Abstract
The focusing of microtubules into mitotic spindle poles in vertebrate somatic cells has been assumed to be the consequence of their nucleation from centrosomes. Contrary to this simple view, in this article we show that an antibody recognizing the light intermediate chain of cytoplasmic dynein (70.1) disrupts both the focused organization of microtubule minus ends and the localization of the nuclear mitotic apparatus protein at spindle poles when injected into cultured cells during metaphase, despite the presence of centrosomes. Examination of the effects of this dynein-specific antibody both in vitro using a cell-free system for mitotic aster assembly and in vivo after injection into cultured cells reveals that in addition to its direct effect on cytoplasmic dynein this antibody reduces the efficiency with which dynactin associates with microtubules, indicating that the antibody perturbs the cooperative binding of dynein and dynactin to microtubules during spindle/aster assembly. These results indicate that microtubule minus ends are focused into spindle poles in vertebrate somatic cells through a mechanism that involves contributions from both centrosomes and structural and microtubule motor proteins. Furthermore, these findings, together with the recent observation that cytoplasmic dynein is required for the formation and maintenance of acentrosomal spindle poles in extracts prepared from Xenopus eggs (Heald, R., R. Tournebize, T. Blank, R. Sandaltzopoulos, P. Becker, A. Hyman, and E. Karsenti. 1996. Nature (Lond.). 382: 420–425) demonstrate that there is a common mechanism for focusing free microtubule minus ends in both centrosomal and acentrosomal spindles. We discuss these observations in the context of a search-capture-focus model for spindle assembly.
Chromosome segregation during both mitosis and meiosis is mediated by a complex microtubule-based structure called the spindle (McIntosh and Koonce; 1989; Mitchison, 1989a ; Rieder, 1991). The spindle is assembled in a spatially and temporally regulated manner during the cell cycle, and its assembly and function are intimately associated with microtubule dynamics (Inoué and Salmon, 1995; Hyman and Karsenti, 1996; Nicklas, 1997). The organization of microtubules into spindles is governed largely by the interaction of microtubules and microtubule ends with accessory proteins that regulate microtubule dynamics. These accessory proteins are located on the chromosomes (kinetochores), derived from the cytosol (some motor proteins), and/or found at the microtubule minus ends (centrosomes and peri-centrosomal region in somatic cells). The result of these complex interactions is a typical fusiform microtubule array in both mitotic and meiotic cells with microtubule plus ends attached to the chromosomes and minus ends focused into spindle poles.
One striking difference between spindles in vertebrate somatic cells and some types of meiotic and plant cells is that microtubules in vertebrate somatic cells are nucleated from centrosomes, whereas plant cells and some meiotic cells lack bonafide centrosomes. This single structural difference has spurred two different hypotheses regarding the mechanism by which microtubule minus ends are focused at spindle poles (for discussions see Wilson, 1925; Schrader, 1953; Rieder et al., 1993; Waters and Salmon, 1997). In acentrosomal spindles, microtubules associate with chromatin and are drawn into two focused poles through the action of minus end-directed microtubule motors (Bastmeyer et al., 1986; Steffen et al., 1986; Theurkauf and Hawley, 1992; McKim and Hawley, 1995; Vernos and Karsenti, 1995; Heald et al., 1996; Matthies et al., 1996; Merdes et al., 1996). In contrast, in mitotic spindles in vertebrate cells the predominant view holds that microtubule minus ends are focused at the poles as a consequence of their nucleation from the centrosomes (Kirschner and Mitchison, 1986; Hayden et al., 1990; Holy and Leibler, 1994; Rieder and Salmon, 1995). It is currently unclear, however, if mitotic spindles containing centrosomes, like acentrosomal spindles, also use minus end-directed motor activity to promote the focusing of microtubule minus ends at spindle poles despite the presence of centrosomes.
The dynein-specific monoclonal antibody 70.1 (Steuer et al., 1991) was recently shown to perturb the function of cytoplasmic dynein during spindle assembly in meiotic extracts prepared from Xenopus eggs (Heald et al., 1996). It blocked the formation of spindle poles as well as induced the disorganization of the polar regions of preassembled spindles, suggesting that dynein function was important to establish and maintain these spindle poles. Spindles assembled under those conditions, however, do not contain centrosomes, and the spindle poles are focused through an acentrosomal mechanism (Lohka and Maller, 1985; Sawin and Mitchison, 1991; Heald et al., 1996; Merdes et al., 1996). Thus, in this article we have used the 70.1 antibody to investigate whether the organization of microtubules at the polar ends of the mitotic spindle also relies on the action of cytoplasmic dynein despite the inherent focusing activity of centrosomes. We report that perturbation of cytoplasmic dynein function with the 70.1 antibody in somatic cells leads to the disruption of mitotic spindle poles and the separation of the centrosomes from the body of the spindle. Furthermore, the 70.1 antibody prevents the assembly of mitotic asters when added to a cell-free mitotic extract, and in both cases, reduces the efficiency with which dynactin associates with microtubules. These data indicate that microtubule minus ends are focused at mitotic spindle poles through contributions from both centrosomes and accessory proteins, including the minus end-directed motor cytoplasmic dynein and dynactin, and suggest that there are common aspects to the mechanism by which free microtubule minus ends are focused into poles in centrosomal and acentrosomal spindles. These results are discussed in the context of a search-capture-focus model for mitotic spindle assembly.
Materials and Methods
Cell Culture
The human HeLa cell line and the monkey CV-1 cell line were both maintained in DME containing 10% fetal calf serum, 2 mM glutamine, 100 IU/ml penicillin, and 0.1 μg/ml streptomycin. Cells were grown at 37°C in a humidified incubator with a 5% CO2 atmosphere.
Immunological Techniques
The control (mAb 154; Compton et al., 1991) and dynein-specific (mAb 70.1; Steuer et al., 1991) IgMs were purified from ascites fluid by mannose-binding protein affinity chromatography (Pierce, Rockford, IL). The purified antibodies were dialyzed into 0.1 M Tris, pH 7.4, and concentrated using centricon-30 concentrators (Amicon, Beverly, MA) to 8–16 mg/ml. The remaining antibodies used in this study were a rabbit anti- nuclear mitotic apparatus (NuMA)1 (Gaglio et al., 1995), mouse anti-tubulin (DM1A; Blose et al., 1984), rabbit anti-Eg5 stalk-tail (Sawin et al., 1992), mouse anti-Arp1 (45A; Schafer et al., 1994), mouse anti-p150 dynactin (150B; Gaglio et al., 1996), and mouse anti-dynein (74.1; Dillman and Pfister, 1994).
Indirect immunofluorescence microscopy was performed on cultured cells by immersion in microtubule stabilization buffer (MTSB: 4 M glycerol, 100 mM PIPES, pH 6.9, 1 mM EGTA, and 5 mM MgCl2) for 1 min at room temperature, extraction in MTSB plus 0.5% Triton X-100 for 2 min, followed by MTSB for 2 min. Cells were then fixed in −20°C methanol for 10 min. Indirect immunofluorescence microscopy on mitotic asters assembled in the cell-free mitotic extract was performed by dilution of 5 μl of the extract into 25 μl of KHM buffer (78 mM KCl, 50 mM Hepes, pH 7.0, 4 mM MgCl2, 2 mM EGTA, 1 mM DTT; Burke and Gerace, 1986). The diluted sample was then spotted onto a poly–l-lysine coated glass coverslip and fixed by immersion in −20°C methanol. Both the fixed cells and mitotic asters were rehydrated in TBS (10 mM Tris-HCl, pH 7.5, 150 mM NaCl) containing 1% albumin, and all antibody incubations and washes were performed in TBS plus 1% albumin. Each primary antibody was incubated on the coverslip for 30 min except for the 45A antibody against dynactin and the 74.1 antibody against cytoplasmic dynein, which were incubated on the coverslip for 2 h. After primary antibody treatment, the coverslips were washed for 5 min in TBS plus 1% albumin, and the bound antibodies were detected using either fluorescein- or Texas red-conjugated species- and antibody isotype-specific secondary antibodies at dilutions of 1:500 (Vector Labs, Burlingame, CA). DNA was detected using DAPI (4′,6-diamidino-2-phenylindole) at 0.4 μg/ml (Sigma Chemical Co., St. Louis, MO). After a final wash the coverslips were mounted in FITC-guard mounting medium (Testog, Inc., Chicago, IL) and observed on a Nikon Optiphot microscope equipped for epifluorescence (Nikon, Inc., Meliville, NY).
Proteins from the mitotic extracts were solubilized directly with SDS-PAGE sample buffer. The proteins were then separated by size using SDS-PAGE and transferred to PVDF (polyvinylidene difluoride) membrane (Millipore Corp., Bedford, MA). The membranes were blocked in TBS containing 5% nonfat milk for 30 min at room temperature and the primary antibody incubated for 6 h at room temperature in TBS containing 1% nonfat milk. Nonbound primary antibody was removed by washing five times for 3 min each in TBS, and the bound antibody was detected using either horseradish peroxidase-conjugated protein A or horseradish peroxidase-conjugated goat anti–mouse (Bio Rad, Hercules, CA). The nonbound secondary reagent was removed by washing five times for 3 min each in TBS and the signal detected using enhanced chemiluminescence (Amersham Corp., Arlington Heights, IL).
