Abstract
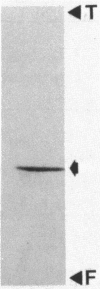
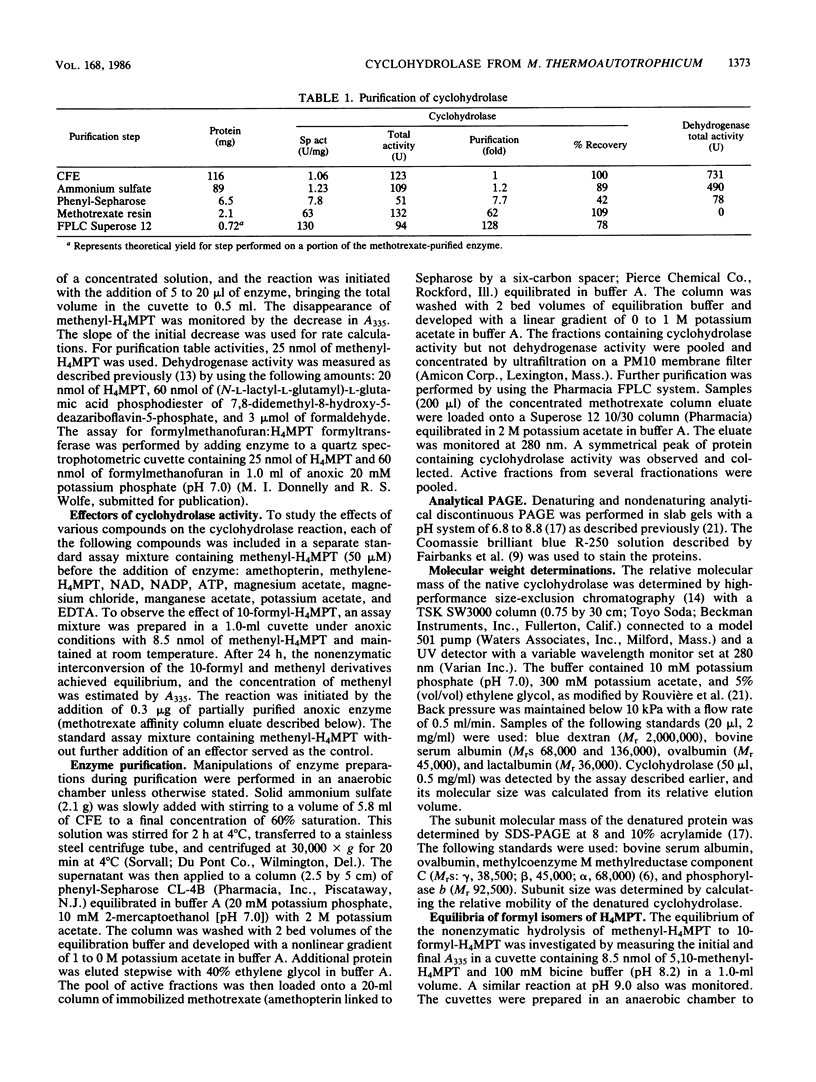
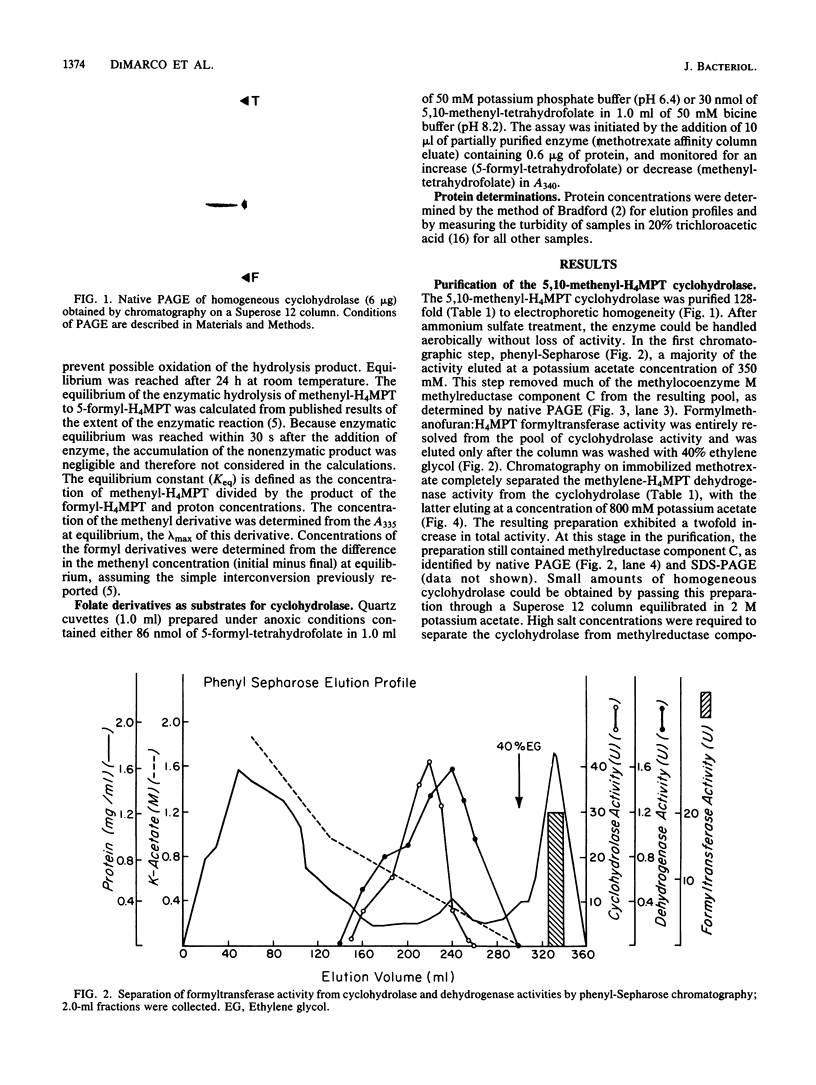
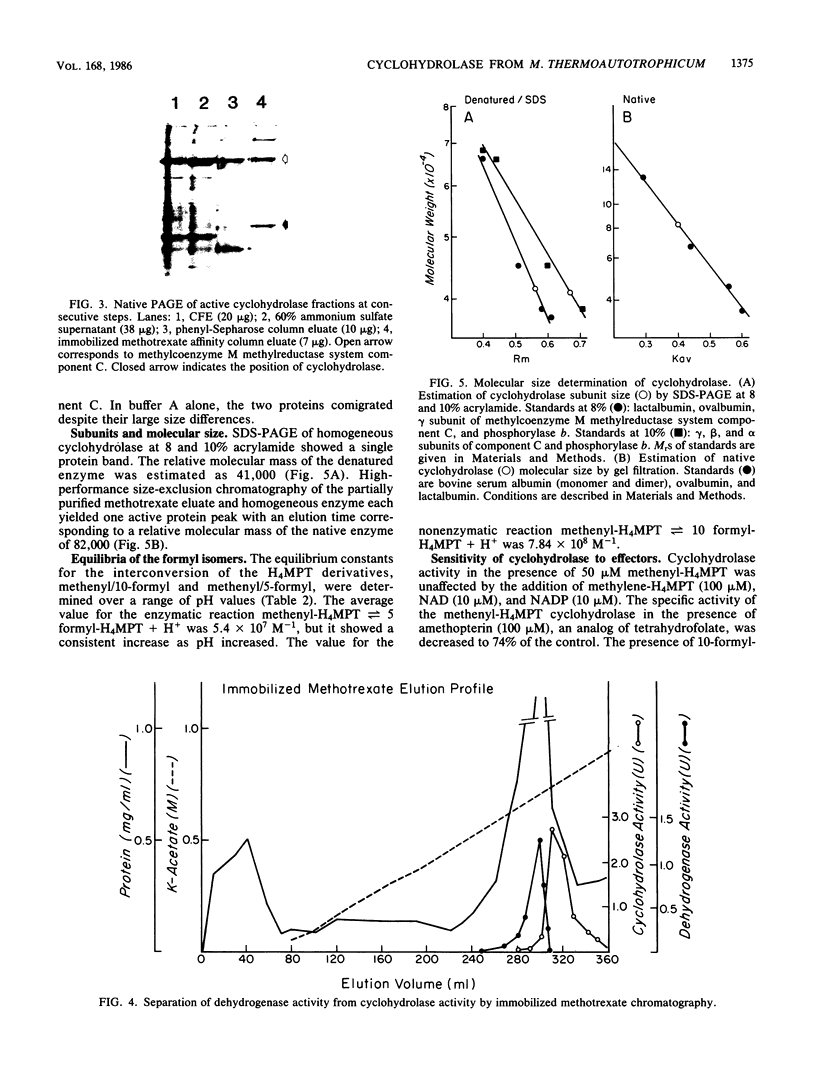
The 5,10-methenyltetrahydromethanopterin cyclohydrolase of Methanobacterium thermoautotrophicum was purified 128-fold to homogeneity. The enzyme had a subunit Mr of 41,000 as indicated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. From high-performance size exclusion chromatography of the native protein, an Mr of 82,000 was determined, suggesting a dimer of identical subunits. The enzyme was inhibited by 10-formyltetrahydromethanopterin and stimulated by Mg2+. Evaluation of the reaction equilibrium indicated that the methenyl derivative was favored over 5-formyltetrahydromethanopterin, with a much higher equilibrium constant than for the analogous reaction of tetrahydrofolate derivatives. Folate derivatives did not serve as substrates for this enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark J. E., Ljungdahl L. G. Purification and properties of 5,10-methenyltetrahydrofolate cyclohydrolase from Clostridium formicoaceticum. J Biol Chem. 1982 Apr 10;257(7):3833–3836. [PubMed] [Google Scholar]
- Dev I. K., Harvey R. J. A complex of N5,N10-methylenetetrahydrofolate dehydrogenase and N5,N10-methenyltetrahydrofolate cyclohydrolase in Escherichia coli. Purification, subunit structure, and allosteric inhibition by N10-formyltetrahydrofolate. J Biol Chem. 1978 Jun 25;253(12):4245–4253. [PubMed] [Google Scholar]
- Donnelly M. I., Escalante-Semerena J. C., Rinehart K. L., Jr, Wolfe R. S. Methenyl-tetrahydromethanopterin cyclohydrolase in cell extracts of Methanobacterium. Arch Biochem Biophys. 1985 Nov 1;242(2):430–439. doi: 10.1016/0003-9861(85)90227-9. [DOI] [PubMed] [Google Scholar]
