the tissue-specific inactivation of individual histone modifying enzymes, determination of how signaling cascades regulate these enzymes, mutagenesis of specific residues in core histones and the introduction of mutated histones and histone code readers using transgenes.
2.5. Chromatin and development
2.5.1. DNA-binding or remodeling: What comes first?
Earlier we stated that chromatin remodelers are recruited to promoters and enhancers via specific-DNA binding proteins such as c-Maf, CREB and Pax6 and/or by reading the histone code. As this remodeling actually facilitates the binding of these transcription factors to DNA, the question is; How does this process start? The answer to this question depends on the chromatin structure of each individual locus that is inherited through the cellular memory. As we show in the next section, lineage-specifying regulatory genes have a very unique structure in ES cells. Thus, it appears that these genes are poised for transcription from the beginning of embryonic development. In contrast, genes that are found in closed chromatin require “decisive/pioneering” DNA-binding factors (see Cosma, 2002; Lomvardas and Thanos, 2002), such as Fox/HNF3 and GATA4 which can initiate the opening of closed chromatin during liver cell differentiation. As the known decisive/pioneering factors are in the group of genes with poised chromatin structure, the cell possess the capacity to open closed chromatin when needed. However, it remains to be determined which ocular cell lineage-specifying proteins posses this important activity. Notably, Lhx2, Pax6, Rx, Six3 and Sox2 all act at early stages of eye development and should be considered as candidate “decisive/pioneering” factors as we proposed earlier for Pax6 and lens lineage formation (Yang et al., 2006).
2.5.2. Regulation of chromatin structure at the beginning of embryonic development
Mammalian development requires the specification of over 260 unique cell types from a single totipotent cell. The initial division and subsequent expansion of cells from the fertilized egg results in the formation of a blastocyst (Boyer et al., 2005). A group of cells, the inner cell mass, can be propagated in culture in an undifferentiated state as embryonic stem (ES) cells which have the potential to give rise to every cell lineage of the adult organism. Hence, ES cells can be used for studies of chromatin structure and epigenetic regulatory mechanisms corresponding to the beginning of embryonic development.
A series of recent studies provided novel insights into the transcriptional regulation of key regulatory (master/selector) genes of development including those that control lens and ocular development (see chapter 3) in human ES cells (Bernstein et al., 2006; Boyer et al., 2005; Lee et al., 2006). It was shown that the ES cell identity is determined by a regulatory network in which central roles are played by a small number of genes encoding transcription factors including OCT4, SOX2, NANOG and c-MYC (see Chambers, 2004). ChIP on chip studies identified 353 genes co-occupied by OCT4, SOX2 and NANOG, a significant proportion of which encode homeodomain transcription factors including PAX6, LHX2, OTX1, MEIS1 and DLX5 which are all involved in the formation of the eye as described in detail in Chapter 3. Despite this occupancy, many of these lineage specific genes were not expressed, in fact their repression in ES cells is essential to maintain ES cell pluripotency (Boyer et al., 2005). Recently, it was demonstrated that the occupied, but repressed genes are also occupied by SUZ12, and by inference, the Polycomb Repressive Complex 2 (PRC2) (T. I. Lee et al., 2006), which, in combination with the histone H3K27 methyltransferase EZH2 causes H3 K27 methylation of these loci resulting in transcriptional repression. The important conclusions of these studies are that the key regulatory genes of development are already occupied by specific DNA-binding transcription factors in chromatin of ES cells and that their repressed state is due to a specific type of core histone H3 modification recognized by the specific multiprotein PcG complex, PCR2.
Another important property of the chromatin structure of genes encoding lineage-specific transcription factors emerged from the co-analysis of histone H3 K27 (associated with transcriptional silencing) and H3 K4 (associated with transcriptional activity) methylation profiles in mouse ES cells. Notably, about three quarters of the H3 K27 methylated, and transcriptionally silenced regions simultaneously contained H3 K4 methylated domains (Bernstein et al., 2006). These “bivalent domains” were found in 119 genes that control development including the regulators of eye development Pitx3, Pax6, Otx2, Pax2, Six3, Shh, FGF8, Pitx2 and Prox1. When more differentiated cell lines were analyzed, these bivalent domains largely resolved into expanded regions of either H3 K27 or K4 methylation depending on the transcriptional status of individual genes. This indicates that the transcriptional activation of these key regulatory genes as ES cells undergo further development does not start from the ground state of heterochromatin, but rather from chromatin domains “poised” for transcription in response to internal and external signals that govern individual differentiation programs (Bernstein et al., 2006).
2.5.3. Chromatin remodeling and terminal differentiation
Genes encoding products of terminal differentiation are transcriptionally repressed in both ES cells and early progenitors of the differentiated lineage (Bernstein et al., 2006). As their “ground” state is heterochromatin, the coordinated action of distinct chromatin remodeling activities is thought to be necessary for their robust activation during cellular differentiation. Although the number of genes studied at this level of precision is very small compared to the number of individual cell types, some generalizations are possible.
Chromatin remodeling of the myogenin (Myog) locus during skeletal muscle differentiation (de la Serna et al., 2006) and the β-globin locus during erythropoiesis (Bultman et al., 2005; Im et al., 2005) serve as representative models to illustrate the interplay between distinct chromatin remodeling activities. The Myog locus is constitutively occupied by a heterodimer of Meis/Pbx homeodomain-containing proteins in undifferentiated cells (see de la Serna et al., 2006). Muscle-specific expression of MyoD is triggered with the onset of differentiation and is first recruited to the myogenin promoter via protein-protein interactions with Pbx while the E-box binding sites of the myogenin promoter are in an inaccessible chromatin environment. MyoD then sequentially recruits HATs to the myogenin locus that acetylate both the promoter histones and MyoD itself (see de la Serna et al., 2005). Acetylation of lysine 8 in histone H4 recruits the chromatin remodeling complex SWI/SNF, via a bromodomain in the Brg1 subunit which decondenses the chromatin of the Myog locus. The transcription factors MyoD and MEF2 then can access their multiple E-box targets in the Myog locus facilitating transcription (Ohkawa et al., 2006). In contrast, three different chromatin remodeling complexes have been demonstrated to be important for the regulation of the mammalian β-globin locus. The erythroid-specific factor ELKF recruits the SWI/SNF complex to this locus (Bultman et al., 2005; Kim et al., 2007; Lee et al., 1999) while the DNA-binding hematopoietic-regulator Ikaros is a component of the PYR complex which also contains SWI/SNF and NuRD proteins (O’Neill et al., 2000). Antisense knockout of Snf2h in cultured cells also interfered with normal erythropoiesis although the precise mechanism of this enzyme remains to be determined (Stopka and Skoultchi, 2003). However, studies of more loci are required to fully understand complexity and logic of chromatin remodeling processes.
3.Embryonic lens development
Lens development has been a very attractive model for embryological studies for over one hundred years (see Grainger, 1992). With the advent of molecular biology complemented with mouse genetics, studies of lens development are progressing towards a full understanding of the genetic and epigenetic regulatory mechanisms that underlie its formation. The vertebrate lens first appears as a disk of ectodermal cells, the lens placode, recruited from the head ectoderm of the neurula stage embryo. At this developmental stage, the columnar lens progenitor cells are in close apposition with the outgrowing optic vesicle, an evaginated structure of the embryonic forebrain (diencephalon) (see Lang and McAvoy, 2004). Genetic studies in mouse have shown that optic vesicles are essential for normal embryonic lens formation (see Medina-Martinez and Jamrich, 2007) but are not necessary for ectopic lens formation (see Donner et al., 2006b).
3.1. Establishment of lens progenitor cells
3.1.1. Formation of neural plate and general pre-placodal region
Patterning events during gastrulation and early neuralization (see Dawid, 2004; Weinstein and Hemmati-Brivanlou, 1999) lead to the subdivision of the ectoderm into at least four distinct domains: neural plate, neural crest, pre-placodal region (PPR) and primitive epidermis (see Fig. 5) (see Litsiou et al., 2005). The PPR is defined as a transient structure comprised of multipotent progenitor cells that exhibit “placodal competence” (see Streit, 2004), the ability of any portion of the PPR to develop into any of the placode derivatives, if placed under the influence of specific signals that are generated at specific regions of the developing embryo (Jacobson, 1963a; Jacobson, 1963b; Jacobson, 1963c). The lens lineage originates from the PPR which also gives rise to the anterior pituitary, olfactory neurons, inner ear and the trigeminal and epibranchial cranial placodes (see Streit, 2004) as shown in Figs. 4 and 5. All cranial placodes, with the exception of anterior pituitary and lens placodes, are “neurogenic”, a term indicating that these placodes generate neuronal cells such as olfactory-receptor neurons and vestibuloaccoustic ganglion cells (see Graham and Begbie, 2000). In addition, some of the neurogenic placodes also generate sensory receptor cells and sensory epithelia. Thus, the number of distinct types that originate from the PPR cells is diverse except for the lens placode that give rise only to undifferentiated lens epithelial cells and differentiated lens fibers.
Figure 5. Lens development: the earliest stages.
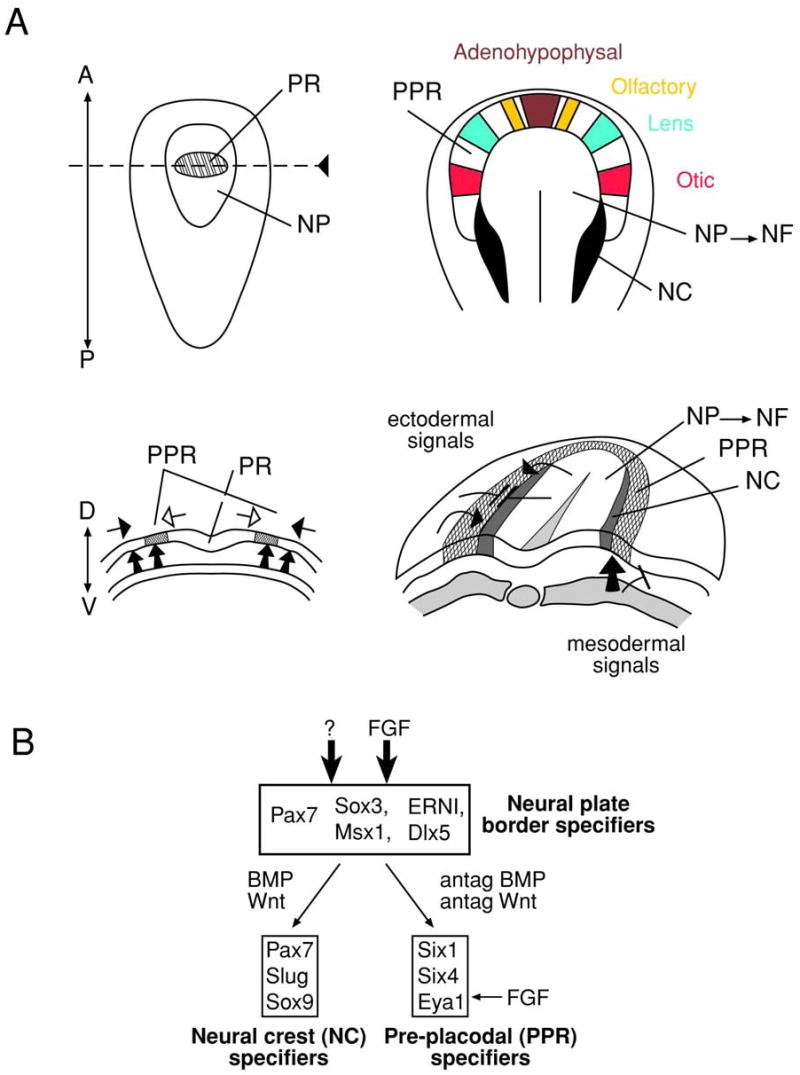
(A) Neural plate stage (left) and transition from neural plate to neural folds (right). The position of future placodes in the PPR is indicated by colors: adenohypophysal (brown), olfactory (yellow), lens (green) and otic (red). The signals that are thought to form the PPR are shown by arrows. The candidate signaling pathways/molecules are discussed in text. Adopted from (Litsiou et al., 2005). Neural crest, NC; neural folds, NF; neural plate, NP; pre-placodal region, PPR; prospective retina, PR.
Figure 4. Schematic diagram of ocular-cell lineage formation during vertebrate development.

Tissues with ectopic lens formation (eLens) and a possibility of lens transdifferentiation (tdLens) are shown in blue and green, respectively. The ectopic lens formation can occur as a result of perturbed sonic hedgehog (see anterior pituitary) and Wnt signaling (see ectoderm). Missexpression of Pax6 (see ectoderm) and Six3 (see otic placode) can also produce ectopic lenses. Transdifferentiations of corneal epithelium, iris and RPE into lens illustrate reversibility of cellular memory and of epigenetic regulatory mechanisms.
The PPR is first established at the neural plate stage as a narrow band of cells that encircles the anterior neural plate (Fig. 5) by active FGF signaling in parallel with the inhibition of BMP and Wnt signaling provided by both the lateral mesoderm and neuroectoderm. It is hypothesized that the same anti-BMP factors secreted from the dorsal midline mesoderm, noggin and Cerberus, that establish the neural plate also participate (Kuroda et al., 2004) in the formation of PPR at the dorso-ventral axis (Brugmann et al., 2004; Litsiou et al., 2005). These signals influence expression of Six1 (see below), a homeodomain-containing transcriptional activator and repressor (Brugmann et al., 2004).
At present, the only known molecular markers of the PPR are members of the Six and Eya families of regulatory genes. Six 1 and Six4 are the PPR markers in chick, Xenopus and zebrafish (Bailey and Streit, 2006; Baker and Bronner-Fraser, 2001; Schlosser and Ahrens, 2004) while the phosphatase co-activator Eya1 marks the Xenopus PPR while Eya2 is expressed in the chick (Mishima and Tomarev, 1998) and zebrafish PPR (Schlosser, 2005). It should be noted that published reports state that the expression of all of these genes is initiated much later in the mouse embryo so PPR markers in mouse are not well described (Oliver et al., 1995b; Xu et al., 1997; Zou et al. 2004). However, a recent study detected expression of Six3 in the PPR of five somite mouse embryos (~E8.0) (Liu et al., 2006) although earlier studies of this gene reported its earliest expression in the lens placode (Oliver et al., 1995a). In addition, the forkhead transcription factor Foxg1/BF-1 is expressed in a broad region corresponding to the PPR in one to three somite mouse embryos (Hatini et al., 1999) and Foxg1-/- mice exhibit a plethora of eye patterning defects (Huh et al., 1999). However, the functional role of these markers in the PPR of all species studied (see Schlosser, 2005) is not known since they are expressed in other structures and tools to specifically inactivate gene function in the PPR have not been developed (Bhattacharyya et al., 2004). Thus, studies to understand the process of PPR cell lineage formation will require the identification of key signals that traffic between the neural plate, naïve ectoderm and mesoderm that dictate the specific gene expression profile that is required to establish the PPR.
3.1.2. Formation of the neural tube, optic vesicles and lens progenitor cells
Neural fold elevation occurs simultaneously with the formation of the foregut from the endoderm and the cardiac mesoderm from the naïve mesoderm. The three-dimensional positioning of the foregut endoderm/cardiac mesoderm/head ectoderm generates a novel arrangement of tissues and signaling sources that are likely to control the developmental fates of the PPR. Embryological experiments in Xenopus have shown that a broad domain of surface head ectoderm of neural fold stage embryos acquires a lens-forming bias (see Fisher and Grainger, 2004) that may coincide with the entire PPR defined above (see Streit, 2004).
Next, the neural folds form an open neural tube (Fig. 6A) subdivided into four prospective regions corresponding to the forebrain, midbrain, hindbrain and spinal cord. Mesoderm and endoderm derived tissues retract from the head region resulting in the neural tube and neural crest derived mesenchyme underlying the PPR (Fig. 6). Consequently, the most anterior part of the PPR forms a single Rathke’s pouch, a future anterior pituitary, and a pair of olfactory placodes due to the influence of the anterior neural ridge (see Baker and Bronner-Fraser, 2001). The remainder of the PPR becomes bilaterally restricted and additional region-specific signals direct the PPR to develop into the appropriate cell lineages (see Bailey and Streit, 2006; Baker and Bronner-Fraser, 2001; Noramly and Grainger, 2002; Streit, 2004; Torres and Giraldez, 1998).
Figure 6. Lens development: formation of lens progenitor cells and the lens placode.

(A) The part of PPR shown here give rise to the prospective lens ectoderm and may be already specified as a lens lineage, see text. The optic sulci (OS) are morphologically distinct regions of the prospective forebrain. The precise contact between the optic vesicle with the surface ectoderm determines location of the lens placode. (B) The optic vesicle (OV) reaches the prospective lens ectoderm (PLE) and mesenchymal cells (Me) are excluded from the region of contact of OV and PLE (E9.5). The lens placode (LP) invaginates (iLP) and is engaged with the optic vesicle in a reciprocal process to form the lens vesicle and optic cup (OC).
The lens placodes are formed bilaterally when the optic vesicle outpouchings from the early diencephalon come into close proximity to the PPR (see Medina-Martinez and Jamrich, 2007). Lens placode formation in mouse requires a sequential activation of Six3 (Liu et al., 2006), Pax6 (Li et al., 1994; Walther and Gruss, 1991) and Sox2 (Furuta and Hogan, 1998; Kamachi et al., 1998; Kamachi et al., 2001) expression that is ultimately co-localized in the prospective lens placode (see Lang, 2004). Pax6 expression in the prospective lens ectoderm (PLE) is dependent upon BMP7 expression. BMP4 is expressed in the surface ectoderm and the directly adjacent mesenchymal cells prior to the appearance of the lens placode (Wawersik et al., 1999). The expression of Sox2 (Wawersik et al., 1999), a gene with a range of roles during development as described for ES cells (see 2.5.2.) and in other systems including the retina (Taranova et al., 2006) is dependent on BMP4 produced in the optic vesicle (Furuta and Hogan, 1998). In mice, αB-crystallin (Robinson and Overbeek, 1996) and N-cadherin (van Raamsdonk and Tilghman, 2000) expression are the hallmarks of lens placode formation while chickens first express δ-crystallin in this structure (Kamachi et al., 2001). In contrast, the more caudal otic placodes, formed prior to the lens placodes, are marked by Pax8 and Sox2 expression (see Baker and Bronner-Fraser, 2001). The otic placodes are formed under the influence of signals sent by the prospective hindbrain to the more posterior PPR (Noramly and Grainger, 2002; Silver and Rebay, 2005; Torres and Giraldez, 1998).
The diversity of cell lineages originating from the PPR cells may result from a relatively homogenous population of common progenitors, or from a number of smaller patches of cells that are biased towards a specific lineage depending on their location within the pre-placodal field (Bailey and Streit, 2006; Graham and Begbie, 2000; Streit, 2004; Whitlock, 2004). To address this issue, cell-fate mapping experiments examining position of cells during individual placode formation have been performed in chicken and zebrafish. Using caged fluorescein injected at the 50% epiboly stage of the zebrafish embryo, cell-fate mapping generated a picture of partially overlapping cell territories supporting the model postulating that the PPR undergoes modest anterio-posterior patterning (Kozlowski et al., 1997). Injection of single cells with a mixture of fluorescein/rhodamine dyes into the neural plate of zebrafish embryos revealed that the olfactory placodes arise from a broad field of cells posteriorly limited by the emerging neural crest cells (Whitlock and Westerfield, 2000). No evidence for the formation of a discrete group of cells and their expansion by proliferation was found.
In chicken, cell-fate mapping of cells destined to form the otic (Streit, 2002) and lens/olfactory (Bhattacharyya et al., 2004) placodes was examined using the fluorescent dyes DiI and DiO injected into small groups of epiblast (ectoderm) cells at the neural plate stage (0-1 somite, stage HH6). Large movements of cells within the PPR were observed supporting a model in which cells segregate over time to converge into their final positions within individual placodes by a set of directed movements (Bhattacharyya et al., 2004). In general, prospective lens cells migrated against the main stream of cells suggesting the presence of local signals. However, as lens placodes were assembled from cells coming from both anterior and more posterior points of origin, it is unclear how the local signals could have worked. One possibility is to assume that the signals controlling cell lineage commitments within the PPR are limited in their amounts. Thus, each individual cell “competes” for these limited amounts of growth factors with other cells and sequesters them from its microenvironment. As a result of this stochastic process, a heterogeneous population of biased cells for each individual placode emerge at different points of origin. Next, these cells initiate the expression of specific cell-surface receptor molecules that are engaged in directed cellular migration and/or cell-sorting events.
The epigenetic/chromatin approach to this problem shows that ectoderm/endoderm/mesoderm lineage-specific genes in ES cells are “poised” for expression (see 2.5). With the onset of gastrulation, the chromatin of these genes is cleared of their repressive histone marks. The current data generated with ES cells predict that the placode-specific genes such as DLX5, MEIS1 and PAX6 maintain their “bivalent” domain status during gastrulation (Bernstein et al., 2006) while waiting for the appropriate extracellular signals to shed off H3 K27me and possibly other repressive histone marks. This transitional state can be linked operationally to the state of developmental “competence” for lens and otic placode formation as precisely evaluated by Grainger and co-workers using Xenopus as model (Gallagher et al., 1996; Henry and Grainger, 1987; Henry and Grainger, 1990). Lens-forming competence was observed at the ectoderm of mid to late gastrula stages, even prior to the formation of the neural plate. Lens forming bias, observed during elevation of the neural folds, should correspond to the onset of expression of the lens lineage-specifying genes, Six3, Pax6 and Sox2, accompanied by formation of active euchromatin within these loci and by heterochromatization of those genomic regions that contain regulatory genes for other developmental programs.
Lens and otic placode formation share common and distinct modalities (Gallagher et al., 1996). The naïve (unbiased) ectoderm first becomes “competent” to form the lens (Henry and Grainger, 1987; Henry and Grainger, 1990) and otic placodes (Gallagher et al., 1996), after which follows the bias-stage of their cell fate commitment. The differences between the two developmental programs include timing, duration and the relative intensity of their respective “inducing” signals. Otic placode formation takes place earlier than lens placode induction, and ear-inducing signaling persists at least throughout the neural tube stages. In contrast, the lens forming response, as evaluated by percentages of lens cells generated from the transplanted ectoderm, begin later and is attenuated in embryos reaching the neural tube stage (Gallagher et al., 1996). It appears that cell-sorting mechanisms coincide with the early “specification” phase of individual placode formation, while the arrival of cells to their final destination can be considered as the “late” specification/determination phase. Consistent with this, expression of Pax6 is initially observed in a broad domain of PPR that includes the prospective adenohypophysal/olfactory/lens placode region (Bhattacharyya et al., 2004; Walther and Gruss, 1991) and restriction of this Pax6 expression domain represents the “specification/inhibition” phase of lens formation (see Grainger, 1992; Fisher and Grainger, 2004). Although Pax6’s expression domain initially overlaps with the Dlx5 domain in future chicken olfactory and lens cells, expression of Pax6 is lost in the olfactory placode in conjunction with loss of Dlx5 expression in the presumptive lens surface ectoderm (Bhattacharyya et al., 2004). Thus, these regional changes of specific gene expression are likely to govern cell movements prior the formation of individual placodes.