Microinjection
CV-1 cells growing on photo-etched α-numeric glass cover slips (Bellco Glass Co., Vineland, NJ) were microinjected following the procedures of Compton and Cleveland (1993) and Capecchi (1980). Interphase cells were microinjected in the cytoplasm with either the control antibody or the dynein-specific antibody and followed by phase contrast microscopy as they progressed into mitosis. Metaphase cells were selected for injection by phase contrast microscopy by virtue of a clearly identifiable bipolar mitotic spindle. Injected cells were followed for up to 4 h after injection unless otherwise stated in the text and were processed for immunofluorescence microscopy as described above.
Mitotic Extracts
Mitotic extracts from HeLa cells were prepared according to Gaglio et al. (1995). HeLa cells were synchronized in the cell cycle by double block with 2 mM thymidine. After release from thymidine block the cells were allowed to grow for 6 h, and then nocodazole was added to a final concentration of 40 ng/ml. The mitotic cells that accumulated over the next 4 h were collected by mitotic shake off and incubated for 30 min at 37°C with 20 μg/ml cytochalasin B. The cells were then collected by centrifugation at 1,500 rpm and washed twice with cold PBS containing 20 μg/ml cytochalasin B. Cells were washed one last time in cold KHM buffer containing 20 μg/ml cytochalasin B and finally Dounce homogenized (tight pestle) at a concentration of ∼3 × 107 cells/ml in KHM buffer containing 20 μg/ml cytochalasin B, 20 μg/ml phenylmethylsulfonyl fluoride, and 1 μg/ml each of chymostatin, leupeptin, antipain, and pepstatin. The crude cell extract was then subjected to sedimentation at 100,000 g for 15 min at 4°C. The supernatant was recovered and supplemented with 2.5 mM ATP (prepared as Mg2+ salts in KHM buffer) and 10 μM taxol, and the mitotic asters were stimulated to assemble by incubation at 30°C for 30–60 min. After incubation, samples were processed for indirect immunofluorescence microscopy as described above, and the remainder of the extract containing the assembled mitotic asters was subjected to sedimentation at 10,000 g for 15 min at 4°C. The supernatant and pellet fractions were both recovered and solubilized in SDS-PAGE sample buffer for immunoblot analysis.
Immunodepletions from the extract before aster assembly was carried out using 20–50 μg of either an anti-Eg5 affinity-purified rabbit polyclonal IgG or the monoclonal antibody 70.1, which is an IgM specific for the IC74 intermediate chain of cytoplasmic dynein. Each antibody was adsorbed onto ∼25 μl of either protein A- or protein G-conjugated agarose (Boehringer Mannheim, Indianapolis, IN). The 70.1 monoclonal antibody against cytoplasmic dynein intermediate chain was coupled to protein G-conjugated agarose using goat anti–murine IgM specific antibody (Vector Lab. Burlingame, CA). The antibody-coupled agarose was washed in KHM buffer and then packed by centrifugation to remove the excess fluid. Efficient depletion of each target protein was routinely achieved by sequential depletion reactions in which the total quantity of packed agarose did not exceed 15 μl per 100 μl of extract. First, half of the antibody-coupled agarose was resuspended with the mitotic extract and incubated with agitation for 1 h at 4°C. After this incubation the agarose was removed from the extract by sedimentation at 15,000 g for 10 s and saved. Next, the extract was recovered and used to resuspend the other half of the antibody-coupled agarose and another incubation performed with agitation for 1 h at 4°C. After this incubation the agarose was removed by sedimentation at 15,000 g for 10 s and pooled with the agarose pellet from the initial depletion reaction. In all cases, immunoblot analysis indicates that this depletion protocol results in nearly 100% efficient depletion of the target protein as described previously (Gaglio et al., 1996). The depleted extract was recovered and microtubule polymerization induced by the addition of taxol and ATP and incubation at 30°C. Each depletion experiment was performed at least two times and in all cases the efficiency of mitotic aster formation (as determined by counting the average number of asters per microscope field) was not significantly different from the values determined previously (Gaglio et al., 1996).
Results
The Dynein-specific Antibody 70.1 Perturbs Mitotic Spindle Assembly In Vivo
Heald et al. (1996) recently showed that an antibody raised against the 74-kD subunit of cytoplasmic dynein purified from chicken embryo fibroblast cells (mAb 70.1; Steuer et al., 1991) perturbed the formation and maintenance of the polar ends of spindles assembled in meiotic extracts prepared from Xenopus eggs. To determine the specificity of this antibody in primate somatic cells, we performed immunoblot analysis of total cell protein from the primate cell lines HeLa and CV-1. Fig. 1 shows that this antibody reacts specifically with a single polypeptide of apparent molecular mass of 74 kD. This band most likely represents the light intermediate chain of cytoplasmic dynein derived from these two cell lines, because this immunoreactive polypeptide has the same apparent molecular weight as the original chicken protein, and we have previously shown that this antibody specifically immunoprecipitates cytoplasmic dynein from HeLa cell mitotic extracts (Gaglio et al., 1996). In some cases where we performed immunoblot analysis with this antibody on enriched cellular fractions, we observed three reactive species that ranged in molecular mass from 70–76 kD (see below). This result is consistent with both the expression of multiple isoforms and the complex post-translational regulation of this subunit of cytoplasmic dynein (Paschal et al., 1992; Niclas et al., 1996; Pfister et al., 1996). Thus, mAb 70.1 is specific for the light intermediate chain of cytoplasmic dynein in these primate cell types in accordance with its specificity for this subunit of cytoplasmic dynein in avian cells (Steuer et al., 1991).
Figure 1.
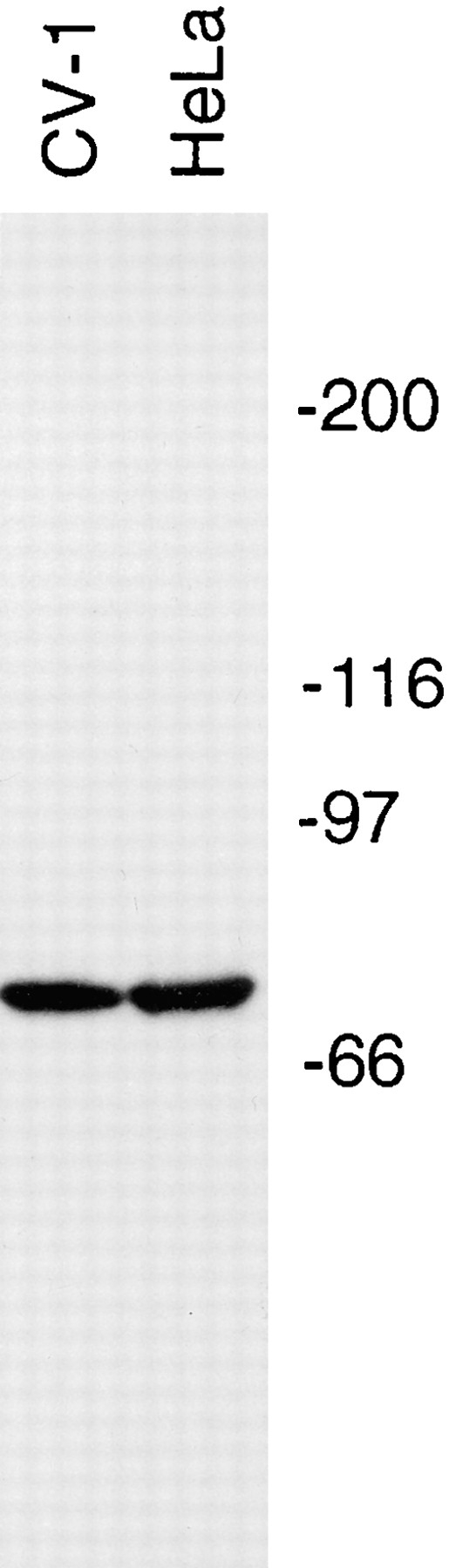
mAb 70.1 specifically recognizes the light intermediate chain of cytoplasmic dynein in primate cells. Immunoblot analysis of total cell protein from 100,000 HeLa or CV-1 cells (∼50 μg protein) using the 70.1 monoclonal antibody. The migration position of myosin (200), β-galactosidase (116), phosphorylase B (97), and BSA (66) are indicated in kD.
To determine if the dynein-specific antibody is capable of disrupting the assembly of the vertebrate mitotic spindle, we microinjected CV-1 cells during interphase with the dynein-specific monoclonal antibody (70.1) and followed the fate of each cell as it entered mitosis. 60% (n = 20) of cells that entered mitosis after microinjection with the dynein-specific antibody were significantly delayed in their completion of mitosis (>2 h). In contrast, 96% (n = 25) of cells that entered mitosis after microinjection with a control antibody completed mitosis normally within 1 h. The control antibody recognizes CENP-E. We specifically chose it as a control because it is a monoclonal IgM that recognizes a known spindle component, and we have previously determined that it does not perturb the progression of mitosis when injected into cultured cells (Compton et al., 1991). Examination of the mitotic spindles in cells injected with the control antibody by immunofluorescence microscopy showed a typical fusiform microtubule array with NuMA concentrated in a characteristic crescent-like position at the polar ends of the spindle (Fig. 2 A). In contrast, cells that entered mitosis in the presence of the dynein-specific antibody have disorganized mitotic spindles that lack well organized poles with NuMA localized along the length of many of the microtubules (Fig. 2 B). These results suggest that this antibody perturbs an essential function of cytoplasmic dynein during the formation of a normal bipolar spindle in somatic cells. This conclusion is consistent with the results previously reported by Heald et al. (1996) using this antibody to perturb acentrosomal spindle formation in extracts prepared from Xenopus eggs as well as evidence supporting a role for cytoplasmic dynein during spindle assembly in somatic cells (Pfarr et al., 1991; Steuer et al., 1991; Vaisberg et al., 1993; Echeverri et al., 1996; Gaglio et al., 1996).
