Abstract
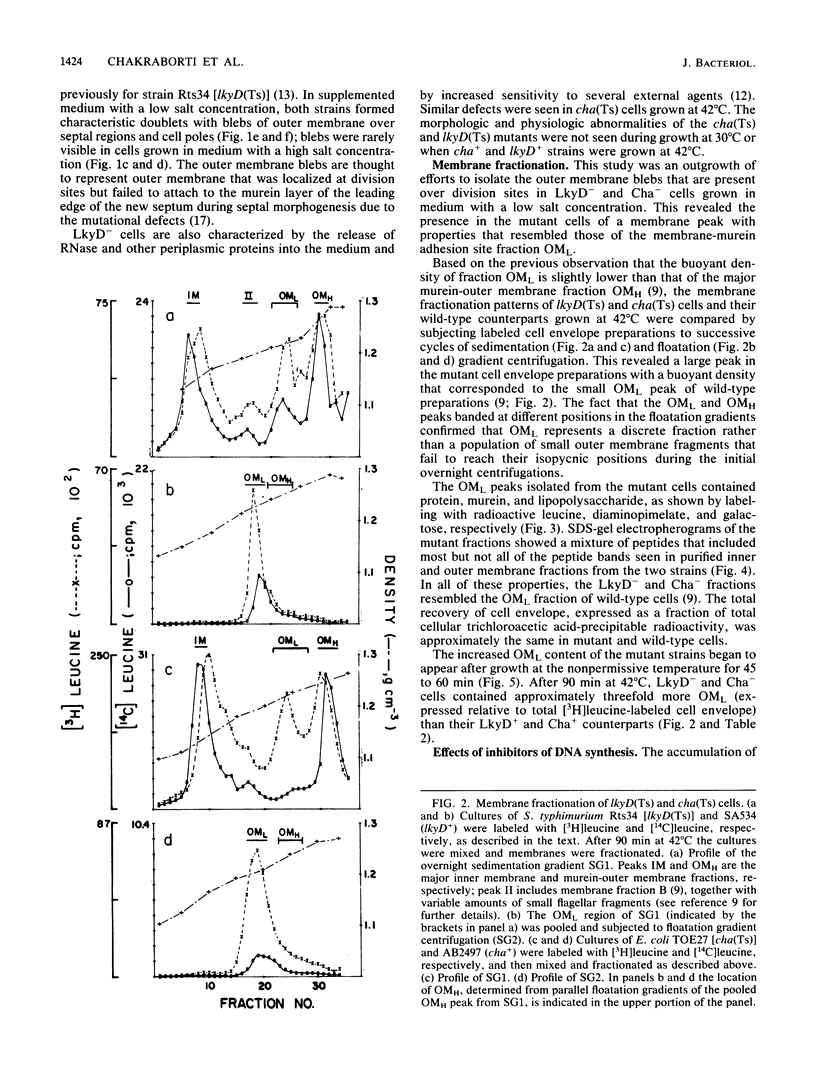
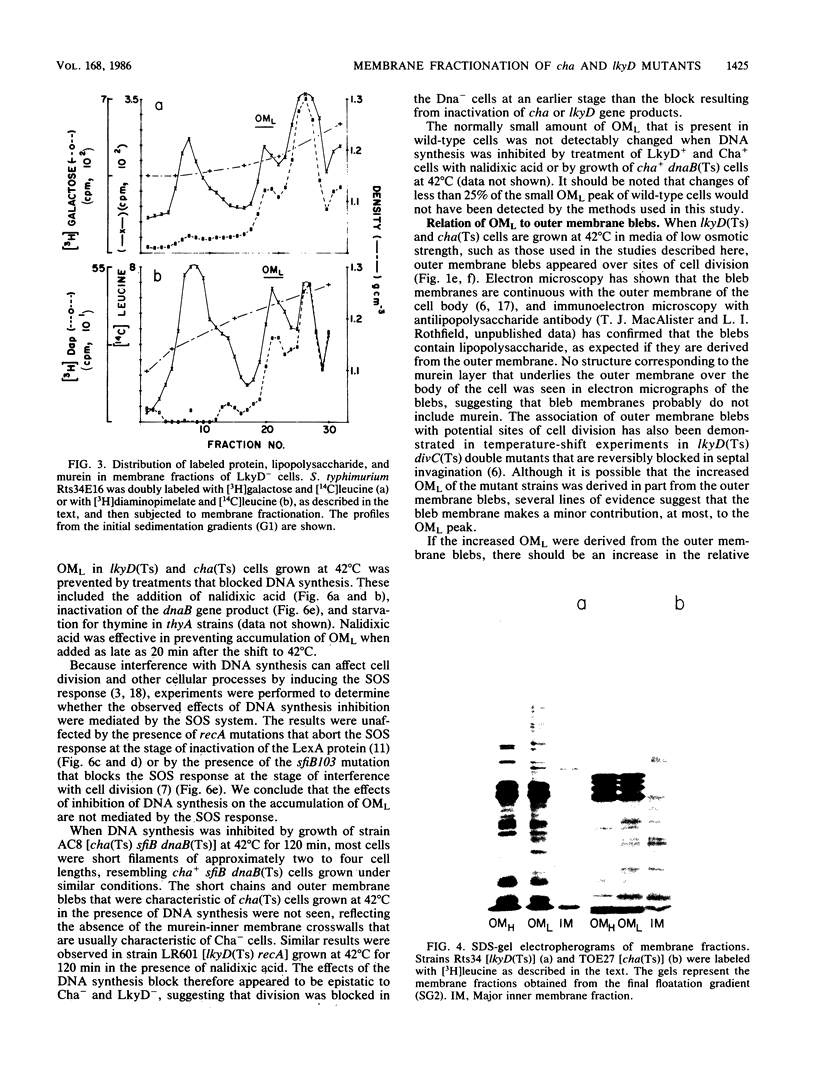
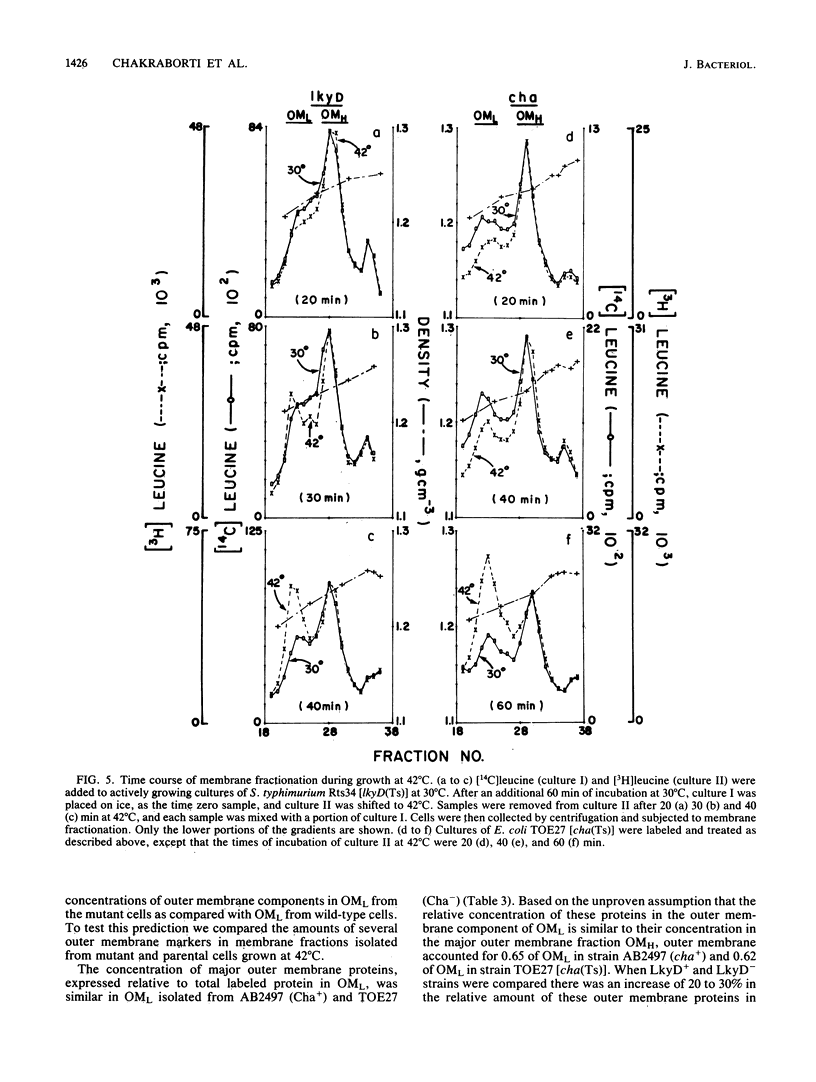
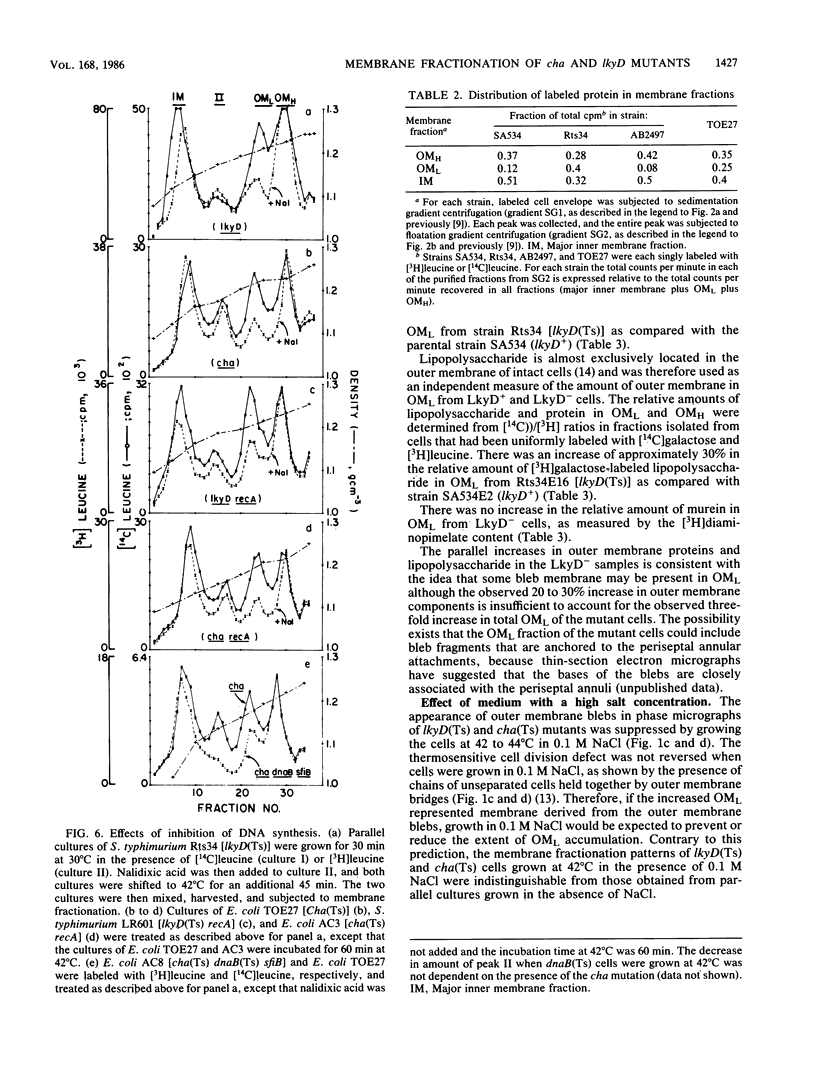
Membrane fractionation studies were performed on Salmonella typhimurium lkyD(Ts) and E. coli cha(Ts) mutants that appeared to be blocked at a late stage of the cell division cycle. In both cases growth of the mutant strains at nonpermissive temperatures was associated with accumulation of a characteristic cell envelope fraction (fraction OML) that contained inner membrane, murein, and outer membrane components. The isolated fraction corresponded in composition and bouyant density to a fraction from wild-type strains that had previously been suggested (M. H. Bayer, G. P. Costello, and M. E. Bayer, J. Bacteriol. 149:758-767, 1982; K. Ishidate, E. S. Creeger, J. Zrike, S. Deb, B. Glauner, T. J. MacAlister, and L. I. Rothfield, J. Biol. Chem. 261:428-443, 1986) to contain adhesion sites between inner membrane, murein, and outer membrane. The accumulation of OML in LkyD- and Cha- cells was prevented by treatments that blocked DNA synthesis. The effects of interference with DNA synthesis did not appear to involve the SOS response.