Recently, Bailey and co-workers separated the chicken PPR from head fold stage embryos (HH6, 23-25 hours) into four regions, each containing a mixture of precursors for adenohypophysal, olfactory, lens, otic, trigeminal and epibranchial placodes (Bailey et al., 2006). After 72 hours in explant culture, they found that all types of explants generated lentoid bodies expressing δ-crystallins. In normal chick embryos, the onset of δ-crystallin expression occurs at about 42 hours of development, which is just a few hours after the optic vesicle contacts the surface ectoderm (see Piatigorsky, 1987). The authors propose that the placode precursors are initially specified as lens progenitors (Bailey et al., 2006). These data were used to support a two-step model in which the PPR ground state established at the neural plate stage (and prior to the formation of optic vesicles) is sufficient for lens formation, and suppression of lens fate is required for the formation of other placodal derivatives (Bailey et al., 2006). However, this model contradicts the analysis of transplantation studies in Xenopus showing that otic bias is established prior to lens placode bias (Gallagher et al., 1996). The fact that these studies were conducted in different species under separate embryological manipulations may account for the discrepancies found in the above studies.
The suppression activities against the “default” lens fate are thought to be required for both the correct positioning of the lens and the optic cup and for the continuation of other developmental programs originating from the cells forming the PPR (Bailey et al., 2006). Although FGF signaling is required for the formation of the PPR (see 3.1.1.), data from chicken, fish and mouse suggest that FGF signaling transiently represses lens specification in major parts of the PPR (“the first step in repression”) and, in parallel, is required for the expression of early markers of adenohypophysis and of both the olfactory and otic placodes (Bailey et al., 2006). It has been shown earlier from analysis of Frs2α2F/2F mice that FGF/MAPK signaling is required for up-regulation of Pax6 in the lens placode (Gotoh et al., 2004). The Frs2α encodes a docking protein that orchestrates the assembly of a Ras-Raf-MAPK arm of FGF signaling (Fig. 7 and section 3.3.3.).
Figure 7. Schematic diagram of FGF signaling in lens differentiation.

FGF binding to the outside of FGFR induces FGFR dimerization, receptor autophosphorylation followed by phosphorylation of the lipid-anchored docking protein Frs2α. Tyrosine-phosphorylated Frs2α serves as a focal protein for a multiprotein complex assembly that signals via two branches of FGF signaling, PI3K/Akt and FGF/MAPK. Additional components of this pathway such as heparin, N-cadherin, MKP3 and Sprouty (not shown) are discussed in text.
Loss-of-function studies of Lhx2 (Porter et al., 1997), Rx (Mathers et al., 1997), Hes1 (Lee et al., 2005) and Mab21like2 (Yamada et al., 2004) in mouse have clearly shown that the optic vesicle is essential for normal lens placode formation (see Medina-Martinez and Jamrich, 2007). It is thought that these genes modulate FGF and BMP signaling in the optic vesicle; however, no mechanistic data are available to confirm this proposal. In contrast, we do know that broad expression of Pax6 in the PPR is gradually restricted only to the lens-fated cells and its expression in adenohypophysal and olfactory placodes is transient.
“The second repressive stage” is executed by signals produced by neural crest cells that migrate through the space between the anterior neural tube and the surface ectoderm (see Gammill and Bronner-Fraser, 2003). The advanced embryological model of lens formation postulates repression of lens fate outside of the prospective lens ectoderm (see Fisher and Grainger, 2004). There are a number of other data from multiple species that show the repression of lens fate outside of the future lens primordium (see Donner et al., 2006b). In the most recent work using chickens, Grainger and co-workers have shown that close contact between the optic vesicle and the prospective lens surface ectoderm prevents the infiltration of neural crest cells (Sullivan et al., 2004). Earlier studies in the mouse have shown that mesenchymal cells are present between the optic vesicle and surface ectoderm and only after the contact between these structures is established is the lens placode formed (Furuta and Hogan, 1998). Contact between these tissues is mediated by interconnecting cellular processes and extracellular matrices comprised of specific proteoglycans and glycosaminoglycans (see Lang and McAvoy, 2004; Menko and Walker, 2004).
The idea that lens fate is established prior to olfactory/adenohypophysal lineage formation (Bailey et al., 2006) is indirectly supported by abnormal lens formation in model systems with abnormal hedhehog (hh) and Wnt-signaling. In zebrafish mutant you-too (yot), isolated lenses develop from the adenohypophysal placode (Kondoh et al., 2000). The molecular mechanism of this mutation is the formation of a dominant negative Gli-2 mutant that attenuates hh signaling (see Kondoh et al., 2004). In addition, the primordium of the chicken anterior pituitary transiently expresses δ1-crystallin (Kamachi et al., 1998). In the mouse, αB-crystallin is also expressed in the anterior adenohypophysis primordium (Haynes et al., 1996). The chicken mutant talpid3 which is defective in a separate branch of hh signaling, also produces ectopic lenses (Lewis et al., 1999). These observations suggest that in the most anterior part of the PPR, the “early” lens program is being suppressed while the pituitary-lineage program is proceeds.
The repression of lens fate in the peri-ocular head surface ectoderm also involves Wnt-signaling (see Donner et al., 2006b). Lens formation does not require active canonical Wnt signaling in the PPR. Deletion of β-catenin and two Wnt co-receptors Lrp5 or Lrp6 does not perturb lens development (Gong et al., 2001; Smith et al., 2005). However, loss of β-catenin function in a specific region of peri-ocular ectoderm leads to the formation of small ectopic lentoid bodies (Smith et al., 2005; Kreslova et al. 2007). Interestingly, abnormal activation of β-catenin in the lens placode mimicked by producing a stabilized, constitutively active β-catenin, prevented lens formation and repressed Pax6 expression (Miller et al., 2006; Smith et al., 2005).
Collectively, studies of BMP, FGF, hh and Wnt-signaling during early stages of lens development show an extensive traffic of signals between the PPR and lateral and/or underlying cells and tissues such as neural crest cells and the regionalized neural tube. These signals establish the PPR and induce in these cells multiple cell fates via local cues that organize cell movements and cell-sorting to assemble the individual placodes. One of the most urgent experiments is to generate better gene expression maps in the mouse PPR and surrounding head tissues prior to lens placode formation and compare these maps with other model organisms (see Medina-Martinez and Jamrich, 2007).
3.2. Proliferation and movement of lens progenitor cells
After the lens placode is specified from the PPR, both the lens placode and the optic vesicle buckle inward, producing two invaginations. The invagination of the lens placode produces the lens pit and simultaneous invagination of the optic vesicle forms the bilayered optic cup which gives rise to the neural retina and retinal pigmented epithelium (see Cvekl and Piatigorsky, 1996). This step is necessary to both set up the future optics of the camera type eye and to position signaling centers correctly to lead to appropriate cell differentiation. Lens epithelial cell movements during this process are believed to be driven by at least three factors: the tightening of the actin belt followed by rearrangement of the cytoskeleton and a corresponding change in cell shape, the movement of adhesive molecules within individual cells, and local cell proliferation. Hence, the cells forming the lens placode are more wedge shaped in cross section and the morphological arrangement of this cell layer will change from flat to indented (see Piatigorsky, 1981).
Micro- and anophthalmia will result if the cells of the lens placode either exhibit a reduced proliferative rate or inappropriate levels of apoptosis. For example, Pax6 haploinsufficiency in mice causes one less cell cycle than normal in the lens placode resulting in smaller lens vesicles and small lenses in adults (van Raamsdonk and Tilghman, 2000). In contrast, inactivation of the Mab21like1 or Six3 genes in the lens placode results in the massive apoptosis of lens progenitor cells (Liu et al., 2006; Yamada et al., 2003). However, it is not known how any of these mutations result in the observed defects in proliferation and cell survival.
As the lens pit is formed and the lens vesicle closes, the cells destined to become the lens separate from the remaining head ectoderm and establish epithelial contacts to form the lens epithelium. The remaining surface ectoderm must also reestablish appropriate epithelial connections and create borders between cells destined to become the cornea, conjunctival epithelium and skin epithelium (see Piatigorsky, 1981). The incomplete separation of the lens vesicle from the surface ectoderm results is the most common developmental abnormality originating during this process and leads to the formation of a stalk between the lens vesicle and the surface ectoderm. There are a number of genes that play specific roles in lens placode invagination, lens vesicle formation and its separation from the surface ectoderm such as AP-2α (Pontoriero et al. Submitted), β-catenin (Smith et al., 2005), Foxe3 (Brownell et al., 2000), N-cadherin (van Raamsdonk and Tilghman, 2000), Ndst1 (Pan et al., 2006) and Pax6 (Baulmann et al., 2002). It seems likely that these genes are either playing direct roles in the modulation of cell adhesion or control the expression of such genes (see Collinson et al., 2004). Notably, Pax6 has been shown to modulate the expression levels of the cell adhesion molecules α4 (Zaniolo et al., 2004) and α5 (Duncan et al., 2000) integrin in eye development while N-cadherin (van Raamsdonk and Tilghman, 2000), Ndst-1 (Pan et al., 2006) and β-catenin (Smith et al., 2005) all have known roles in cell adhesion and its resulting signal processing. Although chick, human, mouse and rat, all form the lens vesicle from invagination of the lens placode, lens formation in Xenopus (Ishibashi and Yasuda, 2001) and zebrafish proceeds via delamination of the lens placodal cells (see Soules and Link, 2005). Thus, the function of regulatory genes that control this stage of lens development and their target genes may be different depending on species studied.
Lens placode invagination is dependent on retinoic acid signaling, another important pathway used in parallel for patterning of the forebrain and retina (Lupo et al., 2006; Wilson and Houart, 2004). The molecular components of this pathway include DNA-binding nuclear receptors RAR (with 9-cis and all-trans retinoic acid acting as ligands) and RXR (with 9-cis retinoic acid as a ligand), each receptor has three different gene variants α, β and γ, encoded by different genes (see Mark et al., 2006). In addition, cellular retinol (CRBP) and retinaldehyde (CRALBP) binding proteins and three retinaldehyde dehydrogenases (RALDH1/2/3) regulate metabolism and storage of the retinoic acids and their derivates (Mark et al., 2006). Transgene reporters driven by three copies of the retinoic acid responsive element (RARE) are active in the PLE from the 8-12 somite stage, E8.75 in mouse (Balkan et al., 1992; Enwright and Grainger, 2000; Rossant et al., 1991). The inhibition of retinoid signaling using antisense oligonucleotides against the CRBP in 3- to 12-somite cultured mouse embryos caused a failure of lens placode invagination (Bavik et al., 1996). Inactivation of Raldh2, expressed in the optic vesicle between E8.5 and E9.5, disrupted optic cup and lens vesicle formation (Mic et al., 2004) and corneal-lenticular stalks are found in RXRα-/-; RARγ-/- compound mice (Kastner et al., 1994). Interestingly, the activity of a retinoic acid activated reporter construct is reduced in the lens placode of the Pax6-/- mouse (Enwright and Grainger, 2000) and one of the possible targets of retinoic acid signaling in lens is the transcription factor AP-2α (West-Mays et al., 1999).
During lens placode formation, expression of bZIP transcription factor c-Maf (see Yang and Cvekl, 2007) and homeodomain-containing factor Prox1 (Duncan et al., 2002; Wigle et al., 1999) are first observed, however, their function is important later to direct the crystallin-dependent lens differentiation program (Wigle et al. 1999; Kawauchi et al., 1999; Ring et al., 2000) as described in detail in section 4.2. In summary, growth of the lens placode and its transition to the lens vesicle is directed by many of the same genes (such as Pax6 and Six3) that first act during lens lineage-specification. Their main functions are to produce lens progenitor cells and protect them from apoptosis, and to directly or indirectly, via Foxe3, Mab21like1 and retinoic acid signaling, to control the adhesive properties of lens cells. The resulting structure, the lens vesicle, provides lens precursor cells that differentiate into the functional lens.
3.3. Lens fiber cell differentiation
3.3.1. Cell cycle exit, signaling and lens-specific proteins
Lens differentiation commences as soon as the lens vesicle, comprised of a single layer of cuboidal epithelial cells, pinches off from the surface ectoderm. The lens vesicle is polarized with its anterior cells retaining their epithelial morphology and proliferative capacity, whereas the posterior lens precursor cells exit the cell cycle (see Griep, 2006) and initiate terminal differentiation forming the primary lens fibers. Cell cycle exit is linked to the upregulation of p57Kip2 and pRb, and downregulation of E2Fs and cyclin B1 (see Griep, 2006). Lens differentiation is regulated by secreted growth factors including members of the FGF family originating from the prospective retina (see Lovicu and McAvoy, 2005; Robinson, 2006). Secreted FGFs interact with four types of tyrosine kinase receptors, FGFR1 to 4 (Robinson, 2006). Recent studies also suggested roles of Wnt (Lyu and Joo, 2004; Stump et al., 2003) and TGFβ-signaling (de Iongh et al., 2001) in the “late” stages of lens fiber cell differentiation.
Lens fiber cell differentiation is marked by cellular elongation, expression and accumulation of crystallins (see 3.3.2.) and other lens-specific proteins such as DNaseIIβ (Nishimoto et al., 2003), the water transport channel MIP/aquaporin0 (see Chepelinsky, 2003), and the intermediate filament proteins filensin and CP49 (see Bassnett and Beebe, 2004; Quinlan and Prescott, 2004). Lens fiber cell differentiation culminates with the regulated degradation of subcellular organelles as these structures would otherwise cause light scattering (see Bassnett, 2002). The nucleus is the last organelle to be eliminated, and, consequently, the entire lens developmental program terminates with the self-destruction of this particular epigenome. The adult lens is comprised of three compartments: anterior lens epithelium, secondary lens fibers and a central syncytium of lens fibers that have lost their organelles (Shestopalov and Bassnett, 2000; Shestopalov and Bassnett, 2003). Detailed information about lens structure and cellular physiology can be found elsewhere (see Lovicu and Robinson, 2004).
3.3.2. Temporal and spatial expression of crystallins in mouse and chicken lens
In mouse, the initiation of αB-crystallin expression marks the formation of the lens placode (Haynes et al., 1996; Robinson and Overbeek, 1996) and its levels upregulate in the lens vesicle. αA-crystallin mRNA appears first in the invaginating lens placode followed by uniform expression in the lens vesicle (Robinson and Overbeek, 1996). Dramatic upregulation of both αA- and αB-crystallin expression is associated with primary lens fiber cell differentiation although, in mammalian lenses, αA-crystallin represents from 20-40% of total lens crystallin while αB-crystallin is present at only 5% of total crystallins (Lampi et al., 1997; Ueda et al., 2002). In contrast, αB-crystallin is the most abundant crystallin found in the lens epithelium. The expression patterns of individual mammalian β/γ-crystallin (βA3/A1, βA2, βA4, βB1, βB2, βB3, γA, γB, γC, γD, γE, γF and γS) genes have not been determined with the full precision. In general, β/γ-crystallin genes are highly expressed in lens fiber cells with no detectable or low expression in lens epithelium (see Cvekl et al., 2004). Expression of rat and mouse βB1-crystallin, probed by in situ hybridization, was found in fully elongated primary lens fiber cells and no expression was found in the embryonic lens epithelium (Duncan et al., 1996; Van Leen et al., 1987) while βB2-crystallin expression does not initiate until after birth in rodents (Ueda et al., 2002). Expression of the γ-crystallin genes, probed by the γE-crystallin probe, identified first signals in the posterior part of the lens vesicle while the same stage showed uniform αA-crystallin expression in the lens vesicle (Van Leen et al., 1987). Recent RNA microarray studies of gene expression in primitive lens tissues obtained using laser capture microdissection from E10 and E12 mouse embryos confirmed significant expression of γ-crystallins in the lens vesicle (Xiao et al., 2006). Examination of lens epithelium obtained from 3-day old rats identified transcripts encoding the βB2-, βB3-, γC-, γD- and γS-crystallins (Wang et al., 2004a). The βB1- and γS-crystallin proteins were detected in the adult lens epithelium and fibers (Wang et al., 2004a) although γS-crystallin expression is absent in the embryonic lens (Wistow et al., 2000). Thus, lens fiber cell differentiation in rodents is marked by the sequential appearance of αB-, αA-, γ- and βB1-crystallins with βB2- and γS-crystallin expression initiating after birth. The expression levels of individual γ-crystallins varies within an order of magnitude both in rodents and human (Lampi et al., 1997; Ueda et al., 2002). The precise temporal and spatial expression of the majority of β-crystallins requires further clarification.
In chicken, expression of δ1- and δ2-crystallins is detected early in the lens placode and transiently in the adenohypophysal placode (Kondoh et al., 2004). Expression of β-crystallins, excluding βB1-crystallin, slightly precedes the expression of the αA-crystallin gene in the lens vesicle (see Piatigorsky, 1981). The temporal and spatial pattern of chicken αB-crystallin expression remains to be determined. The expression levels of δ-crystallins decreases in late embryonic development in birds while β-crystallin expression upregulates leading to dramatically different crystallin ratios between fiber cells formed in the embryo and the adult (see Piatigorsky, 1987). The molecular basis of differential crystallin gene expression between rodents and chicken originates from their genome differences, the presence of lens-specific enhancers in the avian δ-crystallin/argininosuccinate lyase (ASL) locus and variable expression patterns of large Maf genes during lens development termed “expression domain shuffling” (Coolen et al., 2005) as illustrated in chapter 4.
3.4. Signal transduction pathways regulating lens fiber cell differentiation
A large body of evidence demonstrates that lens fiber cell differentiation is regulated by a variety of growth factors present in the vitreous humor such as members of the FGF family, insulin, IGF-I and IGF-II. In addition, growth factors of the BMP/TGFβ, PDGF, EGF and Wnt families were implicated more generally in the process of lens differentiation. There are a number of recent reviews that provide detailed information about these growth factors and the expression of their receptors and co-receptors during embryonic lens formation (Chen et al., 2006; Lang and McAvoy, 2004; Lovicu and McAvoy, 2005; Robinson, 2006). Here, we provide conceptual background on FGF-, BMP/TGFβ- and Wnt-signaling and discuss the potential nuclear targets of these pathways as this area of lens research lags behind the advanced work on receptors, co-receptors and the cytoplasmic components of these pathways.
3.4.2. FGF signaling in lens differentiation
A schematic diagram of two major branches of the FGF-signaling pathway, i.e. PI3K/Akt and MAPK pathways, adapted for lens fiber cell differentiation are shown in Fig. 7. FGFs are self-dimerizing heparin-binding growth factors that associate with high affinity tyrosine kinase receptors (FGFRs) and low affinity receptors, the heparan sulfate proteoglycans (HSPGs) which are localized in the extracellular matrix. There are at least 13 of the known 22 FGF genes expressed in the eye (see (Robinson, 2006). Alternative splicing of four mammalian genes encoding FGFRs generates a plethora of receptors with different specificities and affinities towards the available ligands (see Eswarakumar et al., 2005). In addition, N-cadherin (strongly expressed in the lens placode, see 3.1.2.) was shown to act as a co-receptor of FGFRs (Suyama et al., 2002). Binding of FGF dimers alone, in complex with heparin, or heparin sulfate proteoglycans induces FGFR dimerization that activates their tyrosine kinase activity. This activity is used for both auto- and trans-phosphorylation of the cytoplasmic domains of the FGFRs. The phosphorylated residues in this domain direct assembly and recruitment of signaling complexes that include either of two myristyl-anchored docking proteins, FRS2α and FRS2β, docking protein Gab1, adapter protein Grb2, protein tyrosine phosphatase Shp2 and guaninine nucleotide exchange factor Sos1 (see Bottcher and Niehrs, 2005; Eswarakumar et al., 2005).
The most common pathway regulated by FGF signaling is the MAPK pathway (see Bottcher and Niehrs, 2005; Chang and Karin, 2001). The membrane-bound Frs2α/Gab1/Grb2/Shp2/Sos1 complex allows Sos1 to activate proximally located membrane-bound Ras by GTP exchange. Once in the active GTP-bound state, Ras interacts with several effector proteins such as Raf leading to the activation of the MAPK signaling cascade (see Bottcher and Niehrs, 2005). Expression of a dominant negative form of Ras in lens caused a delay in primary lens fiber cell differentiation (Xie et al., 2006). The FGF/MAPK cascade ends with ERK1/2 kinases that phosphorylate nuclear transcription factors comprising the AP-1 (see Karin, 1996), Ets (E twenty-six oncogene family, see Sharrock, 2001) and Maf (Benkhelifa et al., 2001) families of transcriptional activators and repressors (Fig. 7). It is important to note that ERK1/2 kinases are also downstream of insulin-mediated signaling in lens (Le and Musil, 2001). The PI3K branch of FGF-signaling associates with the complex via Gab1 that is recognized by the p85 regulatory subunit of phosphatidylinositol-3-kinase (PI3K). The catalytical p110 subunit of PI3K phosphorylates Akt kinase, a powerful inhibitor of pro-apoptotic genes (see Bottcher and Niehrs, 2005). Alternatively, PI3K regulates Rac kinase, a component of the Wnt-pathway (see 3.4.3.). Inhibition of PI3K inhibits the actin filament reorganization that is required for fiber cell differentiation (Weber and Menko, 2006). A third FGF activated transduction pathways proceeds via phospholipase C gamma (PLC-γ), a system studied in lens only in the context of oxidative stress (Lin and Takemoto, 2005). There are at least three distinct mechanisms to attenuate FGF signaling using the transmembrane protein Sef, inhibitory proteins Sprouty 1 to 4 (see Boros et al., 2006) and the dual-specificity protein phosphatase MKP3 (see Tsang and Dawid, 2004).
As introduced earlier, FGF signaling plays multiple sequential roles during the establishment of PPR, maintenance of the lens-lineage and lens fiber cell differentiation. As these signaling events occur between distinct cell types receiving FGFs, e.g. pre-placodal progenitors, lens progenitor and precursor cells, with different cell types producing those FGFs (e.g. mesoderm, undifferentiated neuroepithelium and prospective neuroretina), it is reasonable to hypothesize that different ligands, receptors and co-receptors are engaged in these processes. Thus, the “context-dependent” signaling is likely to culminate in the nucleus by activating different sets of transcription factors. Experiments in primary rat lens cultures have shown that low concentrations of FGF2 such as 0.3 nM promote lens epithelial cell proliferation, moderate concentrations (e.g. 3 nM) stimulate migration, and high concentrations (40 to 100 nM) induce fiber cell differentiation (Chamberlain and McAvoy, 1989). These results support the idea that anteroposterior gradients FGFs and FGFRs concentrations play important roles during primary lens fiber cell differentiation (see Lang and McAvoy, 2004; Lovicu and McAvoy, 2005).
Genetic studies in mice have shown an important role for an enzyme involved in the biosynthesis of heparan sulfate proteoglycans, N-acetylglucoseamine N-deacetylase-N-sulfotransferase 1 (Ndst1) in invagination of the lens placode (Pan et al., 2006). In severely affected lens placodes of Ndst1 null mice, expression of Pax6 was also reduced providing evidence that HSPGs cooperate with FGFs during this phase of lens development (Pan et al., 2006). Importantly, reduced MAPK signaling and expression of the DNA-binding factor Erm, an Ets family member, was shown in abnormal lens tissues in Ndst1 null embryos. Additional studies of this gene suggest that hh and Wnt signaling are also affected by the impairment in heparan sulfate synthesis (Pallerla et al., 2007).
FGF signaling also stimulates both cell cycle exit (see Griep, 2006) and the lens differentiation program via the upregulation of crystallin gene expression. Identification of individual FGFs and their receptors controlling distinct phases of lens development is hampered by a wide repertoire of functional redundancies and compensatory effects (see Robinson, 2006). Nevertheless, context-specific functions of individual components allow insights into this process. For example, FGFR2 is required for cell cycle exit during primary lens fiber differentiation as well as lens cell survival (Garcia et al., 2005). Mechanistic studies of FGF signaling in lens would greatly benefit from the identification of FGF-responsive elements in crystallin genes and the characterization of appropriate nuclear factors in lens chromatin (see 4.2.1.).
