Abstract
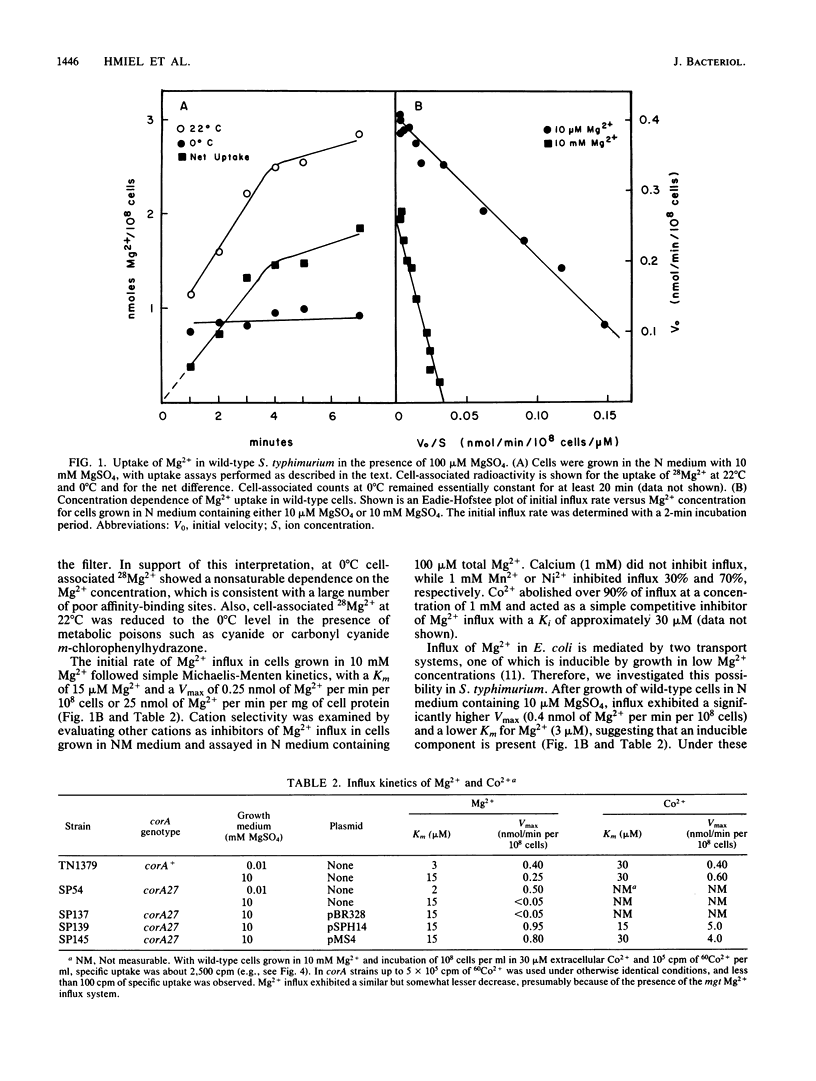
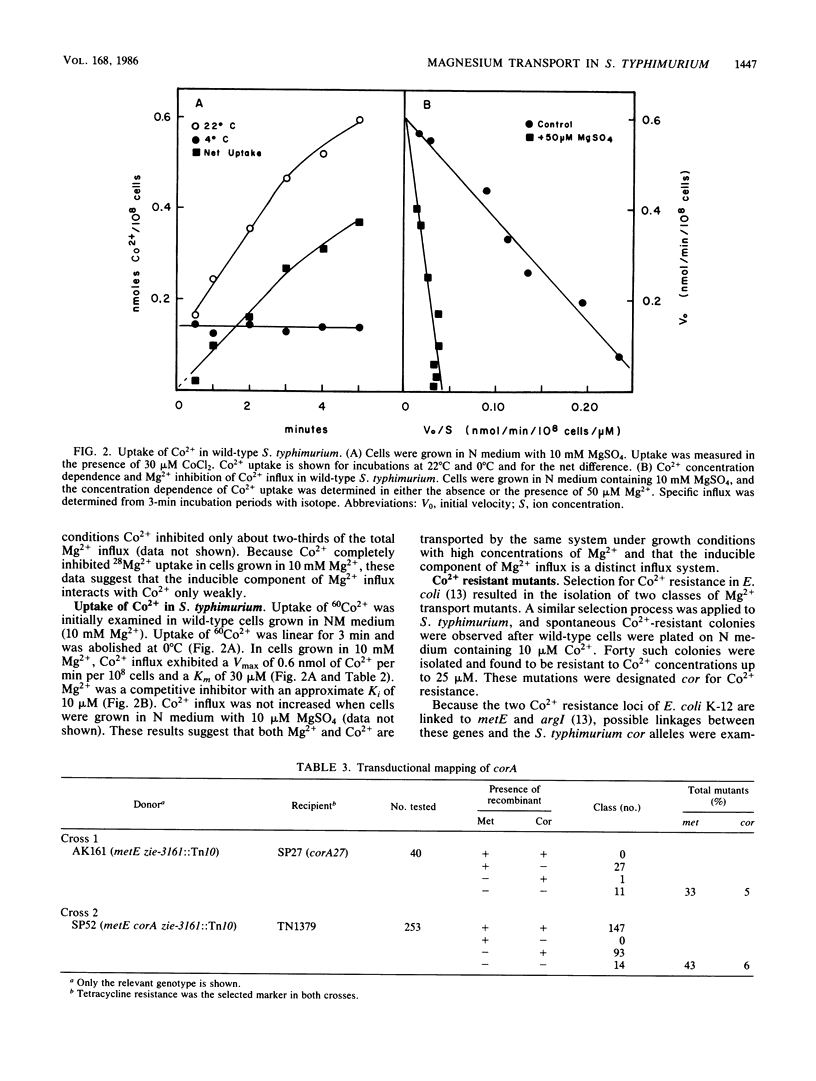
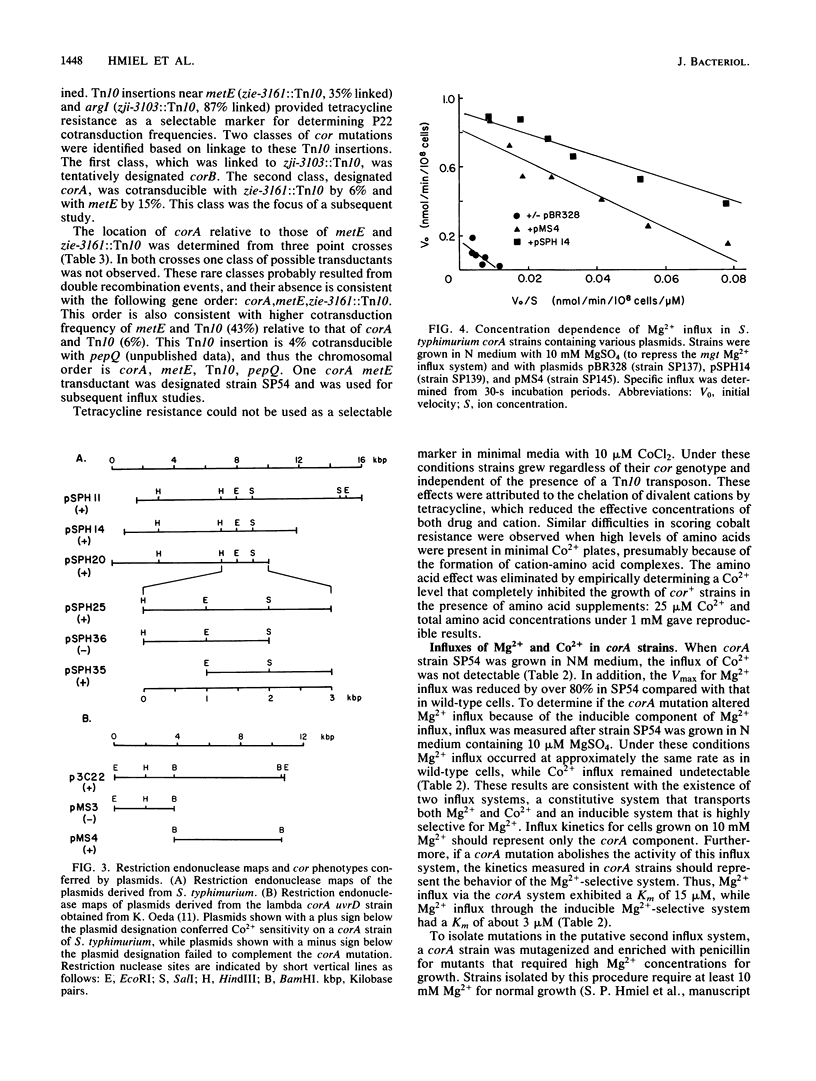
The influx of Mg2+ in Salmonella typhimurium LT-2 was studied by both kinetic and genetic techniques. Wild-type cells grown in a high MgSO4 concentration (10 mM) exhibited a Km of 15 microM for Mg2+ influx, with a Vmax of 0.25 nmol of Mg2+ per min per 10(8) cells. The apparent Km decreased to 3 microM, and the Vmax increased 60% after growth in a low MgSO4 concentration (10 microM). Co2+ was a simple competitive inhibitor (Ki = 30 microM) of Mg2+ influx in cells grown in high Mg2+ concentrations but blocked only a portion of the Mg2+ influx in cells grown in low Mg2+ concentrations. Co2+ influx exhibited kinetics similar to those of Mg2+ influx (Km = 30 microM; Vmax = 0.5 nmol of Co2+ per min per 10(8) cells) but was not affected by growth conditions. Co2+ influx was competitively inhibited by both Mg2+ and Mn2+. Mutations affecting Mg2+ uptake were isolated by selection for spontaneous resistance to toxic levels of Co2+. One class of mutants designated corA mapped at 84 min near metE with the following gene order: corA, metE, zie-3161::Tn10, pepQ. A second class designated corB mapped at 98 min near pyrB. Mg2+ influx was decreased in a corA mutant strain (relative to that of the wild type) when grown in high Mg2+ concentrations but was restored when grown in low Mg2+ concentrations. Co2+ transport was completely abolished by the corA mutation under all growth conditions. Recombinant plasmids carrying the