Figure 2.
The dynein-specific 70.1 antibody blocks the formation of the mitotic spindle. Monkey CV-1 cells were monitored as they progressed through mitosis after microinjection with either a control antibody (A) or the dynein-specific 70.1 monoclonal antibody (B). The mitotic cells were fixed and processed for immunofluorescence microscopy using the DNA-specific dye DAPI, and antibodies specific for tubulin and NuMA as indicated. Bar, 10 μm.
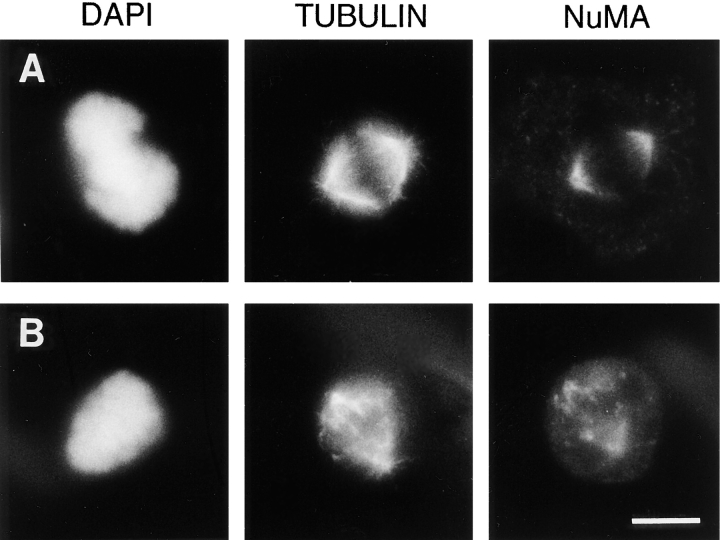
The data presented in Fig. 2 suggest that the 70.1 antibody prevents mitotic spindle formation but does not directly demonstrate whether cytoplasmic dynein plays a specific role in maintaining the focused organization of microtubule minus ends at the mitotic spindle pole in the presence of centrosomes. To address this point, we microinjected CV-1 cells during metaphase (i.e., with pre-assembled spindles) with either the control antibody or the dynein-specific antibody. 8 out of 8 metaphase cells injected with the dynein-specific antibody were blocked in mitosis for at least 2 h, whereas 8 out of 10 metaphase cells injected with a control antibody completed mitosis normally within 1 h. This indicates that the injection of the dynein-specific antibody into metaphase cells with pre- assembled spindles delays the completion of mitosis. To explore the effects of this antibody we examined the morphology of the mitotic spindle in cells at various times after antibody microinjection. 95% (n = 21) of metaphase cells injected with the control antibody had typical bipolar mitotic spindles with NuMA concentrated in the characteristic crescent-like shape near the centrosomes (Fig. 3 A). In contrast, 91% (n = 43) of metaphase cells injected with the dynein-specific antibody had abnormal mitotic spindles (Fig. 3, B–D). As rapidly as 5 min after injection with the dynein-specific antibody, the mitotic spindles were barrel shaped and the poles were unusually broad as judged by the organization of the microtubules and the distribution of NuMA (Fig. 3 B). If the injected cells were examined between 15 and 30 min after injection, the spindles lacked a typical fusiform organization. The microtubule minus ends appeared splayed, and NuMA was dislocated from the centrosomal region of the spindle and localized on the microtubules at the splayed ends of the spindle (Fig. 3, C and D). At later time points, NuMA was localized along the length of many of the microtubules (Fig. 3 D, arrows). Importantly, the centrosomes in these injected cells stained positively for γ-tubulin (data not shown) and were nucleating microtubules normally forming small astral microtubule arrays, but were separated from the body of the spindle (Fig. 3, C and D, arrowheads). Thus, despite the presence of functional centrosomes, the minus ends of the microtubules became unfocused and were displaced from the centrosomes after the injection of this dynein-specific antibody into metaphase cells.
Figure 3.
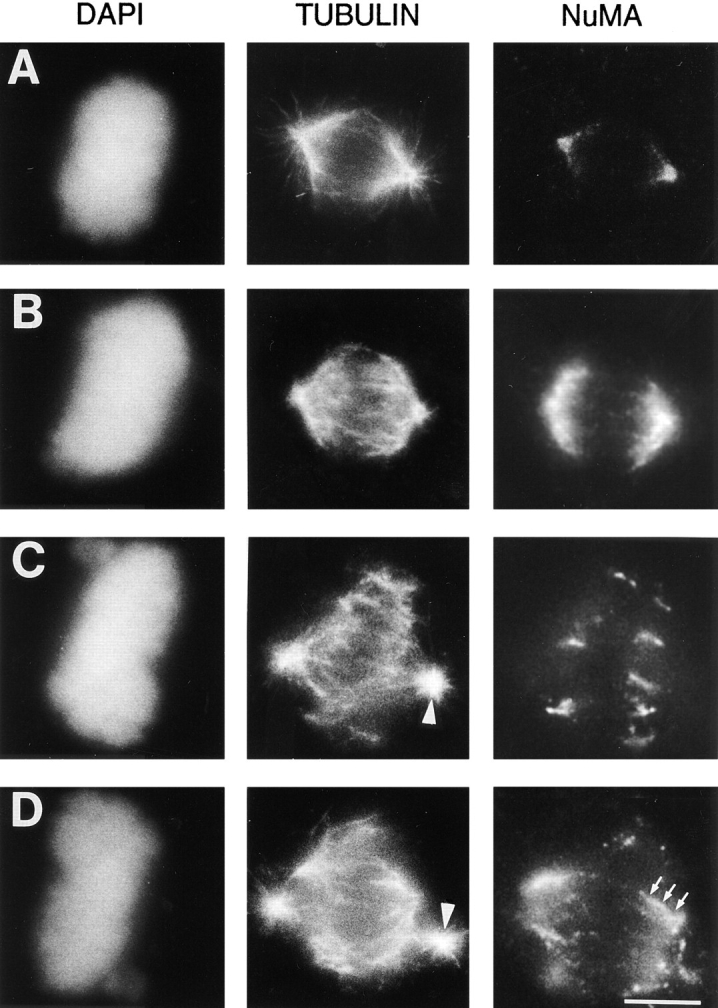
The dynein-specific 70.1 antibody disrupts preassembled mitotic spindles despite the presence of functional centrosomes. Monkey CV-1 cells in metaphase with bipolar mitotic spindles were selected by phase contrast microscopy and microinjected with either a control antibody (A) or the dynein-specific 70.1 monoclonal antibody (B–D). 5 min (B) or 15–30 min (A, C, and D) after microinjection, the cells were fixed and processed for immunofluorescence microscopy using the DNA-specific dye DAPI and antibodies specific for tubulin and NuMA as indicated. Arrowheads in C and D indicate centrosomes and arrows in D indicate NuMA. Bar, 10 μm.
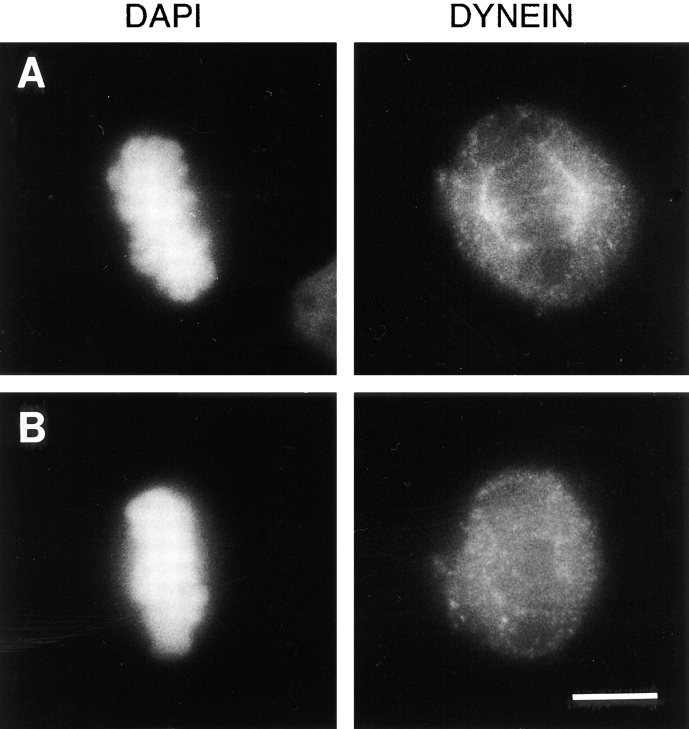
To determine if the 70.1 antibody perturbs the association of cytoplasmic dynein with the mitotic spindle, we microinjected metaphase cells with the 70.1 antibody and stained the cells with the dynein-specific monoclonal antibody 74.1 (Dillman and Pfister, 1994). Cytoplasmic dynein is localized on the mitotic spindle and throughout the cytosol of mitotic cells after injection with the control antibody (Fig. 4 A) consistent with previously published reports (Pfarr et al., 1991; Steuer et al., 1991). In contrast, the signal intensity for cytoplasmic dynein on mitotic spindles of cells injected with the dynein-specific 70.1 antibody is significantly reduced (Fig. 4 B). The intensity of staining of the cytosolic fraction of cytoplasmic dynein is equivalent between these two samples, indicating that the staining efficiency for each sample was similar (Fig. 4, A and B). The possibility that the 70.1 antibody sterically hinders the binding of the 74.1 antibody to cytoplasmic dynein is unlikely, because the 74.1 antibody decorates the cytosolic fraction of cytoplasmic dynein to the same extent in the presence of either the 70.1 or control antibodies (Fig. 4, A and B). Also, preincubation of fixed cells with the 70.1 antibody does not reduce the signal observed using the 74.1 antibody in immunofluorescence microscopy (data not shown). These results indicate that the dynein-specific 70.1 antibody disrupts the interaction of cytoplasmic dynein with the mitotic spindle in somatic cells.