3.4.3. BMP/TGFβ-signaling in lens differentiation
The BMP/TGFβ-signaling pathways also regulate multiple stages of lens development. A number of BMP and TGFβ ligands and their transmembrane Ser/Thr kinase receptors, are expressed in the lens and surrounding tissues (de Iongh et al., 2001; Faber et al., 2002). Upon ligand binding, a heterotetrameric complex forms between homodimers of the type I (e.g. Alk 1 and Alk5 for TGFβ-signals, Alk2, 3 and 6 for BMP signals) and type II receptors (TBRII for TGFβ-signals, and ActRII, BMPRII and MISRII receptors for BMPs), with the type II receptor transphosphorylating the type I receptor (see Derynck and Zhang, 2003; Feng and Derynck, 2005). The activated type I receptor interacts with an adaptor protein, SARA, which recruits Smad proteins. In the TGFβ-cascade, cytoplasmic Smad 2 and 3 are phosphorylated, form cytoplasmic complexes with the common mediator Smad4, followed by their translocation into the nucleus. In the BMP-pathway, phosphorylated Smad1/4/5 binds Smad4. By recognizing Smad-binding sites (5’-GATC-3’), Smad4/Smad3 or Smad4/Smad1 can activate or repress transcription depending on which chromatin remodeling enzymes they associate with. Recruitment of p300, p/CAF and CBP to DNA via Smad3 and Smad4 is known to activate transcription. In contrast, binding of c-Ski, Sno, Evi-1, DACH1 and Sip1 results in transcriptional repression (see Feng and Derynck, 2005). Notably, Sip1 null lenses exhibit defective FoxE3 expression, often have corneo-lenticular stalks and abnormal crystallin expression patterns. Alternate signal transduction cascades can be mediated via membrane bound Ras and its downstream target, ERK1/2 or via TAK1/MEKK1 kinases producing activated JNK and p38 kinases. Studies in different cell types identified AP-1 proteins, c-Myc, Ets factors, Foxo1, Maf proteins, NF-κB, Stat-3 and other transcription factors as targets of these kinases. Negative regulators of this pathway include cytoplasm localized inhibitory Smads, Smad6 and Smad7. In addition, Smads are posttranslational regulated by ubiquitin-proteasome-mediated degradation triggered by the E3 ubiquitin ligase, Smurf1 (see Derynck and Zhang, 2003).
BMP/TGFβ-signaling, like FGF signaling, also operates differently in different cellular contexts. Temporal and spatial regulation of receptors binding various BMP/TGFβs and the distribution of phosphorylated Smads suggest strategies to mechanistically dissect this pathway. Experiments in chicken (Belecky-Adams et al., 2002) and mouse (Faber et al., 2002) showed repression of fiber cell elongation by noggin, a BMP ligand inhibitor. Primary lens fiber differentiation is also inhibited by overexpression of a dominant-negative form of Alk6 using the αA-crystallin promoter (-366/+44) (Faber et al., 2002). Inactivation of the BMP-receptor type I, Alk3, in the presumptive lens ectoderm resulted in defects suggesting a function for this receptor in both the late stages of fiber cell differentiation and in proliferation and/or survival of the lens epithelium (Beebe et al., 2004). In contrast, conditional inactivation of TGFβIRII in the lens lineage did not produce any phenotype (Beebe et al., 2004). The BMP and TGFβ ligands are not only produced by other ocular cells to regulate lens fiber cell differentiation. The lens itself can also act as a TGFβ signaling center to control the development of ocular structures derived from the neural crest (Ittner et al., 2005).
3.4.3. Wnt-signaling in lens differentiation
Like FGF- and BMP/TGFβ-signaling, the Wnt signaling pathways are used during early lens (see 3.1.) and retinal development (see de Iongh et al., 2006) for multiple purposes. A recent study suggested that Wnt signaling enhances FGF-triggered lens fiber cell differentiation including crystallin gene expression (Lyu and Joo, 2004). In mammals, 19 secreted glycoproteins of the Wnt family bind to any of 10 types of Frizzled (Fz) receptors which have seven transmembrane domains and require the structurally less complex co-receptors LRP5/6 for their function. Wnt signaling functions through three pathways: Wnt/β-catenin (“canonical”), Wnt/planar cell polarity (PCP) and Wnt/Ca2+ pathways (see (Cadigan and Liu, 2006; Chen et al., 2006). Activation of Frizzled receptors leads to the phosphorylation of Dishevelled (Dsh) proteins and their binding to the intracellular membrane. Phosphorylated Dsh recruits the β-catenin destruction complex, axin/APC/GSK3β, to the membrane. In the canonical pathway, degradation (with inactive Wnt signaling) or stabilization (caused by formation of Wnt/Fz/LRP5/6 ternary complex) of β-catenin allows some of cytoplasmic β-catenin to translocate into the nucleus. Nuclear β-catenin forms a complex with the DNA-binding transcription factor LEF/TCF (with a consensus binding site 5’-CTTTGWW-3’), a strong transcriptional activator of Wnt-target genes. The Wnt/PCP pathway diverges from the canonical pathway downstream of membrane bound Dsh in complex with Daam1 which interacts with three small GTPases, Rho, Rac and Cdc42. These enzymes participate in cytoskeletal changes used for directional cell migration or activate the c-Jun N-terminal kinase (JNK) pathways that regulate activities of a number of DNA-binding transcription factors (see Aouadi et al., 2006; Yordy and Muise-Helmericks, 2000).
Lens formation does not require active canonical Wnt signaling in the PPR (see 3.1.2.) as indicated by the lack of LEF/TCF function in the lens as measured in the TOPgal reporter transgenic mouse (Smith et al., 2005). In contrast, the Wnt/β-catenin pathway plays important roles in the functional integrity of the lens epithelium as mutations in the co-receptor LRP6 show dismorphogenesis of the lens epithelium (Stump et al., 2003). In cultured rat explants, Wnt3a conditioned medium induced expression of β-crystallins in the absence of cell elongation (Lyu and Joo, 2004). However, incubation of explants in the presence of 50 ng/ml of FGF2 for just 1 hour, followed by addition of Wnt3a-containing medium resulted of elongated lens fibers and accumulation of β-crystallins, MIP/aquaporin0, p57Kip2 and N-cadherin (Lyu and Joo, 2004). Expression of genes that function in Wnt/β-catenin signaling suggest a role for this pathway in crystallin gene regulation (Lyu and Joo, 2004). In contrast, the Wnt/PCP pathway may play roles in the cytoskeletal rearrangements involved in fiber cell elongation (see Chen et al., 2006).
4. Transcriptional control of lens regulatory and structural genes
The pathways that control lens development, summarized in Fig. 8, were derived from genetic studies and corroborated by mechanistic studies into the transcriptional regulation of the most important regulatory (Pax6, Sox2, Six3, c-Maf and FoxE3) and structural (crystallins, filensin, CP49, and MIP/aquaporin0) genes in mouse and chicken lenses. Herein, we provide up-to-date analysis of the architecture of lens-specific promoters and enhancers and their associated transcription factors.
Figure 8. Pax6 networks during lens lineage formation and differentiation.
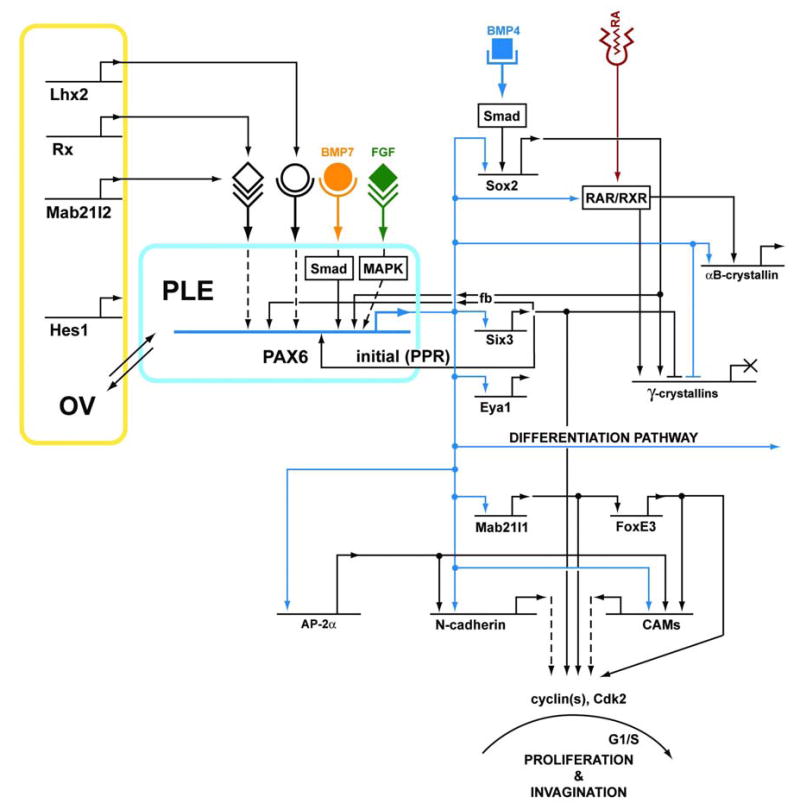


(A) Gene regulated by Pax6 in the prospective lens ectoderm (PLE) and lens placode. Expression of Hes1, Lhx2, Mab21like2 and Rx in the optic vesicle (OV) is essential for mouse lens placode formation; however, the mechanisms of these genes remain to be elucidated. (B) Three genes, c-Maf, Prox1 and Pitx3, are directly or indirectly regulated by Pax6 during the proliferation of lens progenitor cells. Expression of prox1 is required for the cell cycle exit prior the terminal differentiation. (C) A complex regulatory network proposed to control crystallin gene expression in differentiating lens fibers. Dotted lines show proposed links between the FGF/MAPK pathway to regulate expression of Pax6, c-Maf and αA-crystallin during lens differentiation.
4.1. Transcriptional control of lens regulatory genes
Studies of the transcriptional regulation of lens lineage-specific regulatory genes including Pax6, Sox2, Six3, c-Maf and FoxE3 largely explain the molecular mechanisms controlling lens-lineage formation. The common denominator of their transcriptional control is the use of multiple tissue-specific and tissue-preferred enhancers at each specific locus. Some of these enhancers respond differently to a variety of extracellular signaling cascades which controls both their broader/regional and local activities. Note that lens lineage-specific transcription factors genes are often mutually cross-regulated and are used at both the top of the regulatory hierarchy and in the regulation of differentiation specific markers (see Davidson and Erwin, 2006; Gehring and Ikeo, 1999). For instance, Pax6 is crucial for both the formation of the lens placode (see Fig. 8A) and the appropriate expression of crystallin genes which are markers of lens fiber cell terminal differentiation (see Fig. 8C).
4.1.1. Regulation of Pax6 gene expression in lens
The Pax6 gene is transcribed from three promoters: two 5’-promoters, P1 and P0 (Okladnova et al., 1998; Plaza et al., 1993; Xu et al., 1999; Xu and Saunders, 1997), and an internal promoter, Pα (Kim and Lauderdale, 2006) (see Fig. 9). Phylogenetic footprinting of Pax6 loci from human, mouse, frog, zebrafish and fugu revealed high levels of conservation for noncoding sequences implicating their role in Pax6 regulation (Morgan, 2004).
Figure 9. Genomic structure and enhancers of the PAX6 locus.

(A) The PAX6/Pax6 locus is located in the WAGR region of human chromosome 11 (Wilms tumour, aniridia and growth retardation) and in the syntenic region of mouse chromosome 2. ELP4 is a gene partially embedded in the PAX6 locus. Centromere, cen; DNAse I hypersensitivity region, HS; telomere, tel. (B) Three Pax6 promoters (P0, P1 and Pα) are regulated by a variety of tissue-specific distal enhancers.
Expression of Pax6 in lens, retina, cornea, brain, olfactory system, pituitary and pancreas is controlled by tissue-preferred enhancers located both 5’ and 3’ from its promoters (see Fig. 9). The ectodermal enhancer (EE) was shown to direct Pax6 expression in the surface ectoderm, lens and cornea (Kammandel et al., 1999; Williams et al., 1998). The EE contains binding sites for the Meis (Zhang et al., 2002), Sox (Aota et al., 2003; Donner et al., 2006a), Six3 (Liu et al., 2006) and Oct family of proteins (Donner et al., 2006a), as well as a single Pax6-binding site (Aota et al., 2003). Transgenic expression supported by this enhancer is initiated in the surface ectoderm at E8.75 in mouse, i.e. with a short delay compared to that of endogenous Pax6 expression. The second lens-specific enhancer, SIMO, is active from E10.5 and was genetically identified using a group of aniridia patients with presumptive mutations in non-coding sequences (Kleinjan et al., 2001; Lauderdale et al., 2000). The SIMO element contains multiple Six3-binding sites (Liu et al., 2006). It is possible that coordination between the EE and SIMO element is needed to recreate the full expression pattern of Pax6 in the eye although it is possible that additional Pax6 enhancers have yet to be discovered.
4.1.2. Regulation of Sox2 gene expression in chicken lens and cultured mouse cells
Temporal and spatial regulation of chicken Sox2 expression in various compartments of the central nervous system and sensory placodes was examined using a systemic dissection of roughly 50 kb of the Sox2 locus. Eleven enhancers were identified that directed expression of the EGFP reporter gene in the sensory placodes (lens, olfactory and otic) and multiple neural tissues of electroporated chick embryos (Uchikawa et al., 2003). Three enhancers, N-4, N-3 and L (see Fig. 10), triggered reporter gene expression in the lateral head ectoderm, lens placode and differentiating lens, respectively. Phylogenetic footprinting of the chicken, mouse and human N-4 and N-3 Sox2 enhancers identified several putative TAAT core binding sites for Sox and homeodomain proteins (Uchikawa et al., 2003) and Six3 was shown to activate both enhancers (Liu et al., 2006).
Figure 10. Transcriptional regulation of chicken Sox2 locus.

Five enhancers, N-3, NOP-1, NOP-2, N-4 and L and their activities in the developing placodes and lens are shown (Uchikawa et al., 2003).
Sox2 expression in cultured mouse F9 embryonic carcinoma cells originates from a single start site despite the absence of a canonical TATA-box (Wiebe et al., 2000). A promoter fragment of −528/+238 exhibited substantial activity, while a truncated promoter fragment, −72/+4, drives “basal” promoter activity (Wiebe et al., 2000). Studies of lens lineage-specific transcription factors revealed that AP-2α, Pax6 and Prox1 activated the mouse Sox2 -426/+6 promoter in HEK293 fibroblasts (Lengler et al., 2005). In contrast, Msx2 reduced Sox2 promoter activity while Six3 did not affect it at all. Several tentative binding sites for AP-2α, Pax6, Prox1, Six3 and Msx2 were proposed, however, no further data are available about their physiological role in the regulation of the Sox2 gene. Further studies of three distal Sox2 enhancers are expected to generate valuable information about the process of lens lineage determination as the earliest expression of Sox2 may depend on signals that control lens formation and a later stage of Sox2 expression is controlled by BMP4 (Fig. 8, see Lang, 2004).
4.1.3. Regulation of Six3, c-Maf and Foxe3 expression in lens
There have been far fewer transcriptional studies of the Six3, c-Maf and FoxE3 genes in the lens compared to Pax6 and Sox2. A series of co-transfection studies were performed with the human SIX3 promoter in kidney fibroblasts (Lengler and Graw, 2001) to determine whether lens lineage-specific factors regulate expression of Six3. These data suggested that both Pax6 and Prox1 activated this promoter via the −703/-349 region, while Six3 and Msx2 reduced promoter activity presumably via cis-acting sites within the proximal region, -349/+44. Three distal consensus Six3-binding sites containing the core recognition sequence TAAT are present in the Six3 promoter and were confirmed by in vitro DNA-binding assays. Co-transfection experiments performed in NIH3T3 cells revealed that Six3 alone and combinations of Six3 with Grg4 or Grg5 decreased Six3 promoter activity. A distal Pax6-binding site was identified 2 kb upstream from the first coding exon in mouse Six3 (Goudreau et al., 2002) and the zebrafish Six3.1 (Wargelius et al., 2003) while Pax6 was found to activate Six3 expression in mice (Liu et al. 2006). Notably, Six3 also activates the Pax6 gene (Liu et al., 2006) and transgenic overexpression of Six3 rescues the Pax6 haploinsufficiency phenotype (Goudreau et al., 2002).
Expression of c-Maf in the invaginating mouse and rat lens placodes suggested that its expression could also be controlled by lens lineage specific transcription factors. A preliminary study of the regulation of the rat c-Maf promoter in mouse C3H10T1/2 fibroblasts found that a -5.6 kb/+656 fragment was highly active with the -350/+540 region containing multiple Pax6 and Maf (MARE) binding sites (Sakai et al., 2001). Thus, c-Maf is a promising candidate direct target gene for Pax6 (see Fig. 8B and C) and for its own autoregulation although this hypothesis has not yet been tested in a lens environment. Since c-Maf expression is upregulated in primary lens fiber cells concomitant with reduced expression of Pax6 (Ring et al., 2000), it is plausible that Pax6 is required for the initial expression of c-Maf in the invaginating lens placode. However, the high levels of c-Maf expression in the differentiating lens fibers which express reduced amounts of Pax6, may solely depend on the autoregulatory loop and/or involvement of yet unknown proteins, probably regulated by FGF signaling.
Foxe3 expression is lost in the surface ectoderm of Pax6 Sey/Sey embryos indicating that Pax6 is required for FoxE3 expression (Brownell et al., 2000). A 6.2 kb region containing the mouse Foxe3 promoter was sufficient to drive expression of the lacZ reporter gene in the mouse lens. Dissection of this region into four fragments, (5’-D, C, B and A-3’) revealed that fragment C harbors the major and fragment B the minor lens element (Yoshimoto et al., 2005). Studies of Mab21like1 knock out mice established that Foxe3 is downstream of Mab21like1 which is downstream of Pax6 (Yamada et al., 2003). Recently, a novel regulatory mechanism for Foxe3 was proposed that includes the combined activities of Sip1 (a Smad-binding zinc-finger homeodomain transcription factor, see 4.2.6.) and Smad8 via the proximal promoter block A (Yoshimoto et al., 2005).
In summary, more detailed studies of the transcriptional regulation of the Six3, c-Maf and Foxe3 genes coupled with studies of other important genes including AP-2α, Eya1, Hsf4, Mab21like1, Prox1, Six5 and others is essential to completely understand genetic program of mammalian lens development.
4.2. Transcriptional control of lens structural genes
The major lens fiber structural proteins with lens-preferred expression are crystallins, the specialized intermediate filament proteins filensin and CP49, and the membrane channel MIP/MIP28/aquaporin 0. There is an extensive body of evidence supporting the idea that various combinations of lens lineage-regulatory genes including Pax6, c-Maf, Six3, Sox1, Sox2, Prox1 and Hsf4 in combination with more ubiquitously expressed genes (e.g. AP-1, CREB and RARβ/RXRβ and USF) temporally and spatially regulate all mouse crystallin genes. In contrast, the molecular mechanisms controlling lens fiber cell-specific expression of filensin, CP49 and MIP remain to be determined.
As shown in detail below, many lens structural genes are directly regulated using two or more transcription factors with the earlier one regulating the expression of the later one and both regulating the crystallin target gene. This type of regulation, a “feed-forward loop” network motif, provides a number of regulatory options that include speeding up or delaying the response of the target genes (Mangan and Alon, 2003). The possible outcomes of one or a few simple feed-forward loops are in agreement with the diverse temporal and spatial control of gene expression during lens development.
4.2.1. Regulation of the mouse αA-crystallin gene
Analysis of the temporal and spatial regulation of the mouse αA-crystallin gene provides a number of details that are not currently available for other crystallin genes. Three distinct DNA-binding transcription factors, Pax6, c-Maf, and CREB, were implicated as αA-crystallin regulatory factors (Ashery-Padan et al., 2000; Cvekl et al., 1995a; Kawauchi et al., 1999; Ring et al., 2000; Yang and Cvekl, 2005). Chromatin immunoprecipitations (ChIPs) of the mouse αA-crystallin locus confirmed the association of these factors with four regulatory regions of this gene and provided novel mechanistic insights into the regulation of αA-crystallin during lens fiber cell differentiation (Yang et al., 2006).
The mouse αA-crystallin gene contains a lens-specific promoter that is regulated by at least three distal enhancers, DCR1, DCR2 and DCR3. This promoter (-366/+46) was one of the first ever tested in transgenic mice and shown to be lens specific in vivo (Overbeek et al., 1985). A series of follow up studies demonstrated that even shorter fragments, -111/+46 and −88/+46, support lens specific expression while -60/+46 is inactive in transgenic mouse lenses (see Cvekl and Piatigorsky, 1996). Transgenic studies to characterize temporal and spatial expression patterns directed from both −366/+46 (Govindarajan and Overbeek, 2001; Stolen and Griep, 2000) and −1800/+46 (Yang et al., 2006) αA-crystallin promoter fragments found expression only in primary lens fibers that was at least 300-times lower than that of the endogenous αA-crystallin gene. Pax6 activates the -366/+46 promoter in co-transfections and multiple Pax6 binding sites were identified in the lens-specific promoter of the mouse αA-crystallin gene (Yang and Cvekl, 2005) as shown in Fig. 11. A CREB-binding site was at −110/-103 and mediated induction with 8-BrcAMP, forskolin and Tax1 (Cvekl et al., 1995a) and most likely corresponds to the CREB detected at the promoter in vivo by ChIP (Yang et al., 2006). However, expression of αA-crystallin requires additional factors as both Pax6 and CREB are expressed in other tissues not expressing αA-crystallin (Walther and Gruss, 1991). Deletion of the bZIP protein, c-Maf, results in a lack of primary fiber cell elongation and a 99% reduction of αA-crystallin expression levels (Kawauchi et al., 1999; Ring et al., 2000). Like Pax6, c-Maf interacts with multiple regions of the αA-crystallin promoter and can activate its expression in co-transfection tests (Yang and Cvekl, 2005).
Figure 11. Transcriptional regulation of the mouse αA-crystallin locus.

(A) Diagram of distal control regions (DCR1, DCR2 and DCR3; shown by black boxes), promoter and four exons (1, ins, 2 and 3). (B) Transcription factor binding sites for CREB, Mafs and Pax6 (Yang et al., 2006). Putative binding sites for Ets, Pax6, Mafs (MARE) in DCR1. Note that MAREs contain embedded AP-1 binding sites (Yang and Cvekl, 2005). Putative binding sites for CREB, Mafs (MARE), retinoic acid receptors (RARE) and Pax6 in DCR3. (C) Model for moderate levels of expression of αA-crystallin in lens vesicle. (D) Model for high levels of expression of αA-crystallin in differentiating primary lens fibers. Increase expression of αA-crystallin is due to higher abundance of c-Maf and CREB at the promoter and of CREB at DCR3 (Yang et al., 2006).
Our recent studies found that DCR1 in combination with the 1.9 kb αA-crystallin promoter is sufficient to drive reporter gene expression in the lens vesicle and lens epithelium of transgenic mice (Yang et al., 2006), virtually recapitulating the spatial and temporal pattern of expression of the endogenous αA-crystallin gene (Robinson and Overbeek, 1996). In contrast, DCR3/transgenes were activated 24h later compared to DCR1/transgenes and their expression was restricted to more elongated lens primary fiber cells. Notably, DCR1 mediates promoter activation in response to FGF2 in primary rat lens explants while DCR3 did not, consistent with DCR1 as a target for the FGF signaling known to be required for lens fiber cell differentiation. In contrast, DCR3 could be a downstream target for non-canonical Wnt- (Lyu and Joo, 2004; Smith et al., 2005; Stump et al., 2003) and TGFβ- (de Iongh et al., 2001) signaling thought to function after FGF-triggered lens fiber cell differentiation (see Lovicu and McAvoy, 2005).