corA region from either Escherichia coli K-12 or S. typhimurium complemented the corA mutation in S. typhimurium, restoring uptake of both Co2+ and Mg2+ and conferring sensitivity to Co2+. The S. typhimurium corA gene was localized to a restriction fragment of approximately 1.5 kilobases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel-Tsipis J., Botstein D., Fox M. S. Generalized transduction by phage P22 in Salmonella typhimurium. I. Molecular origin of transducing DNA. J Mol Biol. 1972 Nov 14;71(2):433–448. doi: 10.1016/0022-2836(72)90361-0. [DOI] [PubMed] [Google Scholar]
- Eggertsson G., Adelberg E. A. Map positions and specificities of suppressor mutations in Escherichia coli K-12. Genetics. 1965 Aug;52(2):319–340. doi: 10.1093/genetics/52.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W. Magnesium transport across cell membranes. J Membr Biol. 1984;80(1):1–14. doi: 10.1007/BF01868686. [DOI] [PubMed] [Google Scholar]
- Lusk J. E., Kennedy E. P. Magneisum transport in Escherichia coli. J Biol Chem. 1969 Mar 25;244(6):1653–1655. [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Kennedy E. P. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J Biol Chem. 1971 May 10;246(9):3042–3049. [PubMed] [Google Scholar]
- Nelson D. L., Kennedy E. P. Transport of magnesium by a repressible and a nonrepressible system in Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1091–1093. doi: 10.1073/pnas.69.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeda K., Horiuchi T., Sekiguchi M. Molecular cloning of the uvrD gene of Escherichia coli that controls ultraviolet sensitivity and spontaneous mutation frequency. Mol Gen Genet. 1981;184(2):191–199. doi: 10.1007/BF00272904. [DOI] [PubMed] [Google Scholar]