Figure 4.
The dynein-specific 70.1 antibody causes a reduction in the efficiency with which cytoplasmic dynein associates with the mitotic spindle in vivo. Monkey CV-1 cells in metaphase with bipolar mitotic spindles were selected by phase contrast microscopy and microinjected with either a control antibody (A) or the dynein-specific 70.1 monoclonal antibody (B). The cells were then fixed and processed for immunofluorescence microscopy using the DNA-specific dye DAPI and the 74.1 antibody, which is specific for the light intermediate chain of cytoplasmic dynein as indicated. Bar, 10 μm.
The Dynein-specific Antibody 70.1 Perturbs Mitotic Aster Assembly In Vitro
We next tested if mitotic asters that form through a centrosome-independent mechanism in somatic cell mitotic extracts (Gaglio et al., 1995, 1996) are perturbed by the addition of this dynein-specific antibody. Addition of the control antibody to the extract either before mitotic aster assembly (Fig. 5 A) or after mitotic aster assembly (data not shown) had no observable effect on the organization of microtubules within the mitotic asters or the localization of NuMA at the central core of each aster. Addition of the dynein-specific antibody, however, prevented the assembly of mitotic asters if added to the extract before induction of aster assembly and perturbed the organization of preassembled mitotic asters if it was added to the extract after the induction of aster assembly (Fig. 5, B and C). In both cases, NuMA was associated with the microtubules but was distributed along the length of many of the microtubules instead of concentrated at any one position. This indicates that addition of this antibody before mitotic aster formation prevented the accumulation of NuMA in any one position, while addition of this antibody to pre- assembled mitotic asters caused the displacement of NuMA from the region near the microtubule minus ends. Thus, perturbation of cytoplasmic dynein function by the addition of this antibody is sufficient to not only prevent the assembly of mitotic asters in this system but also to disrupt the organization of pre-assembled mitotic asters in a manner very similar to the disruption of the polar regions of mitotic spindles in cultured cells (see above) and in acentrosomal spindles assembled in extracts prepared from Xenopus eggs (Heald et al., 1996).
Figure 5.
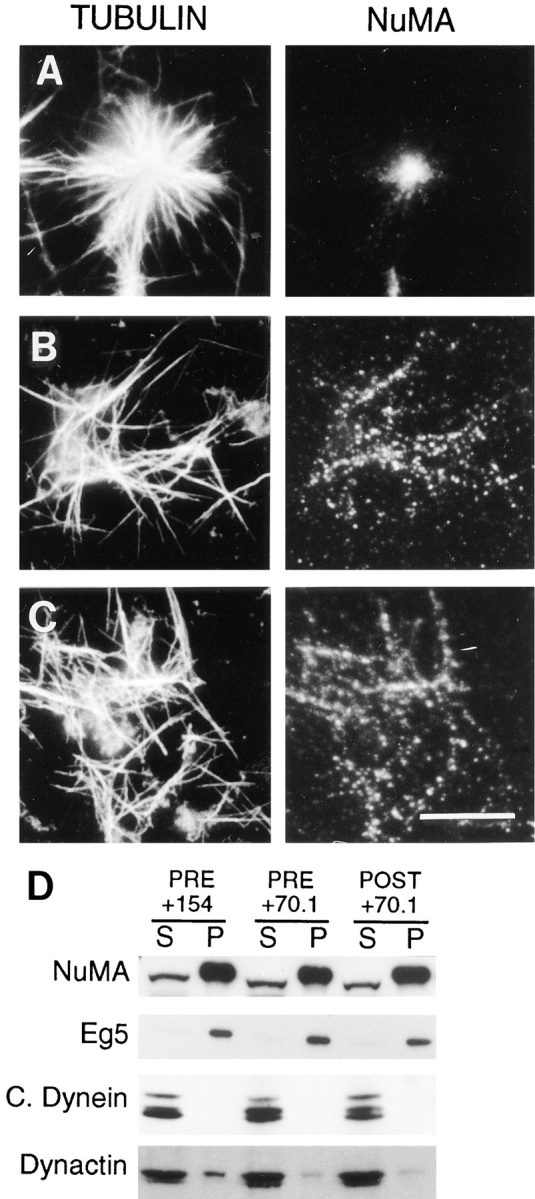
The dynein-specific 70.1 antibody disrupts both the formation and maintenance of mitotic asters assembled in a cell-free mitotic extract. The control antibody (A) and the dynein-specific 70.1 antibody (B and C) were added to a HeLa cell mitotic extract either before (A and B) or after (C) the induction of mitotic aster assembly by the addition of taxol and incubation at 30°C. After incubation, a portion of the sample was fixed and processed for immunofluorescence microscopy (A–C) using antibodies specific for tubulin and NuMA as indicated. The remainder of the sample, in which either the control antibody (154) or the dynein-specific antibody (70.1) were added before (PRE) or after (POST) mitotic aster assembly, was separated into 10,000-g soluble (S) and insoluble (P) fractions. These fractions were subjected to immunoblot analysis using antibodies specific for NuMA, Eg5, cytoplasmic dynein, and dynactin as indicated (D). Bar, 10 μm.
The Dynein-specific Antibody 70.1 Reduces the Efficiency with Which Dynactin Associates with Microtubules
To determine if the presence of these antibodies in the mitotic extract altered the efficiency with which proteins known to be involved in spindle pole and aster assembly associated with microtubules, we performed immunoblot analysis on the soluble and insoluble fractions derived from the mitotic extract containing the control and dynein-specific antibodies (Fig. 5 D). The efficiency with which tubulin (data not shown), NuMA, cytoplasmic dynein, or Eg5 was converted from the soluble fraction to the insoluble fraction during the reaction was not significantly altered by the presence of the dynein-specific antibody, despite the fact that this antibody disrupted the formation of mitotic asters. This was true whether the antibody was added before (Fig. 5 D, PRE) or after (Fig. 5 D, POST) the formation of mitotic asters. In contrast, we consistently observed a reduction in the efficiency with which dynactin associated with the microtubules in the presence of the dynein-specific antibody. Typically, 15–30% of dynactin associates with the mitotic asters in the insoluble fraction (Gaglio et al., 1996). Fig. 5 D shows that 15% of dynactin is associated with the mitotic asters in the insoluble pellet in the presence of the control antibody, but <5% of dynactin is found in the insoluble fraction in the presence of the dynein-specific antibody (Fig. 5 D; percentages determined by densitometry). The alteration in the efficiency with which dynactin associates with the microtubules in the presence of the dynein-specific antibody was not dependent on when the antibody was added to the extract, because the same result was obtained if the dynein-specific antibody was added before mitotic aster assembly or if the antibody was added to pre-assembled mitotic asters. This effect on dynactin, while indirect because the antibody is directed against cytoplasmic dynein (Fig. 1), is specific because the efficiency with which both NuMA and Eg5 associated with microtubules in the presence of this antibody was not appreciably altered. Thus, these data suggest that in addition to its direct effects on cytoplasmic dynein, this dynein-specific antibody perturbs the efficiency with which dynactin associates with microtubules in this system.