Studies of the chromatin structure of the mouse αA-crystallin locus demonstrated that high levels of histone H3 K9 acetylation spanning the entire αA-crystallin coding regions from DCR1 to DCR3 are detectable in lens cells expressing both moderate and high levels of αA-crystallin, but not fibroblasts. In contrast, a shorter domain of increased histone H3 K27 trimethylation (promoter to +4 kb) was detected preferentially in chromatin obtained from a lens cell line expressing moderate levels of αA-crystallin (Yang et al., 2006). These data show that distinct chromatin structures correlate with moderate and high levels of mouse αA-crystallin expression.
ChIP analysis of HAT distribution along the 16kb of the mouse αA-crystallin locus identified a region of CBP abundance in the promoter and a broad distribution of p300 in mouse lens chromatin derived from tissue which expresses high levels of the αA-crystallin gene (Yang et al., 2007). In contrast, little p300 and CBP was found in chromatin of cultured lens epithelial cells which express only moderate amounts of mouse αA-crystallin. The chromatin remodeling enzyme Brg1 is only found at low abundance throughout the 16kb mouse αA-crystallin locus in lens epithelial cells while a key component of ISWI complexes, Snf2h, is detected at high abundance only in the promoter (Yang et al., 2006). Notably, Pax6 directly binds to Brg1, c-Maf associates with Snf2h in the presence of nucleic acids, and CREB interacts with both Brg1 and Snf2h. The broad distribution of Brg1 suggests that Pax6 initially recruits Brg1 to the αA-crystallin locus to prepare chromatin for initial remodeling at the onset of the lens lineage in the lens placode. Once c-Maf becomes expressed in the invaginating lens placode, it binds to the promoter and recruits Snf2h- containing ISWI complexes to activate the promoter. However, this process is thought to require DCR1 as studies of temporal and spatial expression of the 1.9 kb promoter fragment alone never detected expression in the lens vesicle. ChIPs identified binding of Pax6, c-Maf and TBP/TFIID to DCR1 in chromatin obtained from both cultured lens epithelial cells and newborn lenses. The ability of DCR1 to mediate FGF signaling requires additional experimentation including identification of FGF-responsive cis-sites and their corresponding factors (see Fig. 11B).
4.2.2. Regulation of the mouse αB-crystallin gene
The mouse αB-crystallin locus contains a structurally similar proximal gene, HspB2 (Iwaki et al., 1997) only 866 bp upstream in a head-to-head orientation (see Fig. 12). Both αB-crystallin and HspB2 are co-expressed in muscle although only the αB-crystallin gene is active in the lens (Doerwald et al., 2004; Iwaki et al., 1997). Four kilobases of 5’-flanking sequence of the mouse αB-crystallin gene (which also contains the HSPB2 locus) drove lacZ reporter gene expression in a pattern mimicking that of endogenous αB-crystallin in lens, heart, somites and skeletal muscle (Haynes et al., 1996). Two promoter fragments −164/+44 and −115/+44, but not a shorter fragment −68/+44, were sufficient to activate the CAT reporter gene in transgenic lenses (Gopal-Srivastava et al., 1996). The αB-crystallin promoter is activated in lens by an enhancer (-427/-255, MLH enhancer) that is also used for its activation in non-lens tissues, e.g. in skeletal muscle and heart (Gopal-Srivastava et al., 1995). Subsequent studies have shown that the MLH enhancer does not activate the nearby HspB2 promoter. Therefore, it is likely that a genomic boundary element resides next to the MLH enhancer. MyoD, SRF (Gopal-Srivastava et al., 1995) and LEDGF/p75 (Singh et al., 2001) bind to the MLH enhancer in in vitro assays although LEDGF/p75 null mice do not appear to have any lens defects (Sutherland et al., 2006). DNaseI footprinting analysis of proteins interacting with the proximal mouse αB-crystallin promoter revealed two lens-specific protected regions, LSR1 and LSR2. Both LSR1 and LSR2 are comprised of an array of Pax6-binding sites in combination with MAREs (Chauhan et al., 2004; Yang et al., 2004) and RAREs (Gopal-Srivastava et al., 1998) as shown in Fig. 12. In addition, a canonical heat shock element (HSE) was identified near the TATA-box (Somasundaram and Bhat, 2000) which binds HSF4 in vitro although HSF4 null mice do not have attenuated levels of αB-crystallin protein (Fujimoto et al., 2004; Min et al., 2004).
Figure 12. Transcriptional regulation of the mouse αB-crystallin locus.

(A) Schematic diagram of Cryab (exons 1, 2 and 3 shown by black boxes) and Hspb2 (exons 1 and 2 shown by open boxes) genes. Transcriptional start sites are shown by arrows. (B) A series of 5’-flanking sequences of mouse αB-crystallin support expression of reporter genes in various compartments of the lens and in non-lens tissues. Lens placode, LP; Lens epithelium, LE; lens fibers, LF. (C) A diagram of transcription factors implicated for αB-crystallin gene expression in lens placode and lens vesicle. (D) A diagram of transcription factors implicated for αB-crystallin gene expression in primary lens fibers.
A possible model of the temporal and spatial regulation of mouse αB-crystallin gene expression suggests that its expression in the lens placode is triggered by a synergism between Pax6/Pax6(5a) and the retinoic acid nuclear receptors, RARβ/RXRβ. Its transcription could be further bolstered as soon as c-Maf and perhaps MafB appear during the later phase of lens placode growth prior to its invagination (Yang et al., 2004). Maintained expression of αB-crystallin in the lens epithelium would then depend on a combination of Pax6 and c-Maf/MafB. The contribution of the retinoic acid nuclear receptors in mature lens is unclear as expression of RARβ in mouse lens attenuates between E12.5 and E14.5 (Mori et al., 2001). Expression of RXRβ is ubiquitous irrespective of the age of the mouse (Mori et al., 2001).
4.2.3. Regulation of the mouse γ-crystallin cluster
The rodent γ-crystallin gene cluster is comprised of six genes, each encoding a functional protein. Five of these genes are found within approximately 60 kb of genomic DNA with the γF-crystallin gene located 0.6 Mb apart (see Fig. 13A). In contrast, in humans, two genes (ψγE and ψγF) are pseudogenes (Meakin et al., 1987), and only the γC- and γD-crystallins are significantly expressed in lens. Six rodent γ-crystallin genes are simultaneously activated in the embryonic lens but differentially inactivated during postnatal lens development (Klok et al., 1998). Expression of the last active gene, the γB-crystallin gene, is attenuated after 3 months in rat (van Leen et al., 1987). The initial sequence analysis of the 5’-flanking region of each γ-crystallin gene raised the possibility that they operate via a common regulatory mechanism. Alternatively, it is possible that they share a common LCR. Genetic and molecular studies have indeed provided evidence supporting both a common and a gene-specific regulatory mechanism as described below.
Figure 13. Transcriptional regulation of the mouse γ-crystallin cluster.

(A) Schematic diagram of the mammalian γ-crystallin cluster. (B) A diagram of transcription factors implicated for γF-crystallin gene inactivation in lens placode and lens vesicle. (C) A diagram of transcription factors implicated for γF-crystallin gene expression in primary lens fibers.
Most functional studies of γ-crystallin gene regulation were performed using the γD- (Civil et al., 2002; Dirks et al., 1996a; Klok et al., 1998) and γF-crystallin promoters (see Cvekl et al., 2004). A promoter fragment (−759/+45) of the mouse γF-crystallin promoter fused to the lacZ reporter gene essentially reproduced the expression of the endogenous gene (Goring et al., 1987). Shorter fragments, -171/+45 or −67/+45, were expressed only in central lens fibers (Goring et al., 1993). Cell culture experiments identified two enhancer-like regions, -392/-278 and −226/-121; however, they were never tested in transgenic mice (Yu et al., 1990). Transcription factor binding sites for Pax6 (Kralova et al., 2002), RARβ/RXRβ (Tini et al., 1993) Six3, Prox1 (Lengler et al., 2001), Hsf4 (Fujimoto et al., 2004), Sox1 and Sox2 (Kamachi et al., 1995), large Mafs (Rajaram and Kerppola, 2004) and TFIID were identified within the −226 to +45 fragment (see Fig. 13). Based on studies of protein-DNA interactions and expression patterns of these factors, we recently proposed a regulatory model of the mouse γF-crystallin gene (Yang et al., 2004).
During lens placode formation and its transition into the lens vesicle, the γF-crystallin gene is transcriptionally inactive. Co-expression of Pax6, Six3, Sox2, RARβ/RXRβ, c-Maf and perhaps MafB in the lens vesicle suggests that each of these factors associates with the 5’-flanking region of the mouse γF-crystallin promoter, a situation that remains to be experimentally confirmed. Cell culture experiments showed that both Pax6 (Kralova et al., 2002; Lengler et al., 2001; Yang et al., 2004) and Six3 (Lengler et al., 2001; Yang et al., 2004) act as transcriptional repressors of the mouse γF-crystallin promoter fragment −226 to +45. The repressive function of Six3 can be mediated by the recruitment of transcriptional co-repressors Grg4 and Grg5 (Zhu et al., 2002). In addition, Bcl-6 co-repressor, BCOR (Wamstad and Bardwell, 2007), is a potential candidate as a Pax6-bound co-repressor (Horn et al., 2005; Ng et al., 2004). In primary lens fibers, expression of Pax6 is reduced and expression of Six3, RARβ/RXRβ, and Sox2 is eliminated. In contrast, expression of c-Maf, Sox1 and Hsf4 is dramatically upregulated. Both transcriptional co-activators of c-Maf, p300 and CBP, are also co-expressed in lens fiber cells (Yang et al. 2007). Thus, we propose that upregulation of γF-crystallin in primary lens fibers requires a relief from Pax6- and Six3-mediated repression followed by synergistic action of c-Maf and Sox1 (Rajaram and Kerppola, 2004; Yang et al., 2004) via recruitment of HATs, p300 and CBP (Q. Chen et al., 2002). c-Maf and Sox1 are joined by Hsf4 from E12.5 onward (Fujimoto et al., 2004; Min et al., 2004). As Hsf4 recruits Brg1, and, by inference the SWI/SNF complexes (Tu et al., 2006), it is possible that γ-crystallin promoters also use Brg1 for their chromatin remodeling.
The above model is supported by analyses of γF-crystallin gene expression in mice with inactivated c-Maf (Kawauchi et al., 1999; Ring et al., 2000), Sox1 (Nishiguchi et al., 1998) and Hsf4 (Fujimoto et al., 2004) genes since all of these exhibit reductions in γF-crystallin levels in the lens. In addition, γB-, γE- and γF-crystallin levels are also reduced in both c-Maf and Sox1 null lenses. Interestingly, expression of γD- and γB-crystallin was below the detection limits in c-Maf and Sox1 null lenses, respectively. The different sensitivities of each γ-crystallin gene to the lack of either c-Maf, Sox1 or Hsf4 indicates that in spite of their common regulation discussed above, there are specific differences in the transcriptional regulation of individual γ-crystallin genes. This idea is further supported by studies of Prox1 null lenses (Wigle et al., 1999) that showed reduced expression of γB- and γD-crystallin genes but normal expression of γE- and γF-crystallin genes. Sequence variations between γ-crystallin promoters was proposed to account for different levels of their expression including a positively acting element, -15/-4 in the rat γD-crystallin promoter that is absent in the rat γB-crystallin promoter (Klok et al., 1998). Similarly, a putative AP-1 binding site next to the MARE (see Fig. 13) was proposed in the rat γB-crystallin promoter that is absent in the rat γD-crystallin promoter. It would be interesting to find whether the different responses of γ-crystallin genes to c-Maf, Prox1 and Sox1 are caused by single nucleotide variations of their binding sites, methylation dynamics of γ-crystallin promoters (Klok et al., 1998; Peek et al., 1991), or if there are LCRs that preferentially work with specific genes of the γcrystallin locus.
4.2.4. Regulation of mammalian β-crystallin genes
There are six members of the β-crystallin gene family (βA1/A3, βA2, βA4, βB1, βB2, and βB3) dispersed on three mammalian chromosomes. The βA4-crystallin and βB1-crystallin genes are linked head to head and reside in the same general region of the chromosome as the linked βB2-crystallin and βB3-crystallin genes. The human genome has an additional βB2-crystallin derived pseudogene linked to the functional βB2-crystallin locus which can cause gene conversion of the functional locus resulting in human congenital cataract (Vanita et al., 2001). However, transcriptional studies were performed only with the human and mouse βB1-, and the mouse and rat βB2-crystallin genes. Studies on the regulation of the chicken βB1-, βB2- and βA3/A1-crystallin genes are summarized in section 4.2.9.
βB1-crystallin expression is first detectable in elongating lens fiber cell of the lens vesicle and is maintained in lens fiber cells throughout life (Van Leen et al., 1987). Like other crystallin genes, both the human and mouse βB1-crystallin promoters contain canonical TATA-boxes. A series of human βB1-crystallin promoter fragments was examined in transient transgenic assays in zebrafish and the shortest lens-specific promoter region was found in a −90/+61 fragment (Hou et al., 2006). Site-directed mutagenesis of an evolutionary conserved MARE-half site (TGCTGA) within a large genomic fragment (-2966/+61) yielded a significantly decreased expression rate in transgenic zebrafish. Studies of the mouse βB1-crystallin promoter fragment −1493/+44 in transgenic mice found lens fiber cell specific expression albeit at lower levels compared to the shorter −432/+30 chicken βB1-crystallin promoter fragment (Chen et al., 2001). Notably, all fragments of the mouse βB1-crystallin promoter tested in transient transfections in both primary lens epithelial cultures and established lens cell lines were inactive suggesting that the lens fiber cell specificity of this promoter is tightly regulated. It is likely that the mouse βB1-crystallin gene is negatively regulated by Pax6 since endogenous βB1-crystallin levels are repressed in lenses overexpressing Pax6 in lens fiber cells (Duncan et al., 2004b).
βB2-crystallin is expressed at negligible amounts in the embryonic mouse lens, but its expression upregulates sharply at birth and it is the most abundant β-crystallin in the mouse lens by 6 weeks postnatal (van Leen et al., 1987). Endogenous βB2-crystallin gene activity is upregulated in cultured lens cells that overexpress β-catenin suggesting a link between canonical Wnt-signaling and crystallin gene regulation (Lyu and Joo, 2004). The rat βB2-crystallin promoter (Doerwald et al., 2001) was found to have a functional Sox-binding site at position −164/-159 and another cis-acting site (-14/-7) was discovered between the TATA-box and start site of transcription. A possible MARE was also found (-143/-123), however, its mutagenesis did not reduce the promoter activity in differentiating primary lens cells that express c-Maf although activation of the mouse βB2-promoter (−614/+10) by c-Maf was shown in transiently co-transfected fibroblasts (Chen et al., 2002). The proximal promoter also contains a Pax6-binding site (-65/-51) capable of binding Pax6 in vitro (Dirks et al., 1996b) although mutagenesis of this site suggested that this complex was not functionally significant. Since a single Sox-binding site alone is unlikely to be sufficient to confer lens-preferred expression of βB2-crystallin (Chambers et al., 1995); further studies to identify relevant transcription factors are necessary to address this issue. Finally, two additional regulatory regions were identified between −750 and −123 and in the first intron. Individually they act as silencers, together they comprise a stage-specific enhancer (Dirks et al., 1996b). Thus, these distal regulatory elements are prime candidates to study the temporal and spatial regulatory mechanisms of mammalian βB2-crystallin genes.
4.2.5. Regulation of filensin, CP49, integrins and MIP in mammalian lens
Studies of the transcriptional control of non-crystallin structural proteins in the lens are still in their infancy. Filensin (CP94, formerly CP115) and CP49 (formerly phakinin) are lens fiber cell specific intermediate filament proteins which are the major components of the beaded filament lens cytoskeletal network (see Quinlan and Prescott, 2004). The mouse filensin promoter does not contain a TATA-box and transcription starts at either a major site (+1) or a minor site (-136) (Masaki et al., 1998). A minimal promoter region was localized to a short DNA fragment (-70/+40) whose activity is greatly increased in the presence of FGF2. In vitro studies have shown this region to contain AP-2 and Sp1 binding sites and AP-2α interactions with the mouse filensin promoter were recently confirmed in lens chromatin in vivo (Pontoriero et al., 2007). Subsequent studies identified two evolutionary conserved distal enhancer elements (1.7 and 2.1 kb in length); however, their role in vivo remains to be shown (Masaki et al., 2002). The human CP49 promoter is also TATA-less (DePianto et al., 2003b). A promoter fragment −1859/-19 supported lens-preferred expression in transgenic mice (DePianto et al., 2003b). Immunohistochemical and mRNA examination of c-Maf null lenses suggested that c-Maf is not involved in transcriptional control of either mouse filensin or CP49 (DePianto et al., 2003a).
A large number of integrins are expressed in the developing lens, and their expression and distribution marks different stages of lens development (see Menko, 2002). Expression of α5- and β1-integrins was increased in transgenic lenses that overexpressed Pax6(5a) (Duncan et al., 2000). Several Pax6-binding sites were identified in their promoters and binding of Pax6 proteins from lens nuclear extracts were shown. As integrins play diverse roles in signaling during lens differentiation (Simirskii et al., 2007), and, thus, play critical roles in lens homeostasis, studies of their transcriptional control in mouse lens should provide valuable information about the lens differentiation process and extracellular signaling (see Menko and Walker, 2004).
The MIP (major intrinsic protein, also known as MIP26 and aquaporin 0) gene encodes the prevailing fiber cell-specific membrane protein of the ocular lens and is temporally and spatially regulated during development. Lens-specific promoter activity was identified in a -253/+42 fragment of the human MIP gene in lens cell cultures (Wang et al., 1995). Overexpression of the transcription factor AP-2α in lens fiber cells reduced expression of MIP (West-Mays et al., 2002) consistent with the identification of a series of AP-2 binding sites in its promoter (Ohtaka-Maruyama et al., 1998). The molecular basis for lens-fiber cell specific expression of MIP is not well known, although c-Maf can be excluded from the list of potential regulators (DePianto et al., 2003a). Recently, it was shown that FGF2, acting via ERK1/2 and JNK signaling pathways, functions on the extended promoter fragment (-1648/+44) (Golestaneh et al., 2004). Additional studies of the MIP promoter are essential for a full understanding of lens fiber cell differentiation.
4.2.6. Regulation of chicken δ1- and δ2 crystallin loci
Chicken δ1-crystallin is a taxon specific crystallin that was recruited from the argininosuccinate lyase (δ2 crystallin) gene by gene duplication and addition of lens specific regulatory elements. The entire δ1-crystallin locus was found to be methylated in non-lens cells while the locus was hypomethylated in lens cells expressing δ1-crystallin (Sullivan et al., 1989). The 5’ flanking sequence of the δ1-crystallin gene is only weakly active in transfections and lens specific expression was found to depend on an enhancer located in the third intron (Hayashi et al., 1987). The intron 3 enhancer was dissected into three fragments, B3, B4 and B5 which do not have enhancer activity alone but synergistically interact to form the functional enhancer. Notably, this enhancer controls the retinoic acid inducibility of the δ1-crystallin gene although the mechanism of this response is not known (Li et al., 1997).
Multimers of B4 can enhance lens preferred activation of a heterologous promoter and further analysis narrowed the B4 functional element to the DC5 region which harbors a cluster of binding sites (δEF1, δEF2, and δEF3) for transcriptional activators and repressors (Kamachi and Kondoh, 1993). The factor that binds to the δEF1 element was identified and found to repress intron 3 enhancer activity. The δEF1 binding protein was subsequently found to be structurally related to Smad interacting protein 1 (Sip1) and has been given a variety of names including AREB6, TCF8, ZEB1 and Zfhx1a. High levels of δEF1 are present in the chicken lens although no expression has been reported in the mouse lens consistent with the lack of lens phenotype for the δEF1-knockout mouse (Takagi et al., 1998). However, Sip1 is present in the mouse lens and deletion of the mouse Sip1 gene results in profound defects in early lens development.
Binding sites for the SRY-related proteins, Sox-1 and Sox-2, were identified in the δEF2 element although Sox proteins can only activate a multimerized δEF2 element (Kamachi et al., 1995). Two Pax-6 binding sites are located directly 5’ of δEF2 and Pax-6 activated the expression of a reporter construct driven by the δ1-crystallin promoter linked to the entire intron 3 enhancer (Cvekl et al., 1995b). Subsequently, Pax6 and Sox2 were found to form a complex that binds to the δ1-crystallin enhancer and activates transcription (Kamachi et al., 2001) consistent with the expression patterns of δ1-crystallin, Pax6 and Sox proteins in the developing chicken lens (Kamachi et al., 1998). Little is known about the regulation of the B3 and B5 regions of the chicken δ1-crystallin enhancer although both of these elements contain functional Maf sites (Ogino and Yasuda, 1998).
4.2.7. Regulation of the chicken αA-crystallin gene
The initial transgenic mouse experiments have shown that a −242/+77 fragment of the chicken αA-crystallin gene is expressed in whole lens (Klement et al., 1989). A proximal enhancer (-162/-88) was identified as lens-specific (Matsuo et al., 1991) and was dissected into three synergistically-acting elements, αCE1/2/3 (Matsuo and Yasuda, 1992). Expression of this gene studied in chicken primary cells was linked to large Mafs, initially to MafA/L-Maf (Ogino and Yasuda, 1998), and Pax6 (Cvekl et al., 1994), and later to c-Maf and MafB (Yoshida and Yasuda 2002). The temporal and spatial expression of four large Mafs, MafA/L-Maf, MafB, c-Maf and NRL, in lens varies in chicken, mouse, Xenopus and zebrafish (Coolen et al., 2005). In chick, MafA is first expressed in the PLE followed by expression of c-Maf in the lens placode (Reza et al., 2007). During primary lens fiber cell differentiation, MafA is more expressed in the differentiation lens fibers compared to the lens epithelium. Expression of MafB appears in the lens vesicle (Lecoin et al., 2004). However, expression of MafA is attenuated in fully formed lens of the 14-day old chick embryo (Yoshida and Yasuda, 2002). Expression of MafB and c-Maf persists in the chick lens of this stage. Thus the temporal order of large Mafs in the chicken lens is MafA, c-Maf and MafB (Coolen et al., 2005; Yoshida and Yasuda, 2002). In transient transfections, using the multimerized αCE2/MARE, c-Maf and MafB elicited much higher transcriptional activities compared to MafA (Yoshida and Yasuda, 2002). Both in vitro and in vivo DNA-binding studies are necessary to clarify the molecular mechanisms by which large Maf proteins regulate expression of the chicken αA-crystallin gene.