- Ellefson W. L., Wolfe R. S. Component C of the methylreductase system of Methanobacterium. J Biol Chem. 1981 May 10;256(9):4259–4262. [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Leigh J. A., Rinehart K. L., Wolfe R. S. Formaldehyde activation factor, tetrahydromethanopterin, a coenzyme of methanogenesis. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1976–1980. doi: 10.1073/pnas.81.7.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Rinehart K. L., Jr, Wolfe R. S. Tetrahydromethanopterin, a carbon carrier in methanogenesis. J Biol Chem. 1984 Aug 10;259(15):9447–9455. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Grimshaw C. E., Henderson G. B., Soppe G. G., Hansen G., Mathur E. J., Huennekens F. M. Purification and properties of 5,10-methenyltetrahydrofolate synthetase from Lactobacillus casei. J Biol Chem. 1984 Mar 10;259(5):2728–2733. [PubMed] [Google Scholar]
- Gunsalus R. P., Romesser J. A., Wolfe R. S. Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochemistry. 1978 Jun 13;17(12):2374–2377. doi: 10.1021/bi00605a019. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Methyl coenzyme M reductase from Methanobacterium thermoautotrophicum. Resolution and properties of the components. J Biol Chem. 1980 Mar 10;255(5):1891–1895. [PubMed] [Google Scholar]
- Hartzell P. L., Zvilius G., Escalante-Semerena J. C., Donnelly M. I. Coenzyme F420 dependence of the methylenetetrahydromethanopterin dehydrogenase of Methanobacterium thermoautotrophicum. Biochem Biophys Res Commun. 1985 Dec 31;133(3):884–890. doi: 10.1016/0006-291x(85)91218-5. [DOI] [PubMed] [Google Scholar]