The alteration in the efficiency with which dynactin associates with microtubules in the presence of the dynein-specific antibody suggests that addition of this dynein-specific antibody to the extract may have functional consequences that are different from inactivation of cytoplasmic dynein alone. To determine if the reduction of dynactin association with microtubules induced by the addition of the dynein-specific antibody is functionally involved in the disruption of mitotic aster formation, we compared the effects of the addition of the dynein-specific antibody to the depletion of cytoplasmic dynein (Fig. 6). In this experiment, it was necessary to either deplete cytoplasmic dynein or add the 70.1 antibody to extracts depleted of the plus end-directed kinesin-related protein Eg5. The formation of mitotic asters in this system requires cytoplasmic dynein, and asters fail to organize in the absence of cytoplasmic dynein alone due to the imbalance in forces generated by microtubule motors during aster assembly. Mitotic asters will form in the absence of cytoplasmic dynein, however, if the forces generated by microtubule motors are partially equilibrated by the simultaneous depletion of the plus end-directed motor Eg5 (Gaglio et al., 1996). Thus, if the depletion of cytoplasmic dynein is functionally equivalent to the addition of the dynein-specific 70.1 antibody, then mitotic asters should be observed after either the simultaneous depletion of cytoplasmic dynein and Eg5 or addition of the 70.1 antibody to an Eg5-depleted extract. Fig. 6 shows that in the absence of Eg5 alone, microtubules organize into astral arrays that are larger than typical mitotic asters, they lack a well formed central core, and NuMA is diffusely localized at the center (Fig. 6 B; Gaglio et al., 1996). In the absence of both Eg5 and cytoplasmic dynein, mitotic asters form that resemble those formed in the absence of Eg5 alone, although they are somewhat less well organized (Fig. 6 C; Gaglio et al., 1996). Thus, if the 70.1 antibody only affects the function of cytoplasmic dynein, then addition of the 70.1 antibody to an Eg5-depleted extract should also yield asters. Contrary to this prediction, mitotic asters did not form when the 70.1 antibody was added to an Eg5-depleted extract, while addition of the control antibody had no observable effect on the formation of astral microtubule arrays (Fig. 6, D and E). Thus, in this cell-free system the depletion of cytoplasmic dynein is not functionally equivalent to the addition of this dynein-specific antibody. The most likely explanation for this difference is that the dynein-specific antibody both directly affects cytoplasmic dynein and indirectly affects dynactin such that the presence of the 70.1 antibody is analogous to the simultaneous disruption of dynein and dynactin. While we can not rule out the possibility that this antibody has additional deleterious effects beyond the perturbation of cytoplasmic dynein and dynactin, this interpretation is consistent with our previous results showing that the depletion of dynactin is more deleterious to mitotic aster formation than the depletion of cytoplasmic dynein (Gaglio et al., 1996).
Figure 6.
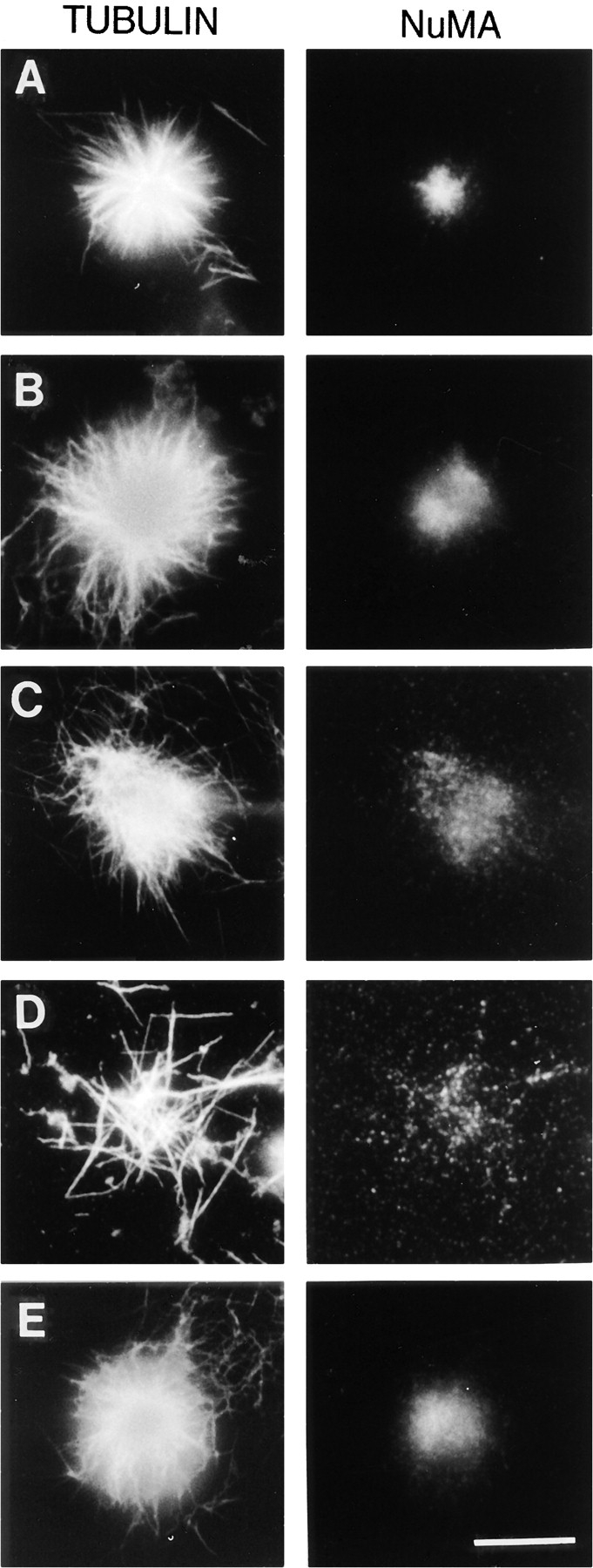
The addition of mAb 70.1 to the cell-free mitotic aster assembly system is more deleterious to mitotic aster assembly than the depletion of cytoplasmic dynein. The cell-free HeLa mitotic extract was depleted using either a preimmune antibody (A) or an Eg5-specific antibody (B–E). The Eg5-depleted samples were further treated by either the depletion of cytoplasmic dynein (C) or the addition of the dynein-specific (D) or control (E) antibodies. After the induction of mitotic aster assembly under these conditions, the samples were fixed and processed for immunofluorescence microscopy using antibodies specific for tubulin and NuMA as indicated. Bar, 10 μm.
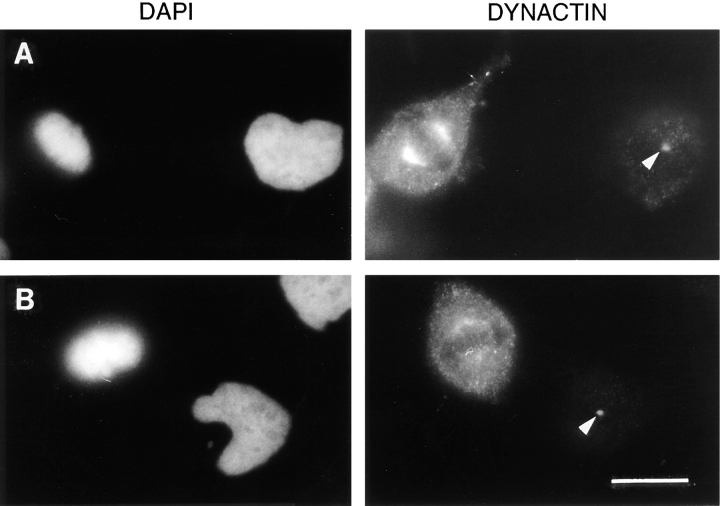
Finally, to determine if the association of dynactin with the mitotic spindle was also altered in vivo by the presence of the 70.1 antibody, we microinjected this dynein-specific antibody into metaphase cells and performed immunofluorescence microscopy with a dynactin-specific antibody (45A, which recognizes the Arp1 subunit; Fig. 7). In metaphase cells that have been microinjected with the control antibody, dynactin is localized in a crescent-like pattern concentrated at the polar regions of the spindle as well as throughout the cytoplasm, consistent with previously published reports (Fig. 7 A; Gill et al., 1991). In contrast, the staining intensity for dynactin at the spindle poles is significantly reduced after the microinjection of the dynein-specific antibody (Fig. 7 B). The staining intensity for dynactin at centrosomes of adjacent, uninjected cells is equivalent, indicating that the staining efficiency was similar for the two samples (Fig. 7, A and B, arrowheads). Thus, while this result is difficult to quantitate, it indicates that the dynein-specific antibody is affecting the efficiency with which dynactin associates with the mitotic spindle in vivo, consistent with our observations using the cell-free system for mitotic aster assembly. The disruption of both cytoplasmic dynein and dynactin by the dynein-specific 70.1 antibody suggests that cytoplasmic dynein and dynactin associate with microtubules in a cooperative manner, which is consistent with the following: the current models for the interaction of dynein and dynactin with microtubules (Allan 1996; Schroer et al., 1996); the original functional characterization of dynactin (Schroer and Sheetz, 1991); the fact that both dynein and dynactin have microtubule binding domains (Waterman-Storer et al., 1995); our previous data showing that the efficiency with which cytoplasmic dynein associates with microtubules in a cell-free mitotic extract can be modulated by manipulating the quantity of dynactin in the extract (Gaglio et al., 1996); and the reduction in the association of cytoplasmic dynein with mitotic structures following the disruption of dynactin (Echeverri et al., 1996). This interpretation offers the most likely explanation for the discrepancy between the present data and the results of Vaisberg et al. (1993), who previously investigated the role of cytoplasmic dynein in the assembly of the mitotic spindle by microinjection of an antibody directed against the cytoplasmic dynein heavy chain. Their antibody, while inhibitory to dynein-mediated motility in vitro, is not known to inhibit dynein-mediated motility in vivo and would not be predicted to disrupt the interaction of dynein with dynactin, which is mediated by the 74-kD light intermediate chain of dynein (Karki and Holzbaur, 1995; Vaughan and Vallee, 1995). The 70.1 antibody, on the other hand, has effects on both cytoplasmic dynein and dynactin, implying that cytoplasmic dynein and dynactin act as a functionally relevant unit that may have structural activities in addition to minus end-directed motor activity.
Figure 7.
The dynein-specific 70.1 antibody causes a reduction in the efficiency with which dynactin associates with the mitotic spindle in vivo. Monkey CV-1 cells in metaphase with bipolar mitotic spindles were selected by phase contrast microscopy and microinjected with either a control antibody (A) or the dynein-specific 70.1 monoclonal antibody (B). The cells were then fixed and processed for immunofluorescence microscopy using the DNA-specific dye DAPI and the 45A antibody, which is specific for the Arp1 subunit of dynactin as indicated. The arrowheads indicate centrosomal staining for dynactin in adjacent uninjected cells, which verifies that these two samples were stained equivalently. Bar, 20 μm.