4.2.8. Regulation of chicken β-crystallin genes
The endogenous chicken βB1-crystallin gene is first expressed in the primary fiber cells of the lens vesicle (Brahma, 1988) and its activity sharply upregulates in late embryonic development to become a major component of the 10 week old chicken lens (Hejtmancik et al., 1985).This gene is linked head to head with the βA4-crystallin gene with a 2147 nt intergenic spacer (see Fig. 14, Duncan et al., 1995). Promoter analysis in transgenic mice revealed that the -432/+30 fragment of the promoter was sufficient to recapitulate both the expression pattern and expression levels of mouse βB1-crystallin (Duncan et al., 1996). A -101/+30 fragment is the minimal lens specific promoter in transgenic mice, although its activity is approximately 1000 fold less than the -432/+30 fragment (Duncan et al., 1996). In primary chicken lens epithelial cell transfections, -126/+30 was found to be the minimal promoter of chicken βB1-crystallin in transfected cells (Roth et al., 1991) although no additional promoter activity was detected with larger fragments. The −126/+30 fragment contains two elements shown to be functional in both transfections and transgenic mice, PL1 (-126/-101) and PL2 (−96 to −76) which share the common core sequence, TGATGA which resembles a consensus Maf responsive element (Fig. 14). EMSA analysis suggests that these elements bind similar factors, although PL2 can confer lens-preferred activation to a heterologous promoter, while PL1 is a more general activator (Duncan et al., 1996). Co-transfection analysis with L-Maf, c-Maf or MafB suggest that Maf proteins can activate the PL2 element (Cui et al., 2004; Ogino and Yasuda, 1998) although the endogenous regulator in the adult chicken lens is likely to be c-Maf since MafB is predominately found in the lens epithelium and L-Maf levels in the lens are decreasing in late embryonic chicken embryos (Ogino and Yasuda, 1998), as βB1-crystallin (β35) mRNA levels increase (Hejtmancik et al., 1985). The chicken βB1-crystallin promoter is activated by Prox1, a homeodomain containing protein essential for lens fiber cell differentiation (Cui et al., 2004; Wigle et al., 1999). This response is partially mediated by the OL2 element, a sequence highly similar to a previously described Prospero binding site (Cui et al., 2004; Hassan et al., 1997). Additional Prox1 binding sites have been identified between -432/-152 although they appear to function as repressive elements in transfected cells (X. Chen et al., submitted). Pax6 has also been shown to interact with the chicken βB1-crystallin promoter and block the activating functions of Mafs and Prox1 (Cui et al., 2004; Duncan et al., 1998). Since Pax6 is predominately expressed in the lens epithelium, while βB1-crystallin is highly lens fiber cell preferred, this leads to a model in which Pax6 prevents inappropriate βB1-crystallin expression in the lens epithelium.
Figure 14. Transcriptional regulation of the chicken βB1-crystallin gene.
(A) Schematic diagram of the human β-crystallin cluster. (B) A diagram of transcription factors implicated for chicken βB1-crystallin gene inactivation in lens placode and lens vesicle. (C) A diagram of transcription factors implicated for βB1-crystallin gene expression in differentiated lens fibers.
The only other chicken β-crystallin gene whose transcriptional regulation has been studied is βA3/A1-crystallin. The minimal promoter necessary for promoter function in transfected chicken patched lens epithelial cells is −382/+22 (McDermott et al., 1996) which contains a T-rich region and an enhancer located between -287/-254. The enhancer contains multiple binding sites for basic leucine zipper transcription factors such as AP-1 and c-Maf although the participation of these factors in βA3/A1-crystallin expression has not been functionally tested (McDermott et al., 1997). The minimal promoter necessary to drive lens specific expression in mice is −143/+22. This fragment binds to Pax6 and this binding leads to transcriptional repression, much as is seen in the chicken βB1-crystallin promoter (JI Haynes, unpublished data).
4.2.9. Regulation of the guinea pig ζ-crystallin gene
ζ-crystallin is a taxon specific crystallin of guinea pigs which is identical to the metabolic enzyme NADPH:quinone oxidoreductase. This gene was recruited to be a crystallin by the addition of a lens specific TATA containing promoter to the TATA-less promoter that controls ubiquitous expression in other tissues. Promoter fragments −756/+70, -533/+70, -498/+70 (+1 counted from the transcription start site controlled by the TATA containing promoter) were found to drive lens specific expression in transgenic mice while the shorter fragments, -385/+70 and −229/+70 also drove gene expression in the brain (Lee et al., 1994; Sharon-Friling et al., 1998).
Nucleotides -207 to −150 (the ZPE element) were found to control lens preferred promoter activity in transient transfections (Lee et al., 1994) that is important for lens preferred promoter activity in transfected cells. Pax-6 binds to ZPE, and mutation of the Pax-6 site in the context of the −756/+70 guinea pig promoter led to the loss of lens specific expression (Richardson et al., 1995). Further, a Maf response element (MARE) resides adjacent to the Pax-6 site (Sharon-Friling et al., 1998) and complexes in vitro with Nrl1, a member of the Maf subfamily of transcription factors (Sharon-Friling et al., 1998).
5. Conclusions and future directions
In this review, we summarized the most recent advances in our understanding of the genetic pathways and epigenetic regulatory mechanisms that govern vertebrate lens development. Earlier studies on gene regulation mechanisms in lens were aimed to elucidate the phenomenon of tissue-specificity. These studies naturally focused on crystallins genes and showed that lens-specific gene expression employs various combinations of lens lineage-specifying factors (Pax6, Six3 and Sox2), factors regulating the lens differentiation process (c-Maf, Hsf4, pRb, Prox1, RARβ/RXRβ and Sox1), and a diverse group of ubiquitously expressed factors (e.g. AP-1, CREB and USF). It is likely that activities and/or amounts of these transcription factors such as c-Maf, Prox1, Sox1, pRb, AP-1 and CREB are directly regulated by the lens differentiation signals mediated by FGF, BMP/TGF-β, and Wnt signaling pathways. In addition, transcription factors from the Ets family, c-Myc, Foxo1, LEF/TCF, NF-κB, Smad family, and Stat-3 are plausible downstream targets of these signaling pathways in lens and future studies have to address these possibilities. For this purpose, the mouse αA-crystallin gene is an ideal model as the FGF-signaling is mediated in vivo by a distinct enhancer, the DCR1 (Yang et al., 2006). In contrast, its downstream enhancer, DCR3, is a candidate region regulated by BMP/TGF-β and Wnt signaling pathways. Molecular dissection of crystallin gene promoters and enhancers identified a series of smaller regulatory modules, comprised of adjacent Pax6/Maf- (see Cvekl et al., 2004), Sox/Pax6- (see Cvekl et al., 2004; Kondoh et al., 2004), Maf/Sox- (Rajaram and Kerppola, 2004), and Pax6/RARE- (Chauhan et al., 2004; Yang et al., 2004) binding sites. We propose that similar arrays of sites regulate expression of other lens-specific genes, CP49, DNaseIIβ, filensin, and MIP/aquaporin0. In summary, the unique temporal and spatial expression patterns of individual crystallin genes originate from the precise location of Pax6/Maf-, Sox/Pax6-, Maf/Sox- and Pax6/RARE-modules, presence of additional cis-elements (e.g. AP-1-sites, CREs, HSEs and Prox1-binding sites) that associate with distinct menus of transcription factors in different compartments of the developing lens at different developmental stages.
The focus of current studies on lens development is on analysis of chromatin bearing lens-regulatory and structural genes during lens differentiation and addressing the plethora of epigenetic aspects of lens development. The epigenetic regulatory mechanisms draw attention of developmental biologists as they help to explain multiple developmental outcomes from common progenitor cells, the gradual specification of individual cell lineages and the differentiation process through the specific mechanisms of gene activation and repression controlled not only be specific DNA-binding transcription factors (see above) but the chromatin remodeling enzymes, methylation of DNA, via untranslated regulatory RNAs and functional compartmentalization of the nucleus (see Margueron et al., 2005; Fig. 1A). There are two special features of the lens differentiation program that make studies of this tissue particularly interesting. Lens differentiation culminates with denucleation, and, thus the final epigenetic code of this program is its self-destruction. Understanding the mechanistic aspects of this “final” stage suggest a possibility to activate and employ this process in other cell types such as cancer cells. Lens can also regenerate in certain species from dorsal iris, RPE and corneal epithelium (Grogg et al., 2005; Tsonis et al., 2004; Alvarado and Tsonis, 2006; see Fig. 4). Lens regeneration proceeds via dedifferentiation to reach a specific stage from which the “rescue” lens-differentiation program begins. It will be interesting to find how this process relates to the normal lens differentiation pathway. Nevertheless, lens regeneration illustrates the reversibility of epigenetic pathways that offer clues to understand genome reprogramming, a process with enormous significance to generate patient-derived stem cells (see Hochedlinger and Jaenisch, 2006).
Herein, we explain emergence of the lens lineage from the PPR as a result of the intersection of multiple intracellular signaling processes with cumulative effects and aided by extensive cell movements and cell-sorting that pattern the PPR. A large body of work remains to be done to characterize these processes at molecular level and to understand involvement of every participating embryonic structure and signaling centers (see Donner et al., 2006b; Medina-Martinez and Jamrich, 2007). Identification of novel mutants with disrupted eye development during the transitional states such as the medaka mutation eyeless (el) (Winkler et al., 2000) and delineation of Pax6, Sox2 and Six3 signal transduction regulated enhancers are thought to provide novel insights into the signaling during that regulate early stages of lens development. Single-cell visualization techniques in living embryos using a repertoire of fluorescent reporters driven by lineage-specific promoters/enhancers and reporters responding to specific extracellular signals are expected to play pivotal roles in these studies as recently shown by Wittbrodt and colleagues (Rembold et al., 2006). Use of each experimental model (chick, fish, frog, and mouse) offers its own advantages and limitations. Combination of data from these models will hopefully address the remaining questions in the molecular biology of eye and lens formation. In conclusion, lens research has reached the point in which transcription factor occupancy can be compared with the profiles of core histone modifications and local chromatin structure at any gene of interest (Yang et al., 2006) as well as the whole genome level (see Blais and Dynlacht, 2005; Ren and Dynlacht, 2004). The future work will ultimately lead to better understanding of embryonic eye development, lens biology and disease.
Acknowledgments
We thank Mrs. Tatyana Harris for preparing the illustrations. We thank Drs. Ruth Ashery-Padan, Milan Jamrich, Louise Wolf and Ying Yang for critical suggestions. Supported by grants from EY12200 (AC), EY012221 (MKD), EY014237 (AC) and EY017296 (AC). AC is a recipient of the Irma T. Hirschl Career Scientist Award.
Abbreviations
- HAT
Histone acetyltransferase
- NC
neural crest
- PPR
pre-placodal region
- (PLE)
prospective lens ectoderm
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarado AS, Tsonis PA. Bridging the regeneration gap: genetic insights from diverse animal models. Nat Rev Genet. 2006;7:873–884. doi: 10.1038/nrg1923. [DOI] [PubMed] [Google Scholar]
- Angelov D, Molla A, Perche PY, Hans F, Cote J, Khochbin S, Bouvet P, Dimitrov S. The histone variant macroH2A interferes with transcription factor binding and SWI/SNF nucleosome remodeling. Mol Cell. 2003;11:1033–1041. doi: 10.1016/s1097-2765(03)00100-x. [DOI] [PubMed] [Google Scholar]
- Aota S, Nakajima N, Sakamoto R, Watanabe S, Ibaraki N, Okazaki K. Pax6 autoregulation mediated by direct interaction of Pax6 protein with the head surface ectoderm-specific enhancer of the mouse Pax6 gene. Dev Biol. 2003;257:1–13. doi: 10.1016/s0012-1606(03)00058-7. [DOI] [PubMed] [Google Scholar]
- Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F. Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie. 2006;88:1091–1098. doi: 10.1016/j.biochi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev Cell. 2006;11:505–517. doi: 10.1016/j.devcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Bailey AP, Streit A. Sensory organs: making and breaking the pre-placodal region. Curr Top Dev Biol. 2006;72:167–204. doi: 10.1016/S0070-2153(05)72003-2. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Balkan W, Colbert M, Bock C, Linney E. Transgenic indicator mice for studying activated retinoic acid receptors during development. Proc Natl Acad Sci U S A. 1992;89:3347–3351. doi: 10.1073/pnas.89.8.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J, Blagojevic J, Carter D, Eskiw C, Fromaget M, Job C, Shamsher M, Trindade IF, Xu M, Cook PR. Specialized transcription factories. Biochem Soc Symp. 2006:67–75. doi: 10.1042/bss0730067. [DOI] [PubMed] [Google Scholar]
- Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Beebe D. Lens fiber differentiation. In: Lovicu FJ, Robinson ML, editors. Development of ocular lens. Cambridge University Press; Cambridge: 2004. pp. 214–244. [Google Scholar]
- Baulmann DC, Ohlmann A, Flugel-Koch C, Goswami S, Cvekl A, Tamm ER. Pax6 heterozygous eyes show defects in chamber angle differentiation that are associated with a wide spectrum of other anterior eye segment abnormalities. Mech Dev. 2002;118:3–17. doi: 10.1016/s0925-4773(02)00260-5. [DOI] [PubMed] [Google Scholar]
- Bavik C, Ward SJ, Chambon P. Developmental abnormalities in cultured mouse embryos deprived of retinoic by inhibition of yolk-sac retinol binding protein synthesis. Proc Natl Acad Sci U S A. 1996;93:3110–3114. doi: 10.1073/pnas.93.7.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D, Garcia C, Wang X, Rajagopal R, Feldmeier M, Kim JY, Chytil A, Moses H, Ashery-Padan R, Rauchman M. Contributions by members of the TGFbeta superfamily to lens development. Int J Dev Biol. 2004;48:845–856. doi: 10.1387/ijdb.041869db. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Benkhelifa S, Provot S, Nabais E, Eychene A, Calothy G, Felder-Schmittbuhl MP. Phosphorylation of MafA is essential for its transcriptional and biological properties. Mol Cell Biol. 2001;21:4441–4452. doi: 10.1128/MCB.21.14.4441-4452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bailey AP, Bronner-Fraser M, Streit A. Segregation of lens and olfactory precursors from a common territory: cell sorting and reciprocity of Dlx5 and Pax6 expression. Dev Biol. 2004;271:403–414. doi: 10.1016/j.ydbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Blais A, Dynlacht BD. Constructing transcriptional regulatory networks. Genes Dev. 2005;19:1499–1511. doi: 10.1101/gad.1325605. [DOI] [PubMed] [Google Scholar]
- Boros J, Newitt P, Wang Q, McAvoy JW, Lovicu FJ. Sef and Sprouty expression in the developing ocular lens: Implications for regulating lens cell proliferation and differentiation. Semin Cell Dev Biol. 2006;17:741–752. doi: 10.1016/j.semcdb.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Bowen NJ, Fujita N, Kajita M, Wade PA. Mi-2/NuRD: multiple complexes for many purposes. Biochim Biophys Acta. 2004;1677:52–57. doi: 10.1016/j.bbaexp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Latek RR, Peterson CL. The SANT domain: a unique histone-tail-binding module? Nat Rev Mol Cell Biol. 2004;5:158–163. doi: 10.1038/nrm1314. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahma SK. Ontogeny of beta B1-crystallin polypeptide during chicken lens development. Exp Eye Res. 1988;47:507–510. doi: 10.1016/0014-4835(88)90060-7. [DOI] [PubMed] [Google Scholar]
- Brownell I, Dirksen M, Jamrich M. Forkhead Foxe3 maps to the dysgenetic lens locus and is critical in lens development and differentiation. Genesis. 2000;27:81–93. doi: 10.1002/1526-968x(200006)27:2<81::aid-gene50>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Pandur PD, Kenyon KL, Pignoni F, Moody SA. Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development. 2004;131:5871–5881. doi: 10.1242/dev.01516. [DOI] [PubMed] [Google Scholar]
- Brun ME, Gasca S, Girard C, Bouton K, De Massy B, De Sario A. Characterization and expression analysis during embryo development of the mouse ortholog of MLL3. Gene. 2006;371:25–33. doi: 10.1016/j.gene.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, Randazzo F, Metzger D, Chambon P, Crabtree G, Magnuson T. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell. 2000;6:1287–1295. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- Bultman SJ, Gebuhr TC, Magnuson T. A Brg1 mutation that uncouples ATPase activity from chromatin remodeling reveals an essential role for SWI/SNF-related complexes in beta-globin expression and erythroid development. Genes Dev. 2005;19:2849–2861. doi: 10.1101/gad.1364105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M. Chromatin unfolding and activation by HMGN(*) chromosomal proteins. Trends Biochem Sci. 2001;26:431–437. doi: 10.1016/s0968-0004(01)01855-2. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- Caron H, van Schaik B, van der Mee M, Baas F, Riggins G, van Sluis P, Hermus MC, van Asperen R, Boon K, Voute PA, Heisterkamp S, van Kampen A, Versteeg R. The human transcriptome map: clustering of highly expressed genes in chromosomal domains. Science. 2001;291:1289–1292. doi: 10.1126/science.1056794. [DOI] [PubMed] [Google Scholar]
- Catez F, Brown DT, Misteli T, Bustin M. Competition between histone H1 and HMGN proteins for chromatin binding sites. EMBO Rep. 2002;3:760–766. doi: 10.1093/embo-reports/kvf156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catez F, Lim JH, Hock R, Postnikov YV, Bustin M. HMGN dynamics and chromatin function. Biochem Cell Biol. 2003;81:113–122. doi: 10.1139/o03-040. [DOI] [PubMed] [Google Scholar]
- Chamberlain CG, McAvoy JW. Induction of lens fibre differentiation by acidic and basic fibroblast growth factor (FGF) Growth Factors. 1989;1:125–134. doi: 10.3109/08977198909029122. [DOI] [PubMed] [Google Scholar]
- Chambers C, Cvekl A, Sax CM, Russell P. Sequence, initial functional analysis and protein-DNA binding sites of the mouse beta B2-crystallin-encoding gene. Gene. 1995;166:287–292. doi: 10.1016/0378-1119(95)00615-x. [DOI] [PubMed] [Google Scholar]
- Chambers I. The molecular basis of pluripotency in mouse embryonic stem cells. Cloning Stem Cells. 2004;6:386–391. doi: 10.1089/clo.2004.6.386. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chauhan BK, Reed NA, Yang Y, Cermak L, Reneker L, Duncan MK, Cvekl A. A comparative cDNA microarray analysis reveals a spectrum of genes regulated by Pax6 in mouse lens. Genes Cells. 2002;7:1267–1283. doi: 10.1046/j.1365-2443.2002.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan BK, Yang Y, Cveklova K, Cvekl A. Functional interactions between alternatively spliced forms of Pax6 in crystallin gene regulation and in haploinsufficiency. Nucleic Acids Res. 2004;32:1696–1709. doi: 10.1093/nar/gkh334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Dowhan DH, Liang D, Moore DD, Overbeek PA. CREB-binding protein/p300 co-activation of crystallin gene expression. J Biol Chem. 2002;277:24081–24089. doi: 10.1074/jbc.M201821200. [DOI] [PubMed] [Google Scholar]
- Chen WV, Fielding Hejtmancik J, Piatigorsky J, Duncan MK. The mouse beta B1-crystallin promoter: strict regulation of lens fiber cell specificity. Biochim Biophys Acta. 2001;1519:30–38. doi: 10.1016/s0167-4781(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Stump RJ, Lovicu FJ, McAvoy JW. A role for Wnt/planar cell polarity signaling during lens fiber cell differentiation? Semin Cell Dev Biol. 2006;17:712–725. doi: 10.1016/j.semcdb.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepelinsky AB. The ocular lens fiber membrane specific protein MIP/Aquaporin 0. J Exp Zoolog A Comp Exp Biol. 2003;300:41–46. doi: 10.1002/jez.a.10307. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Christensen J, Agger K, Cloos PA, Pasini D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE, Helin K. RBP2 Belongs to a Family of Demethylases, Specific for Tri-and Dimethylated Lysine 4 on Histone 3. Cell. 2007;128:1063–1076. doi: 10.1016/j.cell.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Civil A, van Genesen ST, Lubsen NH. c-Maf, the gammaD-crystallin Maf-responsive element and growth factor regulation. Nucleic Acids Res. 2002;30:975–982. doi: 10.1093/nar/30.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapier CR, Langst G, Corona DF, Becker PB, Nightingale KP. Critical role for the histone H4 N terminus in nucleosome remodeling by ISWI. Mol Cell Biol. 2001;21:875–883. doi: 10.1128/MCB.21.3.875-883.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson JM, Hill RE, West JD. Analysis of mouse eye development with chimeras and mosaics. Int J Dev Biol. 2004;48:793–804. doi: 10.1387/ijdb.041885jc. [DOI] [PubMed] [Google Scholar]
- Coolen M, Sii-Felice K, Bronchain O, Mazabraud A, Bourrat F, Retaux S, Felder-Schmittbuhl MP, Mazan S, Plouhinec JL. Phylogenomic analysis and expression patterns of large Maf genes in Xenopus tropicalis provide new insights into the functional evolution of the gene family in osteichthyans. Dev Genes Evol. 2005;215:327–339. doi: 10.1007/s00427-005-0476-y. [DOI] [PubMed] [Google Scholar]
- Cosma MP. Ordered recruitment: gene-specific mechanism of transcription activation. Mol Cell. 2002;10:227–236. doi: 10.1016/s1097-2765(02)00604-4. [DOI] [PubMed] [Google Scholar]
- Cremer T, Kurz A, Zirbel R, Dietzel S, Rinke B, Schrock E, Speicher MR, Mathieu U, Jauch A, Emmerich P, Scherthan H, Ried T, Cremer C, Lichter P. Role of chromosome territories in the functional compartmentalization of the cell nucleus. Cold Spring Harb Symp Quant Biol. 1993;58:777–792. doi: 10.1101/sqb.1993.058.01.085. [DOI] [PubMed] [Google Scholar]