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Bayer M. H., Costello G. P., Bayer M. E. Isolation and partial characterization of membrane vesicles carrying markers of the membrane adhesion sites. J Bacteriol. 1982 Feb;149(2):758–767. doi: 10.1128/jb.149.2.758-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defais M., Caillet-Fauquet P., Fox M. S., Radman M. Induction kinetics of mutagenic DNA repair activity in E. coli following ultraviolet irradiation. Mol Gen Genet. 1976 Oct 18;148(2):125–130. doi: 10.1007/BF00268375. [DOI] [PubMed] [Google Scholar]
- Fung J. C., MacAlister T. J., Weigand R. A., Rothfield L. I. Morphogenesis of the bacterial division septum: identification of potential sites of division in lkyD mutants of Salmonella typhimurium. J Bacteriol. 1980 Aug;143(2):1019–1024. doi: 10.1128/jb.143.2.1019-1024.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung J., MacAlister T. J., Rothfield L. I. Role of murein lipoprotein in morphogenesis of the bacterial division septum: phenotypic similarity of lkyD and lpo mutants. J Bacteriol. 1978 Mar;133(3):1467–1471. doi: 10.1128/jb.133.3.1467-1471.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Ishidate K., Creeger E. S., Zrike J., Deb S., Glauner B., MacAlister T. J., Rothfield L. I. Isolation of differentiated membrane domains from Escherichia coli and Salmonella typhimurium, including a fraction containing attachment sites between the inner and outer membranes and the murein skeleton of the cell envelope. J Biol Chem. 1986 Jan 5;261(1):428–443. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J., Gottfried S., Rothfield L. Leakage of periplasmic enzymes by mutants of Escherichia coli and Salmonella typhimurium: isolation of "periplasmic leaky" mutants. J Bacteriol. 1972 Feb;109(2):520–525. doi: 10.1128/jb.109.2.520-525.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macalister T. J., Macdonald B., Rothfield L. I. The periseptal annulus: An organelle associated with cell division in Gram-negative bacteria. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1372–1376. doi: 10.1073/pnas.80.5.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- Weigand R. A., Rothfield L. I. Genetic and physiological classification of periplasmic-leaky mutants of Salmonella typhimurium. J Bacteriol. 1976 Jan;125(1):340–345. doi: 10.1128/jb.125.1.340-345.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand R. A., Vinci K. D., Rothfield L. I. Morphogenesis of the bacterial division septum: a new class of septation-defective mutants. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1882–1886. doi: 10.1073/pnas.73.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]