Discussion
The data presented here indicate that microtubule minus ends located at mitotic spindle poles in vertebrate somatic cells are organized in a complex manner requiring contributions from both the centrosomes and noncentrosomal protein components. Centrosomes are essential to nucleate microtubules in somatic cells (McIntosh, 1983; Mazia, 1984; Maniotis and Schliwa, 1991; Zhang and Nickals, 1995a,b). Contrary to the traditional view that the formation of a spindle pole in somatic cells is a consequence of this nucleation event, we demonstrate here that the noncentrosomal proteins cytoplasmic dynein and dynactin are also required for both the formation and maintenance of the organization of microtubule minus ends at the mitotic spindle pole. Disruption of their activities using the dynein-specific 70.1 monoclonal antibody leads to the splaying of microtubule minus ends and disruption of the connection between the centrosome and the body of the mitotic spindle. This result underscores the concept that the mitotic spindle pole is not synonymous with a mitotic aster nucleated from a centrosome, but that the spindle pole is a specialized entity of noncentrosomal components that is superimposed onto the centrosomally nucleated microtubule aster.
Based on these data, we propose that mitotic spindle formation in somatic cells proceeds through a search-capture-focus mechanism (Fig. 8). This model expands on the search-capture model (Kirschner and Mitchison, 1986) and begins with the nucleation of microtubule asters from centrosomes. The plus ends of these microtubules “search” the cytoplasm by rapidly converting between growing and shrinking states and are “captured” and stabilized by kinetochores. At some point during the search and capture process microtubules are released (or severed) from the centrosome, and these microtubules become “focused” by structural and motor proteins into a spindle pole with their free minus ends near the centrosome (Fig. 8). The centrosome remains tethered to this newly focused array through a lateral interaction between microtubules within this array and astral microtubules that continue to emanate from the centrosome (Fig. 8).
Figure 8.
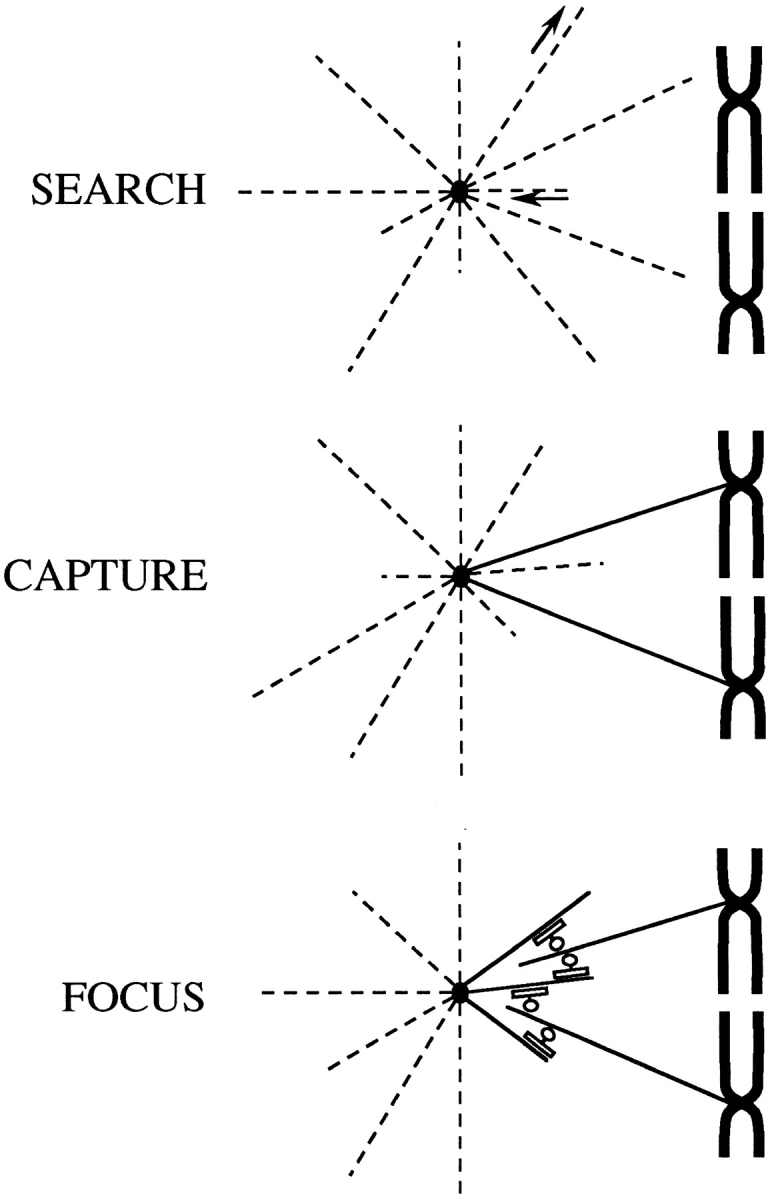
The search-capture-focus model for mitotic spindle assembly. Microtubules in somatic cells are nucleated from centrosomes that form symmetrical mitotic asters. These microtubules are relatively unstable (dashed lines) and “search” the cytoplasm by continuously converting between growing and shrinking states (arrows). Occasionally a microtubule plus end will contact a kinetochore and be “captured” and stabilized (solid lines). At some point during the search and capture events, some of the microtubules will release from the centrosome, resulting in free microtubule minus ends. These free microtubule minus ends are “focused” at the spindle pole by noncentrosomal proteins, including cytoplasmic dynein, dynactin, NuMA, Eg5, and a minus end–directed kinesin-related protein. The centrosome is tethered to this focused group of microtubules by the lateral interaction of microtubules within this array and astral microtubules that emanate from the centrosome.
This search-capture-focus model for spindle assembly accounts for both our observations of spindle pole formation in somatic cells (i.e., centrosomal spindles) as well as spindle pole formation in systems such as plants and some meiotic cells that assemble spindles in the absence of centrosomes. In acentrosomal meiotic systems, microtubules appear to be nucleated from free γ-tubulin ring complexes (Zheng et al., 1996). These short microtubules associate randomly with chromatin in disorganized arrays and are then organized into parallel bundles (Steffen et al., 1986; Theurkauf and Hawley, 1992; Heald et al., 1996). In somatic cells, microtubules are nucleated from γ-tubulin ring complexes that are associated with centrosomes (Moritz et al., 1996), which establishes an oriented microtubule array (mitotic asters) with microtubule plus ends that search the cytoplasm and are captured by kinetochores. Despite these differences in the initial search and capture stages of spindle formation, both systems appear to use a common mechanism to “focus” the free microtubule minus ends into spindle poles. The parallel bundles of microtubules in acentrosomal spindles are focused at their minus ends (Theurkauf and Hawley, 1992; Matthies et al., 1996; Merdes et al., 1996; Heald et al., 1996), while in centrosomal spindles free microtubule minus ends are focused onto the astral microtubule array. This process of focusing free microtubule minus ends is much more pronounced in acentrosomal meiotic systems, because unlike mitotic systems in which microtubules are inherently focused from centrosomes, microtubules in acentrosomal meiotic systems have no organization before the focusing activity exerted by the noncentrosomal components.
According to this search-capture-focus model for spindle assembly it may be possible to separate the microtubule nucleating activity associated with centrosomes in somatic cells from the focusing activity exerted by the noncentrosomal structural and motor proteins. In fact, this possibility has already been confirmed under a variety of different experimental conditions. First, there are several reports in the literature that centrosomes have detached from the body of the spindle while the microtubule minus ends remain focused in a pole (Rieder and Hard, 1990; Mitchison and Salmon, 1992; Murray et al., 1996). These poles are functional because they support chromosome segregation, and in one case it was observed that the microtubule turnover associated with poleward microtubule flux continued to converge towards this pole (Mitchison and Salmon, 1992). Second, centrosomes can be mechanically detached from the body of the mitotic spindle at metaphase, and the minus ends of the mitotic spindle remain focused; and in some cases the chromosomes still migrate toward that focused collection of microtubule minus ends despite the removal of the centrosome (Hiramoto and Nakano, 1988; Nicklas, 1989; Nicklas et al., 1989). Third, in rare cases where microtubules associate with chromosomes in the absence of centrosomes and/or centrioles in cultured cells (Brenner et al., 1977; Keyer et al., 1984; Debec et al., 1995), or if the requirement for centrosomes in microtubule nucleation is bypassed by the addition of taxol both in vivo during mitosis (DeBrabander et al., 1981) and in vitro in mitotic extracts (Gaglio et al., 1995, 1996), then microtubules are still organized into astral and polar arrays. The focusing of microtubule minus ends under these conditions is likely the manifestation of the microtubule minus end focusing activity exerted by the noncentrosomal components. Fourth, electron microscopy has shown that many microtubule minus ends within both mitotic and centrosome-containing meiotic spindles are not associated with centrosomes (Wolf and Bastmeyer, 1991; Mastronarde et al., 1993). Fifth, microtubule release from centrosomes has been documented in cell-free extracts (Belmont et al., 1990) as well as under nonmitotic circumstances in living cells (Keating et al., 1997). Finally, free microtubule minus ends within the mitotic spindle are necessary for tubulin subunit loss during poleward microtubule flux (Mitchison, 1989b ). Taken together, these results clearly discriminate between the processes of microtubule nucleation and minus end focusing during mitosis in somatic cells, and demonstrate that noncentrosomal structural and motor proteins will focus microtubule minus ends independently of centrosomes in somatic cells through a mechanism that is probably related to spindle pole formation in acentrosomal systems.