- Cui W, Tomarev SI, Piatigorsky J, Chepelinsky AB, Duncan MK. Mafs, Prox1, and Pax6 can regulate chicken betaB1-crystallin gene expression. J Biol Chem. 2004;279:11088–11095. doi: 10.1074/jbc.M312414200. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Kashanchi F, Sax CM, Brady JN, Piatigorsky J. Transcriptional regulation of the mouse alpha A-crystallin gene: activation dependent on a cyclic AMP-responsive element (DE1/CRE) and a Pax-6-binding site. Mol Cell Biol. 1995a;15:653–660. doi: 10.1128/mcb.15.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Piatigorsky J. Lens development and crystallin gene expression: many roles for Pax-6. Bioessays. 1996;18:621–630. doi: 10.1002/bies.950180805. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Sax CM, Bresnick EH, Piatigorsky J. A complex array of positive and negative elements regulates the chicken alpha A-crystallin gene: involvement of Pax-6, USF, CREB and/or CREM, and AP-1 proteins. Mol Cell Biol. 1994;14:7363–7376. doi: 10.1128/mcb.14.11.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Sax CM, Li X, McDermott JB, Piatigorsky J. Pax-6 and lens-specific transcription of the chicken delta 1-crystallin gene. Proc Natl Acad Sci U S A. 1995b;92:4681–4685. doi: 10.1073/pnas.92.10.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Yang Y, Chauhan BK, Cveklova K. Regulation of gene expression by Pax6 in ocular cells: a case of tissue-preferred expression of crystallins in lens. Int J Dev Biol. 2004;48:829–844. doi: 10.1387/ijdb.041866ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EH, Erwin DH. Gene regulatory networks and the evolution of animal body plans. Science. 2006;311:796–800. doi: 10.1126/science.1113832. [DOI] [PubMed] [Google Scholar]
- Dawid IB. Organizing the vertebrate embryo--a balance of induction and competence. PLoS Biol. 2004;2:E127. doi: 10.1371/journal.pbio.0020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iongh RU, Abud HE, Hime GR. WNT/Frizzled signaling in eye development and disease. Front Biosci. 2006;11:2442–2464. doi: 10.2741/1982. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Lovicu FJ, Overbeek PA, Schneider MD, Joya J, Hardeman ED, McAvoy JW. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development. 2001;128:3995–4010. doi: 10.1242/dev.128.20.3995. [DOI] [PubMed] [Google Scholar]
- de la Serna IL, Ohkawa Y, Berkes CA, Bergstrom DA, Dacwag CS, Tapscott SJ, Imbalzano AN. MyoD targets chromatin remodeling complexes to the myogenin locus prior to forming a stable DNA-bound complex. Mol Cell Biol. 2005;25:3997–4009. doi: 10.1128/MCB.25.10.3997-4009.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Serna IL, Ohkawa Y, Imbalzano AN. Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers. Nat Rev Genet. 2006;7:461–473. doi: 10.1038/nrg1882. [DOI] [PubMed] [Google Scholar]
- Dean A. On a chromosome far, far away: LCRs and gene expression. Trends Genet. 2006;22:38–45. doi: 10.1016/j.tig.2005.11.001. [DOI] [PubMed] [Google Scholar]
- DePianto DJ, Blankenship TN, Hess JF, FitzGerald PG. Analysis of non-crystallin lens fiber cell gene expression in c-Maf -/- mice. Mol Vis. 2003a;9:288–294. [PubMed] [Google Scholar]
- DePianto DJ, Hess JF, Blankenship TN, FitzGerald PG. Isolation and characterization of the human CP49 gene promoter. Invest Ophthalmol Vis Sci. 2003b;44:235–243. doi: 10.1167/iovs.02-0162. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou MM. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- Dillon N. Gene regulation and large-scale chromatin organization in the nucleus. Chromosome Res. 2006;14:117–126. doi: 10.1007/s10577-006-1027-8. [DOI] [PubMed] [Google Scholar]
- Dillon SC, Zhang X, Trievel RC, Cheng X. The SET-domain protein superfamily: protein lysine methyltransferases. Genome Biol. 2005;6:227. doi: 10.1186/gb-2005-6-8-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks RP, Klok EJ, van Genesen ST, Schoenmakers JG, Lubsen NH. The sequence of regulatory events controlling the expression of the gamma D-crystallin gene during fibroblast growth factor-mediated rat lens fiber cell differentiation. Dev Biol. 1996a;173:14–25. doi: 10.1006/dbio.1996.0003. [DOI] [PubMed] [Google Scholar]
- Dirks RP, Kraft HJ, Van Genesen ST, Klok EJ, Pfundt R, Schoenmakers JG, Lubsen NH. The cooperation between two silencers creates an enhancer element that controls both the lens-preferred and the differentiation stage-specific expression of the rat beta B2-crystallin gene. Eur J Biochem. 1996b;239:23–32. doi: 10.1111/j.1432-1033.1996.0023u.x. [DOI] [PubMed] [Google Scholar]
- Dirscherl SS, Henry JJ, Krebs JE. Neural and eye-specific defects associated with loss of the Imitation Switch (ISWI) chromatin remodeler in Xenopus laevis. Mech Dev. 2005;122:1157–1170. doi: 10.1016/j.mod.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Divina P, Vlcek C, Strnad P, Paces V, Forejt J. Global transcriptome analysis of the C57BL/6J mouse testis by SAGE: evidence for nonrandom gene order. BMC Genomics. 2005;6:29. doi: 10.1186/1471-2164-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JE, Kang YK, Beppu H, Lei H, Li E. Histone H3-K9 methyltransferase ESET is essential for early development. Mol Cell Biol. 2004;24:2478–2486. doi: 10.1128/MCB.24.6.2478-2486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerwald L, Nijveen H, Civil A, van Genesen ST, Lubsen NH. Regulatory elements in the rat betaB2-crystallin promoter. Exp Eye Res. 2001;73:703–710. doi: 10.1006/exer.2001.1077. [DOI] [PubMed] [Google Scholar]
- Doerwald L, van Rheede T, Dirks RP, Madsen O, Rexwinkel R, van Genesen ST, Martens GJ, de Jong WW, Lubsen NH. Sequence and functional conservation of the intergenic region between the head-to-head genes encoding the small heat shock proteins alphaB-crystallin and HspB2 in the mammalian lineage. J Mol Evol. 2004;59:674–686. doi: 10.1007/s00239-004-2659-y. [DOI] [PubMed] [Google Scholar]
- Donner AL, Episkopou V, Maas RL. Sox2 and Pou2f1 interact to control lens and olfactory placode development. Dev Biol. 2006a;303:784–799. doi: 10.1016/j.ydbio.2006.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner AL, Lachke SA, Maas RL. Lens induction in vertebrates: Variations on a conserved theme of signaling events. Semin Cell Dev Biol. 2006b;17:676–685. doi: 10.1016/j.semcdb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Cui W, Oh DJ, Tomarev SI. Prox1 is differentially localized during lens development. Mech Dev. 2002;112:195–198. doi: 10.1016/s0925-4773(01)00645-1. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Cvekl A, Kantorow M, Piatigorsky J. Lens crystallins. In: Lovicu FJ, Robinson ML, editors. Development of ocular lens. Cambridge University Press; Cambridge: 2004a. pp. 119–150. [Google Scholar]
- Duncan MK, Haynes JI, 2nd, Cvekl A, Piatigorsky J. Dual roles for Pax-6: a transcriptional repressor of lens fiber cell-specific beta-crystallin genes. Mol Cell Biol. 1998;18:5579–5586. doi: 10.1128/mcb.18.9.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MK, Haynes JI, 2nd, Piatigorsky J. The chicken beta A4- and beta B1-crystallin-encoding genes are tightly linked. Gene. 1995;162:189–196. doi: 10.1016/0378-1119(95)00363-b. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Kozmik Z, Cveklova K, Piatigorsky J, Cvekl A. Overexpression of PAX6(5a) in lens fiber cells results in cataract and upregulation of (alpha)5(beta)1 integrin expression. J Cell Sci. 2000;113:3173–3185. doi: 10.1242/jcs.113.18.3173. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Li X, Ogino H, Yasuda K, Piatigorsky J. Developmental regulation of the chicken beta B1-crystallin promoter in transgenic mice. Mech Dev. 1996;57:79–89. doi: 10.1016/0925-4773(96)00533-3. [DOI] [PubMed] [Google Scholar]
- Duncan MK, Xie L, David LL, Robinson ML, Taube JR, Cui W, Reneker LW. Ectopic Pax6 expression disturbs lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 2004b;45:3589–3598. doi: 10.1167/iovs.04-0151. [DOI] [PubMed] [Google Scholar]
- Enwright JF, 3rd, Grainger RM. Altered retinoid signaling in the heads of small eye mouse embryos. Dev Biol. 2000;221:10–22. doi: 10.1006/dbio.2000.9652. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Faber SC, Robinson ML, Makarenkova HP, Lang RA. Bmp signaling is required for development of primary lens fiber cells. Development. 2002;129:3727–3737. doi: 10.1242/dev.129.15.3727. [DOI] [PubMed] [Google Scholar]
- Fan Y, Nikitina T, Morin-Kensicki EM, Zhao J, Magnuson TR, Woodcock CL, Skoultchi AI. H1 linker histones are essential for mouse development and affect nucleosome spacing in vivo. Mol Cell Biol. 2003;23:4559–4572. doi: 10.1128/MCB.23.13.4559-4572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Nikitina T, Zhao J, Fleury TJ, Bhattacharyya R, Bouhassira EE, Stein A, Woodcock CL, Skoultchi AI. Histone H1 depletion in mammals alters global chromatin structure but causes specific changes in gene regulation. Cell. 2005;123:1199–1212. doi: 10.1016/j.cell.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Feldman N, Gerson A, Fang J, Li E, Zhang Y, Shinkai Y, Cedar H, Bergman Y. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nat Cell Biol. 2006;8:188–194. doi: 10.1038/ncb1353. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Fisher M, Grainger R. Lens induction and determination. In: Lovicu FJ, Robinson ML, editors. Development of the ocular lens. Cambridge University Press; Cambridge: 2004. pp. 27–47. [Google Scholar]
- Fujimoto M, Izu H, Seki K, Fukuda K, Nishida T, Yamada S, Kato K, Yonemura S, Inouye S, Nakai A. HSF4 is required for normal cell growth and differentiation during mouse lens development. Embo J. 2004;23:4297–4306. doi: 10.1038/sj.emboj.7600435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher BC, Henry JJ, Grainger RM. Inductive processes leading to inner ear formation during Xenopus development. Dev Biol. 1996;175:95–107. doi: 10.1006/dbio.1996.0098. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Bronner-Fraser M. Neural crest specification: migrating into genomics. Nat Rev Neurosci. 2003;4:795–805. doi: 10.1038/nrn1219. [DOI] [PubMed] [Google Scholar]
- Gangloff YG, Romier C, Thuault S, Werten S, Davidson I. The histone fold is a key structural motif of transcription factor TFIID. Trends Biochem Sci. 2001;26:250–257. doi: 10.1016/s0968-0004(00)01741-2. [DOI] [PubMed] [Google Scholar]
- Garcia CM, Yu K, Zhao H, Ashery-Padan R, Ornitz DM, Robinson ML, Beebe DC. Signaling through FGF receptor-2 is required for lens cell survival and for withdrawal from the cell cycle during lens fiber cell differentiation. Dev Dyn. 2005;233:516–527. doi: 10.1002/dvdy.20356. [DOI] [PubMed] [Google Scholar]
- Gaszner M, Felsenfeld G. Insulators: exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet. 2006;7:703–713. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- Gjerset R, Gorka C, Hasthorpe S, Lawrence JJ, Eisen H. Developmental and hormonal regulation of protein H1 degrees in rodents. Proc Natl Acad Sci U S A. 1982;79:2333–2337. doi: 10.1073/pnas.79.7.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestaneh N, Fan J, Fariss RN, Lo WK, Zelenka PS, Chepelinsky AB. Lens major intrinsic protein (MIP)/aquaporin 0 expression in rat lens epithelia explants requires fibroblast growth factor-induced ERK and JNK signaling. J Biol Chem. 2004;279:31813–31822. doi: 10.1074/jbc.M403473200. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Gopal-Srivastava R, Cvekl A, Piatigorsky J. Pax-6 and alphaB-crystallin/small heat shock protein gene regulation in the murine lens. Interaction with the lens-specific regions, LSR1 and LSR2. J Biol Chem. 1996;271:23029–23036. doi: 10.1074/jbc.271.38.23029. [DOI] [PubMed] [Google Scholar]
- Gopal-Srivastava R, Cvekl A, Piatigorsky J. Involvement of retinoic acid/retinoid receptors in the regulation of murine alphaB-crystallin/small heat shock protein gene expression in the lens. J Biol Chem. 1998;273:17954–17961. doi: 10.1074/jbc.273.28.17954. [DOI] [PubMed] [Google Scholar]
- Gopal-Srivastava R, Haynes JI, 2nd, Piatigorsky J. Regulation of the murine alpha B-crystallin/small heat shock protein gene in cardiac muscle. Mol Cell Biol. 1995;15:7081–7090. doi: 10.1128/mcb.15.12.7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goring DR, Bryce DM, Tsui LC, Breitman ML, Liu Q. Developmental regulation and cell type-specific expression of the murine gamma F-crystallin gene is mediated through a lens-specific element containing the gamma F-1 binding site. Dev Dyn. 1993;196:143–152. doi: 10.1002/aja.1001960208. [DOI] [PubMed] [Google Scholar]
- Goring DR, Rossant J, Clapoff S, Breitman ML, Tsui LC. In situ detection of beta-galactosidase in lenses of transgenic mice with a gamma-crystallin/lacZ gene. Science. 1987;235:456–458. doi: 10.1126/science.3099390. [DOI] [PubMed] [Google Scholar]
- Gotoh N, Ito M, Yamamoto S, Yoshino I, Song N, Wang Y, Lax I, Schlessinger J, Shibuya M, Lang RA. Tyrosine phosphorylation sites on FRS2alpha responsible for Shp2 recruitment are critical for induction of lens and retina. Proc Natl Acad Sci U S A. 2004;101:17144–17149. doi: 10.1073/pnas.0407577101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudreau G, Petrou P, Reneker LW, Graw J, Loster J, Gruss P. Mutually regulated expression of Pax6 and Six3 and its implications for the Pax6 haploinsufficient lens phenotype. Proc Natl Acad Sci U S A. 2002;99:8719–8724. doi: 10.1073/pnas.132195699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan V, Overbeek PA. Secreted FGFR3, but not FGFR1, inhibits lens fiber differentiation. Development. 2001;128:1617–1627. doi: 10.1242/dev.128.9.1617. [DOI] [PubMed] [Google Scholar]
- Graham A, Begbie J. Neurogenic placodes: a common front. Trends Neurosci. 2000;23:313–316. doi: 10.1016/s0166-2236(00)01606-4. [DOI] [PubMed] [Google Scholar]
- Grainger RM. Embryonic lens induction: shedding light on vertebrate tissue determination. Trends Genet. 1992;8:349–355. doi: 10.1016/0168-9525(92)90280-h. [DOI] [PubMed] [Google Scholar]
- Gregg RG, Willer GB, Fadool JM, Dowling JE, Link BA. Positional cloning of the young mutation identifies an essential role for the Brahma chromatin remodeling complex in mediating retinal cell differentiation. Proc Natl Acad Sci U S A. 2003;100:6535–6540. doi: 10.1073/pnas.0631813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griep AE. Cell cycle regulation in the developing lens. Semin Cell Dev Biol. 2006;17:686–697. doi: 10.1016/j.semcdb.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogg MW, Call MK, Okamoto M, Vergara MN, Del Rio-Tsonis K, Tsonis PA. BMP inhibition-driven regulation of six-3 underlies induction of newt lens regeneration. Nature. 2005;438:858–862. doi: 10.1038/nature04175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager GL, Elbi C, Johnson TA, Voss T, Nagaich AK, Schiltz RL, Qiu Y, John S. Chromatin dynamics and the evolution of alternate promoter states. Chromosome Res. 2006;14:107–116. doi: 10.1007/s10577-006-1030-0. [DOI] [PubMed] [Google Scholar]
- Hassan B, Li L, Bremer KA, Chang W, Pinsonneault J, Vaessin H. Prospero is a panneural transcription factor that modulates homeodomain protein activity. Proc Natl Acad Sci USA. 1997;94:10991–10996. doi: 10.1073/pnas.94.20.10991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatini V, Ye X, Balas G, Lai E. Dynamics of placodal lineage development revealed by targeted transgene expression. Dev Dyn. 1999;215:332–343. doi: 10.1002/(SICI)1097-0177(199908)215:4<332::AID-AJA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Goto K, Okada TS, Kondoh H. Lens-specific enhancer in the third intron regulates expression of the chicken delta 1-crystallin gene. Genes Dev. 1987;1:818–828. doi: 10.1101/gad.1.8.818. [DOI] [PubMed] [Google Scholar]
- Haynes JI, 2nd, Duncan MK, Piatigorsky J. Spatial and temporal activity of the alpha B-crystallin/small heat shock protein gene promoter in transgenic mice. Dev Dyn. 1996;207:75–88. doi: 10.1002/(SICI)1097-0177(199609)207:1<75::AID-AJA8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Hejtmancik JF, Beebe DC, Ostrer H, Piatigorsky J. δ- and β-crystallin mRNA levels in the embryonic and posthatched chicken lens: temporal and spatial changes during development. Dev Biol. 1985;109:72–81. doi: 10.1016/0012-1606(85)90347-1. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Grainger RM. Inductive interactions in the spatial and temporal restriction of lens-forming potential in embryonic ectoderm of Xenopus laevis. Dev Biol. 1987;124:200–214. doi: 10.1016/0012-1606(87)90472-6. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Grainger RM. Early tissue interactions leading to embryonic lens formation in Xenopus laevis. Dev Biol. 1990;141:149–163. doi: 10.1016/0012-1606(90)90110-5. [DOI] [PubMed] [Google Scholar]
- Ho Y, Elefant F, Liebhaber SA, Cooke NE. Locus control region transcription plays an active role in long-range gene activation. Mol Cell. 2006;23:365–375. doi: 10.1016/j.molcel.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441:1061–1067. doi: 10.1038/nature04955. [DOI] [PubMed] [Google Scholar]
- Horn D, Chyrek M, Kleier S, Luttgen S, Bolz H, Hinkel GK, Korenke GC, Riess A, Schell-Apacik C, Tinschert S, Wieczorek D, Gillessen-Kaesbach G, Kutsche K. Novel mutations in BCOR in three patients with oculo-facio-cardio-dental syndrome, but none in Lenz microphthalmia syndrome. Eur J Hum Genet. 2005;13:563–569. doi: 10.1038/sj.ejhg.5201391. [DOI] [PubMed] [Google Scholar]
- Hou HH, Kuo MY, Luo YW, Chang BE. Recapitulation of human betaB1-crystallin promoter activity in transgenic zebrafish. Dev Dyn. 2006;235:435–443. doi: 10.1002/dvdy.20652. [DOI] [PubMed] [Google Scholar]
- Huh S, Hatini V, Marcus RC, Li SC, Lai E. Dorsal-ventral patterning defects in the eye of BF-1-deficient mice associated with a restricted loss of shh expression. Dev Biol. 1999;211:53–63. doi: 10.1006/dbio.1999.9303. [DOI] [PubMed] [Google Scholar]
- Hussain MA, Habener JF. Glucagon gene transcription activation mediated by synergistic interactions of pax-6 and cdx-2 with the p300 co-activator. J Biol Chem. 1999;274:28950–28957. doi: 10.1074/jbc.274.41.28950. [DOI] [PubMed] [Google Scholar]
- Im H, Grass JA, Johnson KD, Kim SI, Boyer ME, Imbalzano AN, Bieker JJ, Bresnick EH. Chromatin domain activation via GATA-1 utilization of a small subset of dispersed GATA motifs within a broad chromosomal region. Proc Natl Acad Sci U S A. 2005;102:17065–17070. doi: 10.1073/pnas.0506164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioshikhes IP, Albert I, Zanton SJ, Pugh BF. Nucleosome positions predicted through comparative genomics. Nat Genet. 2006;38:1210–1215. doi: 10.1038/ng1878. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Yasuda K. Distinct roles of maf genes during Xenopus lens development. Mech Dev. 2001;101:155–166. doi: 10.1016/s0925-4773(00)00585-2. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Wurdak H, Schwerdtfeger K, Kunz T, Ille F, Leveen P, Hjalt TA, Suter U, Karlsson S, Hafezi F, Born W, Sommer L. Compound developmental eye disorders following inactivation of TGFbeta signaling in neural-crest stem cells. J Biol. 2005;4:11. doi: 10.1186/jbiol29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki A, Nagano T, Nakagawa M, Iwaki T, Fukumaki Y. Identification and characterization of the gene encoding a new member of the alpha-crystallin/small hsp family, closely linked to the alphaB-crystallin gene in a head-to-head manner. Genomics. 1997;45:386–394. doi: 10.1006/geno.1997.4956. [DOI] [PubMed] [Google Scholar]
- Iwase S, Lan F, Bayliss P, de la Torre-Ubieta L, Huarte M, Heng Qi H, Whetstine JR, Bonni A, Roberts TM, Shi Y. The X-Linked Mental Retardation Gene SMCX/JARID1C Defines a Family of Histone H3 Lysine 4 Demethylases. Cell. 2007;128:1077–1088. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Jacobson AG. The Determination and Positioning of the Nose, Lens and Ear. I. Interactions within the Ectoderm, and between the Ectoderm and Underlying Tissues. J Exp Zool. 1963a;154:273–283. doi: 10.1002/jez.1401540303. [DOI] [PubMed] [Google Scholar]
- Jacobson AG. The Determination and Positioning of the Nose, Lens and Ear. Ii. the Role of the Endoderm. J Exp Zool. 1963b;154:285–291. doi: 10.1002/jez.1401540304. [DOI] [PubMed] [Google Scholar]
- Jacobson AG. The Determination and Positioning of the Nose, Lens and Ear. Iii. Effects of Reversing the Antero-Posterior Axis of Epidermis, Neural Plate and Neural Fold. J Exp Zool. 1963c;154:293–303. doi: 10.1002/jez.1401540305. [DOI] [PubMed] [Google Scholar]
- Jacobson RH, Ladurner AG, King DS, Tjian R. Structure and function of a human TAFII250 double bromodomain module. Science. 2000;288:1422–1425. doi: 10.1126/science.288.5470.1422. [DOI] [PubMed] [Google Scholar]
- Jayaraman L, Moorthy NC, Murthy KG, Manley JL, Bustin M, Prives C. High mobility group protein-1 (HMG-1) is a unique activator of p53. Genes Dev. 1998;12:462–472. doi: 10.1101/gad.12.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Kondoh H. Overlapping positive and negative regulatory elements determine lens-specific activity of the delta 1-crystallin enhancer. Mol Cell Biol. 1993;13:5206–5215. doi: 10.1128/mcb.13.9.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Sockanathan S, Liu Q, Breitman M, Lovell-Badge R, Kondoh H. Involvement of SOX proteins in lens-specific activation of crystallin genes. Embo J. 1995;14:3510–3519. doi: 10.1002/j.1460-2075.1995.tb07357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Collignon J, Lovell-Badge R, Kondoh H. Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development. 1998;125:2521–2532. doi: 10.1242/dev.125.13.2521. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Tanouchi A, Sekido R, Kondoh H. Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 2001;15:1272–1286. doi: 10.1101/gad.887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakaka RT, Biggins S. Histone variants: deviants? Genes Dev. 2005;19:295–310. doi: 10.1101/gad.1272805. [DOI] [PubMed] [Google Scholar]
- Kammandel B, Chowdhury K, Stoykova A, Aparicio S, Brenner S, Gruss P. Distinct cis-essential modules direct the time-space pattern of the Pax6 gene activity. Dev Biol. 1999;205:79–97. doi: 10.1006/dbio.1998.9128. [DOI] [PubMed] [Google Scholar]
- Kasper LH, Fukuyama T, Biesen MA, Boussouar F, Tong C, de Pauw A, Murray PJ, van Deursen JM, Brindle PK. Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development. Mol Cell Biol. 2006;26:789–809. doi: 10.1128/MCB.26.3.789-809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, Vonesch JL, Dolle P, Chambon P. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Takahashi S, Nakajima O, Ogino H, Morita M, Nishizawa M, Yasuda K, Yamamoto M. Regulation of lens fiber cell differentiation by transcription factor c-Maf. J Biol Chem. 1999;274:19254–19260. doi: 10.1074/jbc.274.27.19254. [DOI] [PubMed] [Google Scholar]