- Park M. H., Wong B. B., Lusk J. E. Mutants in three genes affecting transport of magnesium in Escherichia coli: genetics and physiology. J Bacteriol. 1976 Jun;126(3):1096–1103. doi: 10.1128/jb.126.3.1096-1103.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. R., Mottur G. P., Bradbeer C. Transport of vitamin B12 in Escherichia coli. Some observations on the roles of the gene products of BtuC and TonB. J Biol Chem. 1980 May 10;255(9):4313–4319. [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S. Active transport of magnesium in escherichia coli. Proc Natl Acad Sci U S A. 1969 Mar;62(3):764–771. doi: 10.1073/pnas.62.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Bhattacharyya P. Cations, antibiotics, and membranes. Methods Enzymol. 1974;32:881–893. doi: 10.1016/0076-6879(74)32090-3. [DOI] [PubMed] [Google Scholar]
- Silver S., Clark D. Magnesium transport in Escherichia coli. J Biol Chem. 1971 Feb 10;246(3):569–576. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wacker W. E., Parisi A. F. Magnesium metabolism. N Engl J Med. 1968 Mar 28;278(13):712–717. doi: 10.1056/NEJM196803282781306. [DOI] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Webb M. The utilization of magnesium by certain Gram-positive and Gram-negative bacteria. J Gen Microbiol. 1966 Jun;43(3):401–409. doi: 10.1099/00221287-43-3-401. [DOI] [PubMed] [Google Scholar]