Finally, prevailing evidence indicates that the mechanism for focusing free microtubule minus ends into spindle poles in both centrosomal and acentrosomal spindles is driven by a common group of noncentrosomal accessory proteins including NuMA, cytoplasmic dynein, dynactin, Eg5, and a minus end-directed kinesin-related protein (Verde et al., 1991; Sawin et al., 1993; Endow et al., 1994; Gaglio et al., 1995, 1996; Blangy et al., 1996; Heald et al., 1996; Matthies et al., 1996; Merdes et al., 1996; Walczak et al., 1997). Experimental data indicate that all of these proteins participate in the organization of spindle poles in both centrosomal and acentrosomal systems. Despite the complex nature of this process and the involvement of numerous components, recent evidence suggests that a trimolecular complex composed of NuMA, cytoplasmic dynein, and dynactin may be crucial for focusing free microtubule minus ends at spindle poles. These three proteins form a stable complex in extracts prepared from Xenopus eggs, and this complex of proteins is essential for the organization of spindle poles in that system (Merdes et al., 1996). While no evidence exists for a stable complex between these proteins in extracts prepared from somatic cells, we show that NuMA fails to concentrate near microtubule minus ends in the absence of dynein and/or dynactin under both in vitro and in vivo conditions, consistent with a dynein/dynactin-dependent movement of NuMA to microtubule minus ends (Figs. 3 and 5, and Gaglio et al., 1996). Indeed, in a striking group of experiments, the perturbation of either cytoplasmic dynein (mAb 70.1 microinjection; present work), dynactin (dynamitin over expression; Echeverri et al., 1996; Gaglio et al., 1996), or NuMA (antibody microinjection; Gaglio et al., 1995) in cultured cells all produced a similar effect on the spindle pole organization characterized by splaying of microtubule minus ends and detachment of centrosomes. Given that the interaction of NuMA with cytoplasmic dynein and dynactin is mitosis-specific in somatic cells (NuMA is nuclear during interphase), it is possible that NuMA confers a unique mitosis-specific property to the minus end-directed motor activity of cytoplasmic dynein and dynactin, which contributes to the essential function of this trimolecular complex during spindle formation.
In the end, we speculate that free microtubule minus ends are necessary for proper spindle function, because they are necessary for the mechanism of poleward microtubule flux, which exerts force through the spindle (Waters et al., 1996) and (depending on the cell type) contributes to poleward chromosome movement (Salmon, 1992; Wilson et al., 1994; Mitchison and Salmon, 1992; Zhai et al., 1995). Free microtubule minus ends are inherently produced in acentrosomal spindles, whereas in centrosomal spindles they must be generated by microtubule release from centrosomes. In both cases, a common pole-forming activity focuses the free microtubule ends. In somatic cells, these free microtubule minus ends that are obligatory to poleward microtubule flux remain attached to the astral microtubules emanating from the centrosome, thus allowing the centrosome-associated astral array to convey positional cues derived from the cell cortex to the body of the spindle.
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM51542) and American Cancer Society (JFRA-635).
Footnotes
1. Abbreviation used in this paper: NuMA, nuclear mitotic apparatus.
Please address all correspondence to Duane A. Compton, Department of Biochemistry, Dartmouth Medical School, Hanover, NH 03755. Tel.: (603) 650-1990; Fax: (603) 650-1128; e-mail: duane.a.compton@dartmouth.edu
T. Gaglio dedicates this paper to his parents Evelio and Josefina. The authors would like to thank Dr. Tim Mitchison and his lab (University of California, San Francisco, CA) for being so generous with their Eg5 antibody, Dr. Yixian Zheng (Carnegie Institute of Washington, Wash. DC) for donating the γ-tubulin antibody, and Dr. Trina Schroer (Johns Hopkins University, Baltimore, MD) for donating the dynactin-specific antibodies. We would also like to recognize the tolerance of Leslie Henderson and Bob Maue during microinjections and thank Alejandro Saredi, Vicki Mountain, Arijit Chakravarty, and Roger Sloboda for stimulating discussion during the preparation of this manuscript.
References
- Allan V. Motor proteins: a dynamic duo. Curr Biol. 1996;6:630–633. doi: 10.1016/s0960-9822(09)00434-5. [DOI] [PubMed] [Google Scholar]
- Bastmeyer M, Steffen W, Fuge H. Immunostaining of spindle components in tipulid spermatocytes using a serum against pericentriolar material. Eur J Cell Biol. 1986;42:305–310. [PubMed] [Google Scholar]
- Belmont LD, Hyman AA, Sawin KE, Mitchison TJ. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990;62:579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Blangy A, Lane HA, d'Herin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Blose SH, Melzer DI, Feramisco JR. 10-nm filaments are induced to collapse in living cells microinjected with monoclonal and polyclonal antibodies against tubulin. J Cell Biol. 1984;98:847–858. doi: 10.1083/jcb.98.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S, Branch A, Meredith S, Berns MW. The absence of centrioles from spindle poles of rat kangaroo (PtK2) cells undergoing meiotic-like reduction division in vitro. J Cell Biol. 1977;72:368–379. doi: 10.1083/jcb.72.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B, Gerace L. A cell free system to study reassembly of the nuclear envelope at the end of mitosis. Cell. 1986;44:639–652. doi: 10.1016/0092-8674(86)90273-4. [DOI] [PubMed] [Google Scholar]
- Capecchi MR. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980;22:479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Compton DA, Cleveland DW. NuMA is required for the proper completion of mitosis. J Cell Biol. 1993;120:947–957. doi: 10.1083/jcb.120.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DA, Yen TJ, Cleveland DW. Identification of novel centromere/kinetochore-associated proteins using monoclonal antibodies generated against human mitotic chromosome scaffolds. J Cell Biol. 1991;112:1165–1175. doi: 10.1083/jcb.112.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debec A, Détraves C, Montmory C, Géraud G, Wright M. Polar organization of γ-tubulin in acentriolar mitotic spindles of Drosophila melanogastercells. J Cell Sci. 1995;108:2645–2653. doi: 10.1242/jcs.108.7.2645. [DOI] [PubMed] [Google Scholar]
- DeBrabander M, Geuens G, Nuydens R, Willebrords R, DeMey J. Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc Natl Acad Sci USA. 1981;78:5608–5612. doi: 10.1073/pnas.78.9.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman JF, III, Pfister KK. Differential phosphorylation in vivo of cytoplasmic dynein associated with anterogradely moving organelles. J Cell Biol. 1994;127:1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow SA, Chandra R, Komma DJ, Yamamoto AH, Salmon ED. Mutants of the Drosophilancd microtubule motor protein cause centrosomal and spindle pole defects in mitosis. J Cell Sci. 1994;107:859–867. doi: 10.1242/jcs.107.4.859. [DOI] [PubMed] [Google Scholar]
- Gaglio T, Saredi A, Compton DA. NuMA is required for the organization of microtubules into aster-like mitotic arrays. J Cell Biol. 1995;131:693–708. doi: 10.1083/jcb.131.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T, Saredi A, Bingham JB, Hasbani MJ, Gill SR, Schroer TA, Compton DA. Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J Cell Biol. 1996;135:399–414. doi: 10.1083/jcb.135.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SR, Schroer TA, Szilak I, Steuer ER, Sheetz MP, Cleveland DW. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden JH, Bowser SS, Rieder CL. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J Cell Biol. 1990;111:1039–1045. doi: 10.1083/jcb.111.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindle around artificial chromosomes in Xenopusegg extracts. Nature (Lond) 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Hiramoto Y, Nakano Y. Micromanipulation studies of the mitotic apparatus in sand dollar eggs. Cell Motil Cytoskel. 1988;10:172–184. doi: 10.1002/cm.970100122. [DOI] [PubMed] [Google Scholar]
- Holy TE, Leibler S. Dynamic instability of microtubule as an efficient way to search in space. Proc Natl Acad Sci USA. 1994;91:5682–5685. doi: 10.1073/pnas.91.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, Karsenti E. Morphogenetic properties of microtubules and mitotic spindle assembly. Cell. 1996;84:401–410. doi: 10.1016/s0092-8674(00)81285-4. [DOI] [PubMed] [Google Scholar]
- Inoué S, Salmon ED. Force generation by microtubule assembly/ disassembly in mitosis and related movements. Mol Biol Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S, Holzbaur ELF. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- Keating TJ, Peloquin JG, Rodionov VI, Momcilovic D, Borisy GG. Microtubule release from the centrosome. Proc Natl Acad Sci USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyer G, Ris H, Borisy GG. Centriole distribution during tripolar mitosis in chinese hamster ovary cells. J Cell Biol. 1984;98:2222–2229. doi: 10.1083/jcb.98.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M, Mitchison TJ. Beyond self-assembly: from microtubule to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Lohka MJ, Maller JL. Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J Cell Biol. 1985;101:518–523. doi: 10.1083/jcb.101.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis A, Schliwa M. Microsurgical removal of centrosomes blocks cell reproduction and centriole generation in BSC-1 cells. Cell. 1991;67:495–504. doi: 10.1016/0092-8674(91)90524-3. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN, McDonald KL, Ding R, McIntosh JR. Interpolar spindle microtubules in PTK cells. J Cell Biol. 1993;123:1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies HJG, McDonald HB, Goldstein LSB, Theurkauf WE. Anastral meiotic spindle morphogenesis: role of the non-claret disjunctional kinesin-like protein. J Cell Biol. 1996;134:455–464. doi: 10.1083/jcb.134.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D. Centrosomes and mitotic poles. Exp Cell Res. 1984;153:1–15. doi: 10.1016/0014-4827(84)90442-7. [DOI] [PubMed] [Google Scholar]