- Kim J, Lauderdale JD. Analysis of Pax6 expression using a BAC transgene reveals the presence of a paired-less isoform of Pax6 in the eye and olfactory bulb. Dev Biol. 2006;292:486–505. doi: 10.1016/j.ydbio.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Kim JK, Huh SO, Choi H, Lee KS, Shin D, Lee C, Nam JS, Kim H, Chung H, Lee HW, Park SD, Seong RH. Srg3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Mol Cell Biol. 2001;21:7787–7795. doi: 10.1128/MCB.21.22.7787-7795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SI, Bultman SJ, Jing H, Blobel GA, Bresnick EH. Dissecting Molecular Steps in Chromatin Domain Activation during Hematopoietic Differentiation. Mol Cell Biol. 2007;27:4551–4565. doi: 10.1128/MCB.00235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Cheong C, Sohn YC, Goo YH, Oh WJ, Park JH, Joe SY, Kang HS, Kim DK, Kee C, Lee JW, Lee HW. Multiple developmental defects derived from impaired recruitment of ASC-2 to nuclear receptors in mice: implication for posterior lenticonus with cataract. Mol Cell Biol. 2002;22:8409–8414. doi: 10.1128/MCB.22.24.8409-8414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinjan DA, Seawright A, Schedl A, Quinlan RA, Danes S, van Heyningen V. Aniridia-associated translocations, DNase hypersensitivity, sequence comparison and transgenic analysis redefine the functional domain of PAX6. Hum Mol Genet. 2001;10:2049–2059. doi: 10.1093/hmg/10.19.2049. [DOI] [PubMed] [Google Scholar]
- Klement JF, Wawrousek EF, Piatigorsky J. Tissue-specific expression of the chicken alpha A-crystallin gene in cultured lens epithelia and transgenic mice. J Biol Chem. 1989;264:19837–19844. [PubMed] [Google Scholar]
- Klok EJ, van Genesen ST, Civil A, Schoenmakers JG, Lubsen NH. Regulation of expression within a gene family. The case of the rat gammaB- and gammaD-crystallin promoters. J Biol Chem. 1998;273:17206–17215. doi: 10.1074/jbc.273.27.17206. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006a;7:715–727. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Yamane K, Bae Y, Zhang D, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature. 2006b;442:312–316. doi: 10.1038/nature04853. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol. 2007 doi: 10.1038/nrm2143. [DOI] [PubMed] [Google Scholar]
- Kondoh H. Transcription factors for lens development assessed in vivo. Curr Opin Genet Dev. 1999;9:301–308. doi: 10.1016/s0959-437x(99)80045-8. [DOI] [PubMed] [Google Scholar]
- Kondoh H, Uchikawa M, Kamachi Y. Interplay of Pax6 and SOX2 in lens development as a paradigm of genetic switch mechanisms for cell differentiation. Int J Dev Biol. 2004;48:819–827. doi: 10.1387/ijdb.041868hk. [DOI] [PubMed] [Google Scholar]
- Kondoh H, Uchikawa M, Yoda H, Takeda H, Furutani-Seiki M, Karlstrom RO. Zebrafish mutations in Gli-mediated hedgehog signaling lead to lens transdifferentiation from the adenohypophysis anlage. Mech Dev. 2000;96:165–174. doi: 10.1016/s0925-4773(00)00387-7. [DOI] [PubMed] [Google Scholar]
- Kosak ST, Groudine M. Form follows function: The genomic organization of cellular differentiation. Genes Dev. 2004;18:1371–1384. doi: 10.1101/gad.1209304. [DOI] [PubMed] [Google Scholar]
- Kozlowski DJ, Murakami T, Ho RK, Weinberg ES. Regional cell movement and tissue patterning in the zebrafish embryo revealed by fate mapping with caged fluorescein. Biochem Cell Biol. 1997;75:551–562. [PubMed] [Google Scholar]
- Kozmik Z, Machon O, Kralova J, Kreslova J, Paces J, Vlcek C. Characterization of mammalian orthologues of the Drosophila osa gene: cDNA cloning, expression, chromosomal localization, and direct physical interaction with Brahma chromatin-remodeling complex. Genomics. 2001;73:140–148. doi: 10.1006/geno.2001.6477. [DOI] [PubMed] [Google Scholar]
- Kralova J, Czerny T, Spanielova H, Ratajova V, Kozmik Z. Complex regulatory element within the gammaE- and gammaF-crystallin enhancers mediates Pax6 regulation and is required for induction by retinoic acid. Gene. 2002;286:271–282. doi: 10.1016/s0378-1119(02)00425-0. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Wessely O, De Robertis EM. Neural induction in Xenopus: requirement for ectodermal and endomesodermal signals via Chordin, Noggin, beta-Catenin, and Cerberus. PLoS Biol. 2004;2:E92. doi: 10.1371/journal.pbio.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KJ, Ma Z, Shih M, Shearer TR, Smith JB, Smith DL, David LL. Sequence analysis of betaA3, betaB3, and betaA4 crystallins completes the identification of the major proteins in young human lens. J Biol Chem. 1997;272:2268–2275. doi: 10.1074/jbc.272.4.2268. [DOI] [PubMed] [Google Scholar]
- Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- Lang RA, McAvoy JW. Growth factors in lens development. In: Lovicu FJ, Robinson ML, editors. Development of ocular lens. Cambridge University Press; Cambridge: 2004. pp. 261–289. [Google Scholar]
- Lauderdale JD, Wilensky JS, Oliver ER, Walton DS, Glaser T. 3’ deletions cause aniridia by preventing PAX6 gene expression. Proc Natl Acad Sci U S A. 2000;97:13755–13759. doi: 10.1073/pnas.240398797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AC, Musil LS. FGF signaling in chick lens development. Dev Biol. 2001;233:394–411. doi: 10.1006/dbio.2001.0194. [DOI] [PubMed] [Google Scholar]
- Lecoin L, Sii-Felice K, Pouponnot C, Eychene A, Felder-Schmittbuhl MP. Comparison of maf gene expression patterns during chick embryo development. Gene Expr Patterns. 2004;4:35–46. doi: 10.1016/s1567-133x(03)00152-2. [DOI] [PubMed] [Google Scholar]
- Lee CH, Murphy MR, Lee JS, Chung JH. Targeting a SWI/SNF-related chromatin remodeling complex to the beta-globin promoter in erythroid cells. Proc Natl Acad Sci U S A. 1999;96:12311–12315. doi: 10.1073/pnas.96.22.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DC, Gonzalez P, Wistow G. Zeta-crystallin: a lens-specific promoter and the gene recruitment of an enzyme as a crystallin. J Mol Biol. 1994;236:669–678. doi: 10.1006/jmbi.1994.1178. [DOI] [PubMed] [Google Scholar]
- Lee HY, Wroblewski E, Philips GT, Stair CN, Conley K, Reedy M, Mastick GS, Brown NL. Multiple requirements for Hes 1 during early eye formation. Dev Biol. 2005;284:464–478. doi: 10.1016/j.ydbio.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Norman J, Shilatifard A, Shiekhattar R. Physical and Functional Association of a Trimethyl H3K4 Demethylase and Ring6a/MBLR, a Polycomb-like Protein. Cell. 2007;128:877–887. doi: 10.1016/j.cell.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, Koseki H, Fuchikami T, Abe K, Murray HL, Zucker JP, Yuan B, Bell GW, Herbolsheimer E, Hannett NM, Sun K, Odom DT, Otte AP, Volkert TL, Bartel DP, Melton DA, Gifford DK, Jaenisch R, Young RA. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengler J, Bittner T, Munster D, Gawad Ael D, Graw J. Agonistic and antagonistic action of AP2, Msx2, Pax6, Prox1 AND Six3 in the regulation of Sox2 expression. Ophthalmic Res. 2005;37:301–309. doi: 10.1159/000087774. [DOI] [PubMed] [Google Scholar]
- Lengler J, Graw J. Regulation of the human SIX3 gene promoter. Biochem Biophys Res Commun. 2001;287:372–376. doi: 10.1006/bbrc.2001.5605. [DOI] [PubMed] [Google Scholar]
- Lengler J, Krausz E, Tomarev S, Prescott A, Quinlan RA, Graw J. Antagonistic action of Six3 and Prox1 at the gamma-crystallin promoter. Nucleic Acids Res. 2001;29:515–526. doi: 10.1093/nar/29.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lercher MJ, Urrutia AO, Hurst LD. Clustering of housekeeping genes provides a unified model of gene order in the human genome. Nat Genet. 2002;31:180–183. doi: 10.1038/ng887. [DOI] [PubMed] [Google Scholar]
- Lewis KE, Drossopoulou G, Paton IR, Morrice DR, Robertson KE, Burt DW, Ingham PW, Tickle C. Expression of ptc and gli genes in talpid3 suggests bifurcation in Shh pathway. Development. 1999;126:2397–2407. doi: 10.1242/dev.126.11.2397. [DOI] [PubMed] [Google Scholar]
- Li HS, Yang JM, Jacobson RD, Pasko D, Sundin O. Pax-6 is first expressed in a region of ectoderm anterior to the early neural plate: implications for stepwise determination of the lens. Dev Biol. 1994;162:181–194. doi: 10.1006/dbio.1994.1077. [DOI] [PubMed] [Google Scholar]
- Li X, Cvekl A, Bassnett S, Piatigorsky J. Lens-preferred activity of chicken delta 1- and delta 2-crystallin enhancers in transgenic mice and evidence for retinoic acid-responsive regulation of the delta 1-crystallin gene. Dev Genet. 1997;20:258–266. doi: 10.1002/(SICI)1520-6408(1997)20:3<258::AID-DVG8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lin D, Takemoto DJ. Oxidative activation of protein kinase Cgamma through the C1 domain. Effects on gap junctions. J Biol Chem. 2005;280:13682–13693. doi: 10.1074/jbc.M407762200. [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–4062. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Liu W, Lagutin OV, Mende M, Streit A, Oliver G. Six3 activation of Pax6 expression is essential for mammalian lens induction and specification. Embo J. 2006;25:5383–5395. doi: 10.1038/sj.emboj.7601398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomvardas S, Thanos D. Opening chromatin. Mol Cell. 2002;9:209–211. doi: 10.1016/s1097-2765(02)00463-x. [DOI] [PubMed] [Google Scholar]
- Lorch Y, Zhang M, Kornberg RD. Histone octamer transfer by a chromatin-remodeling complex. Cell. 1999;96:389–392. doi: 10.1016/s0092-8674(00)80551-6. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lucey M, West KL, Bustin M, Duncan MK. The expression of HMGNs during ocular development. Invest Ophthalmol Vis Sci. 2005;46(1876 Suppl S) [Google Scholar]
- Lupo G, Harris WA, Lewis KE. Mechanisms of ventral patterning in the vertebrate nervous system. Nat Rev Neurosci. 2006;7:103–114. doi: 10.1038/nrn1843. [DOI] [PubMed] [Google Scholar]
- Lyu J, Joo CK. Wnt signaling enhances FGF2-triggered lens fiber cell differentiation. Development. 2004;131:1813–1824. doi: 10.1242/dev.01060. [DOI] [PubMed] [Google Scholar]
- Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proc Natl Acad Sci U S A. 2003;100:11980–11985. doi: 10.1073/pnas.2133841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Trojer P, Reinberg D. The key to development: interpreting the histone code? Curr Opin Genet Dev. 2005;15:163–176. doi: 10.1016/j.gde.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- Marmorstein R, Roth SY. Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev. 2001;11:155–161. doi: 10.1016/s0959-437x(00)00173-8. [DOI] [PubMed] [Google Scholar]
- Martinez-Campa C, Politis P, Moreau JL, Kent N, Goodall J, Mellor J, Goding CR. Precise nucleosome positioning and the TATA box dictate requirements for the histone H4 tail and the bromodomain factor Bdf1. Mol Cell. 2004;15:69–81. doi: 10.1016/j.molcel.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Masaki S, Kamachi Y, Quinlan RA, Yonezawa S, Kondoh H. Identification and functional analysis of the mouse lens filensin gene promoter. Gene. 1998;214:77–86. doi: 10.1016/s0378-1119(98)00230-3. [DOI] [PubMed] [Google Scholar]
- Masaki S, Yonezawa S, Quinlan R. Localization of two conserved cis -acting enhancer regions for the filensin gene promoter that direct lens-specific expression. Exp Eye Res. 2002;75:295–305. [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- Matsuo I, Kitamura M, Okazaki K, Yasuda K. Binding of a factor to an enhancer element responsible for the tissue-specific expression of the chicken alphaA-crystallin gene. Development. 1991;113:539–550. doi: 10.1242/dev.113.2.539. [DOI] [PubMed] [Google Scholar]
- Matsuo I, Yasuda K. The cooperative interaction between two motifs of an enhancer element of the chicken alpha A-crystallin gene, alpha CE1 and alpha CE2, confers lens-specific expression. Nucleic Acids Res. 1992;20:3701–3712. doi: 10.1093/nar/20.14.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JB, Cvekl A, Piatigorsky J. Lens-specific expression of a chicken beta A3/A1-crystallin promoter fragment in transgenic mice. Biochem Biophys Res Commun. 1996;221:559–564. doi: 10.1006/bbrc.1996.0635. [DOI] [PubMed] [Google Scholar]
- McDermott JB, Cvekl A, Piatigorsky J. A complex enhancer of the chicken beta A3/A1-crystallin gene depends on an AP-1-CRE element for activity. Invest Ophthalmol Vis Sci. 1997;38:951–959. [PubMed] [Google Scholar]
- Meakin SO, Du RP, Tsui LC, Breitman ML. Gamma-crystallins of the human eye lens: expression analysis of five members of the gene family. Mol Cell Biol. 1987;7:2671–2679. doi: 10.1128/mcb.7.8.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Martinez O, Jamrich M. Foxe view of lens development and disease. Development. 2007;134:1455–1463. doi: 10.1242/dev.000117. [DOI] [PubMed] [Google Scholar]
- Mellor J. The dynamics of chromatin remodeling at promoters. Mol Cell. 2005;19:147–157. doi: 10.1016/j.molcel.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Mellor J. Dynamic nucleosomes and gene transcription. Trends Genet. 2006a;22:320–329. doi: 10.1016/j.tig.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Mellor J. It takes a PHD to read the histone code. Cell. 2006b;126:22–24. doi: 10.1016/j.cell.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Menko AS. Lens epithelial cell differentiation. Exp Eye Res. 2002;75:485–490. doi: 10.1006/exer.2002.2057. [DOI] [PubMed] [Google Scholar]
- Menko AS, Walker JL. Role of matrix and cell adhesion molecules in lens differentiation. In: Lovicu FJ, Robinson ML, editors. Development of the ocular lens. Cambridge University Press; Cambridge: 2004. pp. 245–260. [Google Scholar]
- Messina DN, Glasscock J, Gish W, Lovett M. An ORFeome-based analysis of human transcription factor genes and the construction of a microarray to interrogate their expression. Genome Res. 2004;14:2041–2047. doi: 10.1101/gr.2584104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Molotkova N, Duester G. Raldh2 expression in optic vesicle generates a retinoic acid signal needed for invagination of retina during optic cup formation. Dev Dyn. 2004;231:270–277. doi: 10.1002/dvdy.20128. [DOI] [PubMed] [Google Scholar]
- Miller LA, Smith AN, Taketo MM, Lang RA. Optic cup and facial patterning defects in ocular ectoderm beta-catenin gain-of-function mice. BMC Dev Biol. 2006;6:14. doi: 10.1186/1471-213X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JN, Zhang Y, Moskophidis D, Mivechi NF. Unique contribution of heat shock transcription factor 4 in ocular lens development and fiber cell differentiation. Genesis. 2004;40:205–217. doi: 10.1002/gene.20087. [DOI] [PubMed] [Google Scholar]
- Mishima N, Tomarev S. Chicken Eyes absent 2 gene: isolation and expression pattern during development. Int J Dev Biol. 1998;42:1109–1115. [PubMed] [Google Scholar]
- Misteli T. The concept of self-organization in cellular architecture. J Cell Biol. 2001;155:181–185. doi: 10.1083/jcb.200108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsouras K, Wong B, Arayata C, Johnson RC, Carey M. The DNA architectural protein HMGB1 displays two distinct modes of action that promote enhanceosome assembly. Mol Cell Biol. 2002;22:4390–4401. doi: 10.1128/MCB.22.12.4390-4401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. Conservation of sequence and function in the Pax6 regulatory elements. Trends Genet. 2004;20:283–287. doi: 10.1016/j.tig.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Mori M, Ghyselinck NB, Chambon P, Mark M. Systematic immunolocalization of retinoid receptors in developing and adult mouse eyes. Invest Ophthalmol Vis Sci. 2001;42:1312–1318. [PubMed] [Google Scholar]
- Nagl NG, Jr, Zweitzig DR, Thimmapaya B, Beck GR, Jr, Moran E. The c-myc gene is a direct target of mammalian SWI/SNF-related complexes during differentiation-associated cell cycle arrest. Cancer Res. 2006;66:1289–1293. doi: 10.1158/0008-5472.CAN-05-3427. [DOI] [PubMed] [Google Scholar]
- Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–487. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- Ng D, Thakker N, Corcoran CM, Donnai D, Perveen R, Schneider A, Hadley DW, Tifft C, Zhang L, Wilkie AO, van der Smagt JJ, Gorlin RJ, Burgess SM, Bardwell VJ, Black GC, Biesecker LG. Oculofaciocardiodental and Lenz microphthalmia syndromes result from distinct classes of mutations in BCOR. Nat Genet. 2004;36:411–416. doi: 10.1038/ng1321. [DOI] [PubMed] [Google Scholar]
- Nightingale KP, O’Neill LP, Turner BM. Histone modifications: signalling receptors and potential elements of a heritable epigenetic code. Curr Opin Genet Dev. 2006;16:125–136. doi: 10.1016/j.gde.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S, Wood H, Kondoh H, Lovell-Badge R, Episkopou V. Sox1 directly regulates the gamma-crystallin genes and is essential for lens development in mice. Genes Dev. 1998;12:776–781. doi: 10.1101/gad.12.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto S, Kawane K, Watanabe-Fukunaga R, Fukuyama H, Ohsawa Y, Uchiyama Y, Hashida N, Ohguro N, Tano Y, Morimoto T, Fukuda Y, Nagata S. Nuclear cataract caused by a lack of DNA degradation in the mouse eye lens. Nature. 2003;424:1071–1074. doi: 10.1038/nature01895. [DOI] [PubMed] [Google Scholar]
- Noramly S, Grainger RM. Determination of the embryonic inner ear. J Neurobiol. 2002;53:100–128. doi: 10.1002/neu.10131. [DOI] [PubMed] [Google Scholar]
- Nusinzon I, Horvath CM. Histone deacetylases as transcriptional activators? Role reversal in inducible gene regulation. Sci STKE. 2005;2005:re11. doi: 10.1126/stke.2962005re11. [DOI] [PubMed] [Google Scholar]
- O’Neill DW, Schoetz SS, Lopez RA, Castle M, Rabinowitz L, Shor E, Krawchuk D, Goll MG, Renz M, Seelig HP, Han S, Seong RH, Park SD, Agalioti T, Munshi N, Thanos D, Erdjument-Bromage H, Tempst P, Bank A. An ikaros-containing chromatin-remodeling complex in adult-type erythroid cells. Mol Cell Biol. 2000;20:7572–7582. doi: 10.1128/mcb.20.20.7572-7582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino H, Yasuda K. Induction of lens differentiation by activation of a bZIP transcription factor, L-Maf. Science. 1998;280:115–118. doi: 10.1126/science.280.5360.115. [DOI] [PubMed] [Google Scholar]
- Ohkawa Y, Marfella CG, Imbalzano AN. Skeletal muscle specification by myogenin and Mef2D via the SWI/SNF ATPase Brg1. Embo J. 2006;25:490–501. doi: 10.1038/sj.emboj.7600943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaka-Maruyama C, Wang X, Ge H, Chepelinsky AB. Overlapping Sp1 and AP2 binding sites in a promoter element of the lens-specific MIP gene. Nucleic Acids Res. 1998;26:407–414. doi: 10.1093/nar/26.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okladnova O, Syagailo YV, Mossner R, Riederer P, Lesch KP. Regulation of PAX-6 gene transcription: alternate promoter usage in human brain. Brain Res Mol Brain Res. 1998;60:177–192. doi: 10.1016/s0169-328x(98)00167-3. [DOI] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995a;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wehr R, Jenkins NA, Copeland NG, Cheyette BN, Hartenstein V, Zipursky SL, Gruss P. Homeobox genes and connective tissue patterning. Development. 1995b;121:693–705. doi: 10.1242/dev.121.3.693. [DOI] [PubMed] [Google Scholar]
- Overbeek PA, Chepelinsky AB, Khillan JS, Piatigorsky J, Westphal H. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A-crystallin promoter in transgenic mice. Proc Natl Acad Sci U S A. 1985;82:7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios D, Puri PL. The epigenetic network regulating muscle development and regeneration. J Cell Physiol. 2006;207:1–11. doi: 10.1002/jcp.20489. [DOI] [PubMed] [Google Scholar]
- Pallerla SR, Pan Y, Zhang X, Esko JD, Grobe K. Heparan sulfate Ndst1 gene function variably regulates multiple signaling pathways during mouse development. Dev Dyn. 2007;236:556–563. doi: 10.1002/dvdy.21038. [DOI] [PubMed] [Google Scholar]
- Pan Y, Woodbury A, Esko JD, Grobe K, Zhang X. Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development. 2006;133:4933–4944. doi: 10.1242/dev.02679. [DOI] [PubMed] [Google Scholar]
- Peek R, Niessen RW, Schoenmakers JG, Lubsen NH. DNA methylation as a regulatory mechanism in rat gamma-crystallin gene expression. Nucleic Acids Res. 1991;19:77–83. doi: 10.1093/nar/19.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AH, O’Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M, Sibilia M, Jenuwein T. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:R546–551. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Gene expression and genetic engineering in the lens. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1987;28:9–28. [PubMed] [Google Scholar]
- Plaza S, Dozier C, Saule S. Quail Pax-6 (Pax-QNR) encodes a transcription factor able to bind and trans-activate its own promoter. Cell Growth Differ. 1993;4:1041–1050. [PubMed] [Google Scholar]
- Plevin MJ, Mills MM, Ikura M. The LxxLL motif: a multifunctional binding sequence in transcriptional regulation. Trends Biochem Sci. 2005;30:66–69. doi: 10.1016/j.tibs.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Pontoriero GF, Deschamps PA, Ashery-Padan R, Wong R, Yang Y, Zavadil J, Sullivan S, Williams T, West-Mays JA. Conditional deletion of AP-2alpha in the lens alters multiple pathways controlling the lens epithelial cell phenotype. 2007 submitted. [Google Scholar]
- Porter FD, Drago J, Xu Y, Cheema SS, Wassif C, Huang SP, Lee E, Grinberg A, Massalas JS, Bodine D, Alt F, Westphal H. Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development. 1997;124:2935–2944. doi: 10.1242/dev.124.15.2935. [DOI] [PubMed] [Google Scholar]
- Postnikov YV, Herrera JE, Hock R, Scheer U, Bustin M. Clusters of nucleosomes containing chromosomal protein HMG-17 in chromatin. J Mol Biol. 1997;274:454–465. doi: 10.1006/jmbi.1997.1391. [DOI] [PubMed] [Google Scholar]
- Quinlan R, Prescott A. Lens cell cytoskeleton. In: Lovicu FJ, Robinson ML, editors. Development of the ocular lens. Cambridge University Press; Cambridge: 2004. pp. 173–187. [Google Scholar]
- Ragoczy T, Bender MA, Telling A, Byron R, Groudine M. The locus control region is required for association of the murine beta-globin locus with engaged transcription factories during erythroid maturation. Genes Dev. 2006;20:1447–1457. doi: 10.1101/gad.1419506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram N, Kerppola TK. Synergistic transcription activation by Maf and Sox and their subnuclear localization are disrupted by a mutation in Maf that causes cataract. Mol Cell Biol. 2004;24:5694–5709. doi: 10.1128/MCB.24.13.5694-5709.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo FM, Khavari P, Crabtree G, Tamkun J, Rossant J. brg1: a putative murine homologue of the Drosophila brahma gene, a homeotic gene regulator. Dev Biol. 1994;161:229–242. doi: 10.1006/dbio.1994.1023. [DOI] [PubMed] [Google Scholar]
- Reeves R. Molecular biology of HMGA proteins: hubs of nuclear function. Gene. 2001;277:63–81. doi: 10.1016/s0378-1119(01)00689-8. [DOI] [PubMed] [Google Scholar]
- Rembold M, Loosli F, Adams RJ, Wittbrodt J. Individual cell migration serves as the driving force for optic vesicle evagination. Science. 2006;313:1130–1134. doi: 10.1126/science.1127144. [DOI] [PubMed] [Google Scholar]
- Ren B, Dynlacht BD. Use of chromatin immunoprecipitation assays in genome-wide location analysis of mammalian transcription factors. Methods Enzymol. 2004;376:304–315. doi: 10.1016/S0076-6879(03)76020-0. [DOI] [PubMed] [Google Scholar]