- Hefti F. High-performance size-exclusion chromatography: a buffer for the reliable determination of molecular weights of proteins. Anal Biochem. 1982 Apr;121(2):378–381. doi: 10.1016/0003-2697(82)90496-1. [DOI] [PubMed] [Google Scholar]
- KAY L. D., OSBORN M. J., HATEFI Y., HUENNEKENS F. M. The enzymatic conversion of N5-formyl tetrahydrofolic acid (folinic acid) to N10-formyl tetrahydrofolic acid. J Biol Chem. 1960 Jan;235:195–201. [PubMed] [Google Scholar]
- KUNITZ M. Crystalline inorganic pyrophosphatase isolated from baker's yeast. J Gen Physiol. 1952 Jan;35(3):423–450. doi: 10.1085/jgp.35.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leigh J. A. Levels of water-soluble vitamins in methanogenic and non-methanogenic bacteria. Appl Environ Microbiol. 1983 Mar;45(3):800–803. doi: 10.1128/aem.45.3.800-803.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukert J. L., Straus L. D., Rabinowitz J. C. Formyl-methyl-methylenetetrahydrofolate synthetase-(combined). An ovine protein with multiple catalytic activities. J Biol Chem. 1976 Aug 25;251(16):5104–5111. [PubMed] [Google Scholar]
- Rouvière P. E., Escalante-Semerena J. C., Wolfe R. S. Component A2 of the methylcoenzyme M methylreductase system from Methanobacterium thermoautotrophicum. J Bacteriol. 1985 Apr;162(1):61–66. doi: 10.1128/jb.162.1.61-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beelen P., Stassen A. P., Bosch J. W., Vogels G. D., Guijt W., Haasnoot C. A. Elucidation of the structure of methanopterin, a coenzyme from Methanobacterium thermoautotrophicum, using two-dimensional nuclear-magnetic-resonance techniques. Eur J Biochem. 1984 Feb 1;138(3):563–571. doi: 10.1111/j.1432-1033.1984.tb07951.x. [DOI] [PubMed] [Google Scholar]