- McIntosh JR. The centrosome as organizer of the cytoskeleton. Mod Cell Biol. 1983;2:115–142. [Google Scholar]
- McIntosh JR, Koonce MP. The mitotic spindle. Science (Wash DC) 1989;246:622–628. doi: 10.1126/science.2683078. [DOI] [PubMed] [Google Scholar]
- McKim KS, Hawley RS. Chromosomal control of meiotic cell division. Science (Wash DC) 1995;270:1595–1601. doi: 10.1126/science.270.5242.1595. [DOI] [PubMed] [Google Scholar]
- Merdes A, Ramyar K, Vechio JD, Cleveland DW. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–458. doi: 10.1016/s0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ. Mitosis: basic concepts. Curr Opin Cell Biol. 1989a;1:67–74. doi: 10.1016/s0955-0674(89)80039-0. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol. 1989b;109:637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Salmon ED. Poleward kinetochore fiber movement occurs during both metaphase and anaphase-A in newt lung cell mitosis. J Cell Biol. 1992;119:569–582. doi: 10.1083/jcb.119.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA. Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature (Lond) 1995;378:637–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Murray AW, Desai AB, Salmon ED. Real time observation of anaphase in vitro. Proc Natl Acad Sci USA. 1996;93:12327–12332. doi: 10.1073/pnas.93.22.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB. The motor for poleward chromosome movement in anaphase is in or near the kinetochore. J Cell Biol. 1989;109:2245–2255. doi: 10.1083/jcb.109.5.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas RB. How cells get the right chromosomes. Science (Wash DC) 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- Nicklas RB, Lee GM, Rieder CL, Rupp G. Mechanically cut mitotic spindles: clean cuts and stable microtubules. J Cell Sci. 1989;94:415–423. doi: 10.1242/jcs.94.3.415. [DOI] [PubMed] [Google Scholar]
- Niclas J, Allan VJ, Vale RD. Cell cycle regulation of dynein association with membranes modulates microtubule-based organelle transport. J Cell Biol. 1996;133:585–593. doi: 10.1083/jcb.133.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal BM, Mikami A, Pfister KK, Vallee RB. Homology of the 74-kD cytoplasmic dynein subunit with a flagellar dynein polypeptide suggests an intracellular targeting function. J Cell Biol. 1992;118:1133–1143. doi: 10.1083/jcb.118.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr CM, Cove M, Grissom PM, Hayes TS, Porter ME, McIntosh JR. Cytoplasmic dynein is localized to kinetochores during mitosis. Nature (Lond) 1991;345:263–265. doi: 10.1038/345263a0. [DOI] [PubMed] [Google Scholar]
- Pfister KK, Salata MW, Dillman JF, III, Vaughan KT, Vallee RB, Torre E, Lye RJ. Differential expression and phosphorylation of the 74-kDa intermediate chains of cytoplasmic dynein in cultured neurons and glia. J Biol Chem. 1996;271:1687–1694. doi: 10.1074/jbc.271.3.1687. [DOI] [PubMed] [Google Scholar]
- Rieder CL. Mitosis: towards a molecular understanding of chromosome behavior. Curr Opin Cell Biol. 1991;3:59–66. doi: 10.1016/0955-0674(91)90166-v. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Hard R. Newt lung epithelial cells: cultivation, use, and advantages for biomedical research. Int Rev Cell Biol. 1990;122:153–220. doi: 10.1016/s0074-7696(08)61208-5. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Salmon ED. Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. J Cell Biol. 1995;124:223–233. doi: 10.1083/jcb.124.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder, C.L., J.G. Ault, U. Eicenlaub-Ritter, and G. Sluder. 1993. Morphogenesis of the mitotic and meiotic spindle: conclusions obtained from one system are not necessarily applicable to the other. In Chromosome Segregation and Aneuploidy. B.K. Vig and A. Kappas, editors. Springer-Verlag, New York. 183–197.
- Sawin KE, Mitchison TJ. Mitotic spindle assembly by two different pathways in vitro. J Cell Biol. 1991;112:925–940. doi: 10.1083/jcb.112.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, LeFuellec K, Philippe M, Mitchison TJ. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature (Lond) 1992;359:540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- Schafer DA, Gill SR, Cooper JA, Heuser JE, Schroer TA. Ultrastructural analysis of the dynactin complex: an actin-related protein is a component of a filament that resembles F-actin. J Cell Biol. 1994;126:403–412. doi: 10.1083/jcb.126.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader, F. 1953. Mitosis, the Movements of Chromosomes in Cell Division. 2nd edition. Columbia Univ. Press, New York. 170 pp.
- Schroer TA, Sheetz MP. Two activators of microtubule-based vesicle transport. J Cell Biol. 1991;115:1309–1318. doi: 10.1083/jcb.115.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer TA, Bingham JB, Gill SR. Actin related protein 1 and cytoplasmic dynein-based motility—what's the connection? . Trends Cell Biol. 1996;6:212–215. doi: 10.1016/0962-8924(96)20014-5. [DOI] [PubMed] [Google Scholar]
- Steuer ER, Schroer TA, Wordemann L, Sheetz MP. Cytoplasmic dynein localizes to mitotic spindles and kinetochores. Nature (Lond) 1991;345:266–268. doi: 10.1038/345266a0. [DOI] [PubMed] [Google Scholar]
- Steffen W, Fuge H, Dietz R, Bastmeyer M, Müller G. Aster-free spindle poles in insect spermatocytes: evidence for chromosome-induced spindle formation. J Cell Biol. 1986;102:1679–1687. doi: 10.1083/jcb.102.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf WE, Hawley RS. Meiotic spindle assembly in Drosophilafemales: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-related protein. J Cell Biol. 1992;116:1167–1180. doi: 10.1083/jcb.116.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisberg EA, Grissom PM, McIntosh JR. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, K.T., and R.B. Vallee. 1995. Cytoplasmic dynein bind dynactin through a direct interaction between the intermediate chains and p150Glued. J. Cell Biol. 131:1507–1516. [DOI] [PMC free article] [PubMed]
- Verde F, Berrez J-M, Antony C, Karsenti E. Taxol-induced microtubule asters in mitotic extracts of Xenopuseggs: requirement for phosphorylated factors and cytoplasmic dynein. J Cell Biol. 1991;112:1177–1187. doi: 10.1083/jcb.112.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernos I, Karsenti E. Chromosomes take the lead in spindle assembly. Trends Cell Biol. 1995;5:297–301. doi: 10.1016/s0962-8924(00)89045-5. [DOI] [PubMed] [Google Scholar]
- Walczak CE, Verma S, Mitchison TJ. XCTK2: a kinesin-related protein that promotes mitotic spindle assembly in Xenopus laevisegg extracts. J Cell Biol. 1997;136:859–870. doi: 10.1083/jcb.136.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Karki S, Holzbaur E. The p150Gluedcomponent of the dynactin complex binds to both microtubules and the actin related protein centractin (Arp-1) Proc Natl Acad Sci USA. 1995;92:1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JC, Salmon ED. Pathways of spindle assembly. Curr Opin Cell Biol. 1997;9:37–43. doi: 10.1016/s0955-0674(97)80149-4. [DOI] [PubMed] [Google Scholar]
- Waters JC, Mitchison TJ, Rieder CL, Salmon ED. The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work. Mol Biol Cell. 1996;7:1547–1558. doi: 10.1091/mbc.7.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, E.B. 1925. The cell in development and inheritance. 2nd edition. Macmillan Co., New York. 483 pp.
- Wilson PJ, Forer A, Leggiadro C. Evidence that kinetochore microtubules in crane-fly spermatocytes disassemble during anaphase primarily at the poleward end. J Cell Sci. 1994;107:3015–3027. doi: 10.1242/jcs.107.11.3015. [DOI] [PubMed] [Google Scholar]
- Wolf KW, Bastmeyer M. Cytology of Lepidoptera. V. The microtubule cytoskeleton in eupyrene spermatocytes of Ephestia kuehniella (pyralidae, Inachis io (Nymphalidae) and Orgyia antiqua(Lymantriidae) Eur J Cell Biol. 1991;55:225–237. [PubMed] [Google Scholar]
- Zhai Y, Kronebusch PJ, Borisy GG. Kinetochore microtubule dynamics and the metaphase-anaphase transition. J Cell Biol. 1995;131:721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Nicklas RB. The impact of chromosomes and centrosomes on spindle assembly as observed in living cells. J Cell Biol. 1995a;129:1287–1300. doi: 10.1083/jcb.129.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Nicklas RB. Chromosomes initiate spindle assembly upon experimental dissolution of the nuclear envelope in grasshopper spermatocytes. J Cell Biol. 1995b;131:1125–1131. doi: 10.1083/jcb.131.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wong ML, Alberts B, Mitchison TJ. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature (Lond) 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]