- Reyes JC, Barra J, Muchardt C, Camus A, Babinet C, Yaniv M. Altered control of cellular proliferation in the absence of mammalian brahma (SNF2alpha) Embo J. 1998;17:6979–6991. doi: 10.1093/emboj/17.23.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza HM, Urano A, Shimada N, Yasuda K. Sequential and combinatorial roles of maf family genes define proper lens development. Mol Vis. 2007;13:18–30. [PMC free article] [PubMed] [Google Scholar]
- Richardson J, Cvekl A, Wistow G. Pax-6 is essential for lens-specific expression of zeta-crystallin. Proc Natl Acad Sci U S A. 1995;92:4676–4680. doi: 10.1073/pnas.92.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring BZ, Cordes SP, Overbeek PA, Barsh GS. Regulation of mouse lens fiber cell development and differentiation by the Maf gene. Development. 2000;127:307–317. doi: 10.1242/dev.127.2.307. [DOI] [PubMed] [Google Scholar]
- Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17:726–740. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA. Differential expression of alpha A- and alpha B-crystallin during murine ocular development. Invest Ophthalmol Vis Sci. 1996;37:2276–2284. [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Roth HJ, Das GC, Piatigorsky J. Chicken βB1-crystallin gene expression: Presence of conserved functional polyomavirus enhancer-like and octamer binding-like promoter elements found in non-lens genes. Mol Cell Biol. 1991;11:1488–1499. doi: 10.1128/mcb.11.3.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AL. Biochemistry and biology of the inducible multifunctional transcription factor TFII-I. Gene. 2001;274:1–13. doi: 10.1016/s0378-1119(01)00625-4. [DOI] [PubMed] [Google Scholar]
- Saha A, Wittmeyer J, Cairns BR. Chromatin remodelling: the industrial revolution of DNA around histones. Nat Rev Mol Cell Biol. 2006;7:437–447. doi: 10.1038/nrm1945. [DOI] [PubMed] [Google Scholar]
- Sakai M, Serria MS, Ikeda H, Yoshida K, Imaki J, Nishi S. Regulation of c-maf gene expression by Pax6 in cultured cells. Nucleic Acids Res. 2001;29:1228–1237. doi: 10.1093/nar/29.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma K, Reinberg D. Histone variants meet their match. Nat Rev Mol Cell Biol. 2005;6:139–149. doi: 10.1038/nrm1567. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Evolutionary origins of vertebrate placodes: insights from developmental studies and from comparisons with other deuterostomes. J Exp Zoolog B Mol Dev Evol. 2005;304:347–399. doi: 10.1002/jez.b.21055. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Ahrens K. Molecular anatomy of placode development in Xenopus laevis. Dev Biol. 2004;271:439–466. doi: 10.1016/j.ydbio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Segal E, Fondufe-Mittendorf Y, Chen L, Thastrom A, Field Y, Moore IK, Wang JP, Widom J. A genomic code for nucleosome positioning. Nature. 2006;442:772–778. doi: 10.1038/nature04979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon-Friling R, Richardson J, Sperbeck S, Lee D, Rauchman M, Maas R, Swaroop A, Wistow G. Lens-specific gene recruitment of zeta-crystallin through Pax6, Nrl-Maf, and brain suppressor sites. Mol Cell Biol. 1998;18:2067–2076. doi: 10.1128/mcb.18.4.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestopalov VI, Bassnett S. Expression of autofluorescent proteins reveals a novel protein permeable pathway between cells in the lens core. J Cell Sci. 2000;113:1913–1921. doi: 10.1242/jcs.113.11.1913. [DOI] [PubMed] [Google Scholar]
- Shestopalov VI, Bassnett S. Development of a macromolecular diffusion pathway in the lens. J Cell Sci. 2003;116:4191–4199. doi: 10.1242/jcs.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Silver SJ, Rebay I. Signaling circuitries in development: insights from the retinal determination gene network. Development. 2005;132:3–13. doi: 10.1242/dev.01539. [DOI] [PubMed] [Google Scholar]
- Simirskii VN, Wang Y, Duncan MK. Conditional deletion of beta1-integrin from the developing lens leads to loss of the lens epithelial phenotype. Dev Biol. 2007;306:658–668. doi: 10.1016/j.ydbio.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh DP, Fatma N, Kimura A, Chylack LT, Jr, Shinohara T. LEDGF binds to heat shock and stress-related element to activate the expression of stress-related genes. Biochem Biophys Res Commun. 2001;283:943–955. doi: 10.1006/bbrc.2001.4887. [DOI] [PubMed] [Google Scholar]
- Sirotkin AM, Edelmann W, Cheng G, Klein-Szanto A, Kucherlapati R, Skoultchi AI. Mice develop normally without the H1(0) linker histone. Proc Natl Acad Sci U S A. 1995;92:6434–6438. doi: 10.1073/pnas.92.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285:477–489. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Somasundaram T, Bhat SP. Canonical heat shock element in the alpha B-crystallin gene shows tissue-specific and developmentally controlled interactions with heat shock factor. J Biol Chem. 2000;275:17154–17159. doi: 10.1074/jbc.M000304200. [DOI] [PubMed] [Google Scholar]
- Soules KA, Link BA. Morphogenesis of the anterior segment in the zebrafish eye. BMC Dev Biol. 2005;5:12. doi: 10.1186/1471-213X-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splinter E, Grosveld F, de Laat W. 3C technology: analyzing the spatial organization of genomic loci in vivo. Methods Enzymol. 2004;375:493–507. doi: 10.1016/s0076-6879(03)75030-7. [DOI] [PubMed] [Google Scholar]
- Stenger JE, Tegtmeyer P, Mayr GA, Reed M, Wang Y, Wang P, Hough PV, Mastrangelo IA. p53 oligomerization and DNA looping are linked with transcriptional activation. Embo J. 1994;13:6011–6020. doi: 10.1002/j.1460-2075.1994.tb06947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolen CM, Griep AE. Disruption of lens fiber cell differentiation and survival at multiple stages by region-specific expression of truncated FGF receptors. Dev Biol. 2000;217:205–220. doi: 10.1006/dbio.1999.9557. [DOI] [PubMed] [Google Scholar]
- Stopka T, Skoultchi AI. The ISWI ATPase Snf2h is required for early mouse development. Proc Natl Acad Sci U S A. 2003;100:14097–14102. doi: 10.1073/pnas.2336105100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Strahl BD, Briggs SD, Brame CJ, Caldwell JA, Koh SS, Ma H, Cook RG, Shabanowitz J, Hunt DF, Stallcup MR, Allis CD. Methylation of histone H4 at arginine 3 occurs in vivo and is mediated by the nuclear receptor coactivator PRMT1. Curr Biol. 2001;11:996–1000. doi: 10.1016/s0960-9822(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Streit A. Extensive cell movements accompany formation of the otic placode. Dev Biol. 2002;249:237–254. doi: 10.1006/dbio.2002.0739. [DOI] [PubMed] [Google Scholar]
- Streit A. Early development of the cranial sensory nervous system: from a common field to individual placodes. Dev Biol. 2004;276:1–15. doi: 10.1016/j.ydbio.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Stump RJ, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev Biol. 2003;259:48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Sullivan CH, Braunstein L, Hazard-Leonards RM, Holen AL, Samaha F, Stephens L, Grainger RM. A re-examination of lens induction in chicken embryos: in vitro studies of early tissue interactions. Int J Dev Biol. 2004;48:771–782. doi: 10.1387/ijdb.041894cs. [DOI] [PubMed] [Google Scholar]
- Sullivan CH, Norman JT, Borras T, Grainger RM. Developmental regulation of hypomethylation of delta-crystallin genes in chicken embryo lens cells. Mol Cell Biol. 1989;9:3132–3135. doi: 10.1128/mcb.9.7.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland HG, Newton K, Brownstein DG, Holmes MC, Kress C, Semple CA, Bickmore WA. Disruption of Ledgf/Psip1 results in perinatal mortality and homeotic skeletal transformations. Mol Cell Biol. 2006;26:7201–7210. doi: 10.1128/MCB.00459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto RK, Clarkson MJ, Tremethick DJ, Luger K. Crystal structure of a nucleosome core particle containing the variant histone H2A.Z. Nat Struct Biol. 2000;7:1121–1124. doi: 10.1038/81971. [DOI] [PubMed] [Google Scholar]
- Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002;2:301–314. doi: 10.1016/s1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- Takagi T, Moribe H, Kondoh H, Higashi Y. DeltaEF1, a zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Development. 1998;125:21–31. doi: 10.1242/dev.125.1.21. [DOI] [PubMed] [Google Scholar]
- Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tini M, Otulakowski G, Breitman ML, Tsui LC, Giguere V. An everted repeat mediates retinoic acid induction of the gammaF-crystallin gene: evidence of a direct role for retinoids in lens development. Genes Dev. 1993;7:295–307. doi: 10.1101/gad.7.2.295. [DOI] [PubMed] [Google Scholar]
- Torres M, Giraldez F. The development of the vertebrate inner ear. Mech Dev. 1998;71:5–21. doi: 10.1016/s0925-4773(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Trieschmann L, Martin B, Bustin M. The chromatin unfolding domain of chromosomal protein HMG-14 targets the N-terminal tail of histone H3 in nucleosomes. Proc Natl Acad Sci U S A. 1998;95:5468–5473. doi: 10.1073/pnas.95.10.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang M, Dawid IB. Promotion and attenuation of FGF signaling through the Ras-MAPK pathway. Sci STKE. 2004;2004:pe17. doi: 10.1126/stke.2282004pe17. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Madhavan M, Tancous EE, Del Rio-Tsonis K. A newt’s eye view of lens regeneration. Int J Dev Biol. 2004;48:975–980. doi: 10.1387/ijdb.041867pt. [DOI] [PubMed] [Google Scholar]
- Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- Tu N, Hu Y, Mivechi NF. Heat shock transcription factor (Hsf)-4b recruits Brg1 during the G1 phase of the cell cycle and regulates the expression of heat shock proteins. J Cell Biochem. 2006;98:1528–1542. doi: 10.1002/jcb.20865. [DOI] [PubMed] [Google Scholar]
- Turner BM. Reading signals on the nucleosome with a new nomenclature for modified histones. Nat Struct Mol Biol. 2005;12:110–112. doi: 10.1038/nsmb0205-110. [DOI] [PubMed] [Google Scholar]
- Turner BM. Defining an epigenetic code. Nat Cell Biol. 2007;9:2–6. doi: 10.1038/ncb0107-2. [DOI] [PubMed] [Google Scholar]
- Uchikawa M, Ishida Y, Takemoto T, Kamachi Y, Kondoh H. Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev Cell. 2003;4:509–519. doi: 10.1016/s1534-5807(03)00088-1. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Duncan MK, David LL. Lens proteomics: the accumulation of crystallin modifications in the mouse lens with age. Invest Ophthalmol Vis Sci. 2002;43:205–215. [PubMed] [Google Scholar]
- Urnov FD, Wolffe AP. Chromatin remodeling and transcriptional activation: the cast (in order of appearance) Oncogene. 2001;20:2991–3006. doi: 10.1038/sj.onc.1204323. [DOI] [PubMed] [Google Scholar]
- van Genderen MM, Kinds GF, Riemslag FC, Hennekam RC. Ocular features in Rubinstein-Taybi syndrome: investigation of 24 patients and review of the literature. Br J Ophthalmol. 2000;84:1177–1184. doi: 10.1136/bjo.84.10.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leen RW, Breuer ML, Lubsen NH, Schoenmakers JG. Developmental expression of crystallin genes: in situ hybridization reveals a differential localization of specific mRNAs. Dev Biol. 1987;123:338–345. doi: 10.1016/0012-1606(87)90392-7. [DOI] [PubMed] [Google Scholar]
- van Leen RW, van Roozendaal KE, Lubsen NH, Schoenmakers JG. Differential expression of crystallin genes during development of the rat eye lens. Dev Biol. 1987;120:457–464. doi: 10.1016/0012-1606(87)90249-1. [DOI] [PubMed] [Google Scholar]
- van Raamsdonk CD, Tilghman SM. Dosage requirement and allelic expression of PAX6 during lens placode formation. Development. 2000;127:5439–5448. doi: 10.1242/dev.127.24.5439. [DOI] [PubMed] [Google Scholar]
- Vanita, Sarhadi V, Reis A, Jung M, Singh D, Sperling K, Singh JR, Burger J. A unique form of autosomal dominant cataract explained by gene conversion between beta-crystallin B2 and its pseudogene. J Med Genet. 2001;38:392–396. doi: 10.1136/jmg.38.6.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wamstad JA, Bardwell VJ. Characterization of Bcor expression in mouse development. Gene Expr Patterns. 2007;7:550–557. doi: 10.1016/j.modgep.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Garcia CM, Shui YB, Beebe DC. Expression and regulation of alpha-, beta-, and gamma-crystallins in mammalian lens epithelial cells. Invest Ophthalmol Vis Sci. 2004a;45:3608–3619. doi: 10.1167/iovs.04-0423. [DOI] [PubMed] [Google Scholar]
- Wang XY, Ohtaka-Maruyama C, Pisano MM, Jaworski CJ, Chepelinsky AB. Isolation and characterization of the 5’-flanking sequence of the human ocular lens MIP gene. Gene. 1995;167:321–325. doi: 10.1016/0378-1119(95)00637-0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fischle W, Cheung W, Jacobs S, Khorasanizadeh S, Allis CD. Beyond the double helix: writing and reading the histone code. Novartis Found Symp. 2004b;259:3–17. discussion 17-21, 163-169. [PubMed] [Google Scholar]
- Wang Y, Wysocka J, Sayegh J, Lee YH, Perlin JR, Leonelli L, Sonbuchner LS, McDonald CH, Cook RG, Dou Y, Roeder RG, Clarke S, Stallcup MR, Allis CD, Coonrod SA. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004c;306:279–283. doi: 10.1126/science.1101400. [DOI] [PubMed] [Google Scholar]
- Wargelius A, Seo HC, Austbo L, Fjose A. Retinal expression of zebrafish six3.1 and its regulation by Pax6. Biochem Biophys Res Commun. 2003;309:475–481. doi: 10.1016/j.bbrc.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Wassarman DA, Sauer F. TAF(II) 250: a transcription toolbox. J Cell Sci. 2001;114:2895–2902. doi: 10.1242/jcs.114.16.2895. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Dev Biol. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Weber GF, Menko AS. Phosphatidylinositol 3-kinase is necessary for lens fiber cell differentiation and survival. Invest Ophthalmol Vis Sci. 2006;47:4490–4499. doi: 10.1167/iovs.06-0401. [DOI] [PubMed] [Google Scholar]
- Weinstein DC, Hemmati-Brivanlou A. Neural induction. Annu Rev Cell Dev Biol. 1999;15:411–433. doi: 10.1146/annurev.cellbio.15.1.411. [DOI] [PubMed] [Google Scholar]
- West KL, Castellini MA, Duncan MK, Bustin M. Chromosomal proteins HMGN3a and HMGN3b regulate the expression of glycine transporter 1. Mol Cell Biol. 2004;24:3747–3756. doi: 10.1128/MCB.24.9.3747-3756.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Mays JA, Coyle BM, Piatigorsky J, Papagiotas S, Libby D. Ectopic expression of AP-2alpha transcription factor in the lens disrupts fiber cell differentiation. Dev Biol. 2002;245:13–27. doi: 10.1006/dbio.2002.0624. [DOI] [PubMed] [Google Scholar]
- West-Mays JA, Zhang J, Nottoli T, Hagopian-Donaldson S, Libby D, Strissel KJ, Williams T. AP-2alpha transcription factor is required for early morphogenesis of the lens vesicle. Dev Biol. 1999;206:46–62. doi: 10.1006/dbio.1998.9132. [DOI] [PubMed] [Google Scholar]
- Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, Spooner E, Li E, Zhang G, Colaiacovo M, Shi Y. Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell. 2006;125:467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Whitehouse I, Flaus A, Cairns BR, White MF, Workman JL, Owen-Hughes T. Nucleosome mobilization catalysed by the yeast SWI/SNF complex. Nature. 1999;400:784–787. doi: 10.1038/23506. [DOI] [PubMed] [Google Scholar]
- Whitlock KE. A new model for olfactory placode development. Brain Behav Evol. 2004;64:126–140. doi: 10.1159/000079742. [DOI] [PubMed] [Google Scholar]
- Whitlock KE, Westerfield M. The olfactory placodes of the zebrafish form by convergence of cellular fields at the edge of the neural plate. Development. 2000;127:3645–3653. doi: 10.1242/dev.127.17.3645. [DOI] [PubMed] [Google Scholar]
- Wiebe MS, Wilder PJ, Kelly D, Rizzino A. Isolation, characterization, and differential expression of the murine Sox-2 promoter. Gene. 2000;246:383–393. doi: 10.1016/s0378-1119(00)00086-x. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999a;21:318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- Williams SC, Altmann CR, Chow RL, Hemmati-Brivanlou A, Lang RA. A highly conserved lens transcriptional control element from the Pax-6 gene. Mech Dev. 1998;73:225–229. doi: 10.1016/s0925-4773(98)00057-4. [DOI] [PubMed] [Google Scholar]
- Wilsker D, Patsialou A, Dallas PB, Moran E. ARID proteins: a diverse family of DNA binding proteins implicated in the control of cell growth, differentiation, and development. Cell Growth Differ. 2002;13:95–106. [PubMed] [Google Scholar]
- Wilson SW, Houart C. Early steps in the development of the forebrain. Dev Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler S, Loosli F, Henrich T, Wakamatsu Y, Wittbrodt J. The conditional medaka mutation eyeless uncouples patterning and morphogenesis of the eye. Development. 2000;127:1911–1919. doi: 10.1242/dev.127.9.1911. [DOI] [PubMed] [Google Scholar]
- Wistow G, Sardarian L, Gan W, Wyatt MK. The human gene for gammaS-crystallin: alternative transcripts and expressed sequences from the first intron. Mol Vis. 2000;6:79–84. [PubMed] [Google Scholar]
- Wolffe AP. Chromatin Structure and Function. Academic Press; San Diego: 1998. [Google Scholar]
- Woodcock CL, Skoultchi AI, Fan Y. Role of linker histone in chromatin structure and function: H1 stoichiometry and nucleosome repeat length. Chromosome Res. 2006;14:17–25. doi: 10.1007/s10577-005-1024-3. [DOI] [PubMed] [Google Scholar]
- Workman JL. Nucleosome displacement in transcription. Genes Dev. 2006;20:2009–2017. doi: 10.1101/gad.1435706. [DOI] [PubMed] [Google Scholar]
- Xiao W, Liu W, Li Z, Liang D, Li L, White LD, Fox DA, Overbeek PA, Chen Q. Gene expression profiling in embryonic mouse lenses. Mol Vis. 2006;12:1692–1698. [PubMed] [Google Scholar]
- Xie L, Overbeek PA, Reneker LW. Ras signaling is essential for lens cell proliferation and lens growth during development. Dev Biol. 2006;298:403–414. doi: 10.1016/j.ydbio.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Xu PX, Woo I, Her H, Beier DR, Maas RL. Mouse Eya homologues of the Drosophila eyes absent gene require Pax6 for expression in lens and nasal placode. Development. 1997;124:219–231. doi: 10.1242/dev.124.1.219. [DOI] [PubMed] [Google Scholar]
- Xu PX, Zhang X, Heaney S, Yoon A, Michelson AM, Maas RL. Regulation of Pax6 expression is conserved between mice and flies. Development. 1999;126:383–395. doi: 10.1242/dev.126.2.383. [DOI] [PubMed] [Google Scholar]
- Xu ZP, Saunders GF. Transcriptional regulation of the human PAX6 gene promoter. J Biol Chem. 1997;272:3430–3436. doi: 10.1074/jbc.272.6.3430. [DOI] [PubMed] [Google Scholar]
- Yadav N, Lee J, Kim J, Shen J, Hu MC, Aldaz CM, Bedford MT. Specific protein methylation defects and gene expression perturbations in coactivator-associated arginine methyltransferase 1-deficient mice. Proc Natl Acad Sci U S A. 2003;100:6464–6468. doi: 10.1073/pnas.1232272100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada R, Mizutani-Koseki Y, Hasegawa T, Osumi N, Koseki H, Takahashi N. Cell-autonomous involvement of Mab21l1 is essential for lens placode development. Development. 2003;130:1759–1770. doi: 10.1242/dev.00399. [DOI] [PubMed] [Google Scholar]
- Yamada R, Mizutani-Koseki Y, Koseki H, Takahashi N. Requirement for Mab21l2 during development of murine retina and ventral body wall. Dev Biol. 2004;274:295–307. doi: 10.1016/j.ydbio.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chauhan BK, Cveklova K, Cvekl A. Transcriptional regulation of mouse alphaB- and gammaF-crystallin genes in lens: opposite promoter-specific interactions between Pax6 and large Maf transcription factors. J Mol Biol. 2004;344:351–368. doi: 10.1016/j.jmb.2004.07.102. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cvekl A. Tissue-specific regulation of the mouse alphaA-crystallin gene in lens via recruitment of Pax6 and c-Maf to its promoter. J Mol Biol. 2005;351:453–469. doi: 10.1016/j.jmb.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cvekl A. Large Maf transcription factors: Cousins of AP-1 proteins and important regulators of cellular differentiation. The Einstein Journal of Biology and Medicine. 2007;23:2–11. doi: 10.23861/ejbm20072347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Stopka T, Golestaneh N, Wang Y, Wu K, Li A, Chauhan BK, Gao CY, Cveklova K, Duncan MK, Pestell RG, Chepelinsky AB, Skoultchi AI, Cvekl A. Regulation of alphaA-crystallin via Pax6, c-Maf, CREB and a broad domain of lens-specific chromatin. Embo J. 2006;25:2107–2118. doi: 10.1038/sj.emboj.7601114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wolf LV, Cvekl A. Distinct Embryonic Expression and Localization of CBP and p300 Histone Acetyltransferases at the Mouse alphaA-Crystallin Locus in Lens. J Mol Biol. 2007;369:917–926. doi: 10.1016/j.jmb.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordy JS, Muise-Helmericks RC. Signal transduction and the Ets family of transcription factors. Oncogene. 2000;19:6503–6513. doi: 10.1038/sj.onc.1204036. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yasuda K. Characterization of the chicken L-Maf, MafB and c-Maf in crystallin gene regulation and lens differentiation. Genes Cells. 2002;7:693–706. doi: 10.1046/j.1365-2443.2002.00548.x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto A, Saigou Y, Higashi Y, Kondoh H. Regulation of ocular lens development by Smad-interacting protein 1 involving Foxe3 activation. Development. 2005;132:4437–4448. doi: 10.1242/dev.02022. [DOI] [PubMed] [Google Scholar]
- Yu CC, Tsui LC, Breitman ML. Homologous and heterologous enhancers modulate spatial expression but not cell-type specificity of the murine gamma F-crystallin promoter. Development. 1990;110:131–139. doi: 10.1242/dev.110.1.131. [DOI] [PubMed] [Google Scholar]
- Zaniolo K, Leclerc S, Cvekl A, Vallieres L, Bazin R, Larouche K, Guerin SL. Expression of the alpha4 integrin subunit gene promoter is modulated by the transcription factor Pax-6 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:1692–1704. doi: 10.1167/iovs.03-0908. [DOI] [PubMed] [Google Scholar]
- Zhang X, Friedman A, Heaney S, Purcell P, Maas RL. Meis homeoproteins directly regulate Pax6 during vertebrate lens morphogenesis. Genes Dev. 2002;16:2097–2107. doi: 10.1101/gad.1007602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Dean A. An insulator blocks spreading of histone acetylation and interferes with RNA polymerase II transfer between an enhancer and gene. Nucleic Acids Res. 2004;32:4903–4919. doi: 10.1093/nar/gkh832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CC, Dyer MA, Uchikawa M, Kondoh H, Lagutin OV, Oliver G. Six3-mediated auto repression and eye development requires its interaction with members of the Groucho-related family of co-repressors. Development. 2002;129:2835–2849. doi: 10.1242/dev.129.12.2835. [DOI] [PubMed] [Google Scholar]
- Zupkovitz G, Tischler J, Posch M, Sadzak I, Ramsauer K, Egger G, Grausenburger R, Schweifer N, Chiocca S, Decker T, Seiser C. Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol Cell Biol. 2006;26:7913–7928. doi: 10.1128/MCB.01220-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


