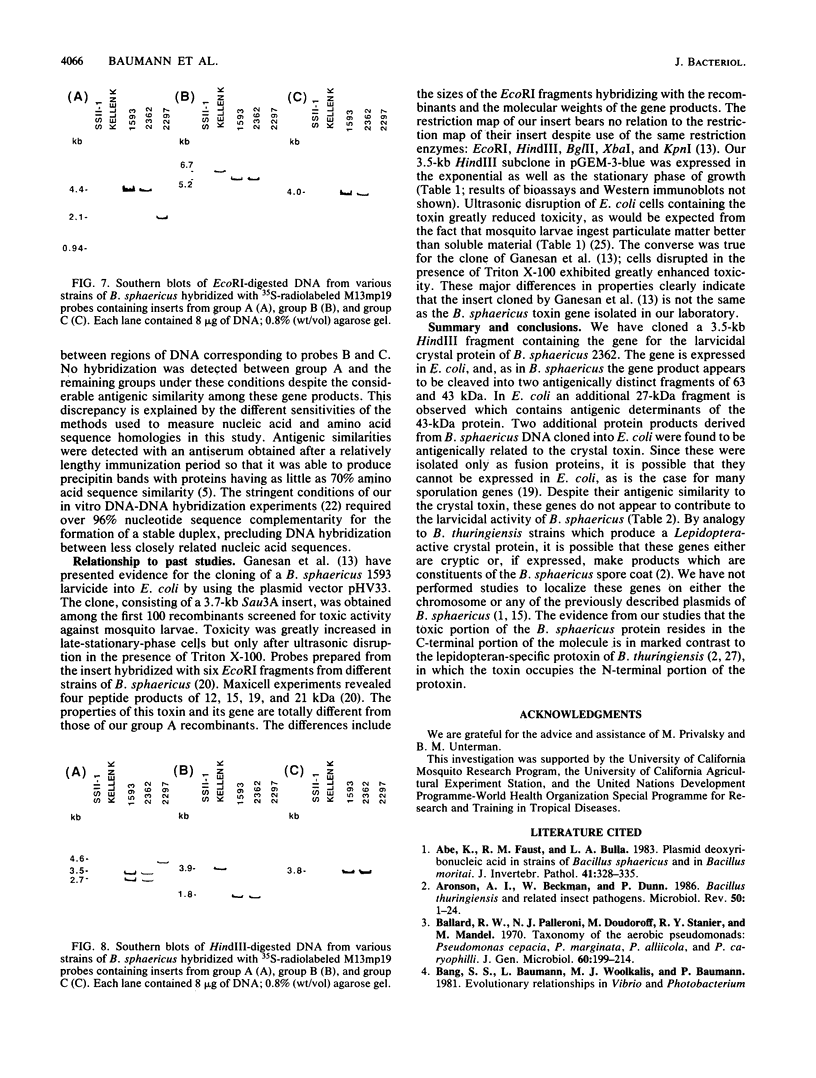
Abstract
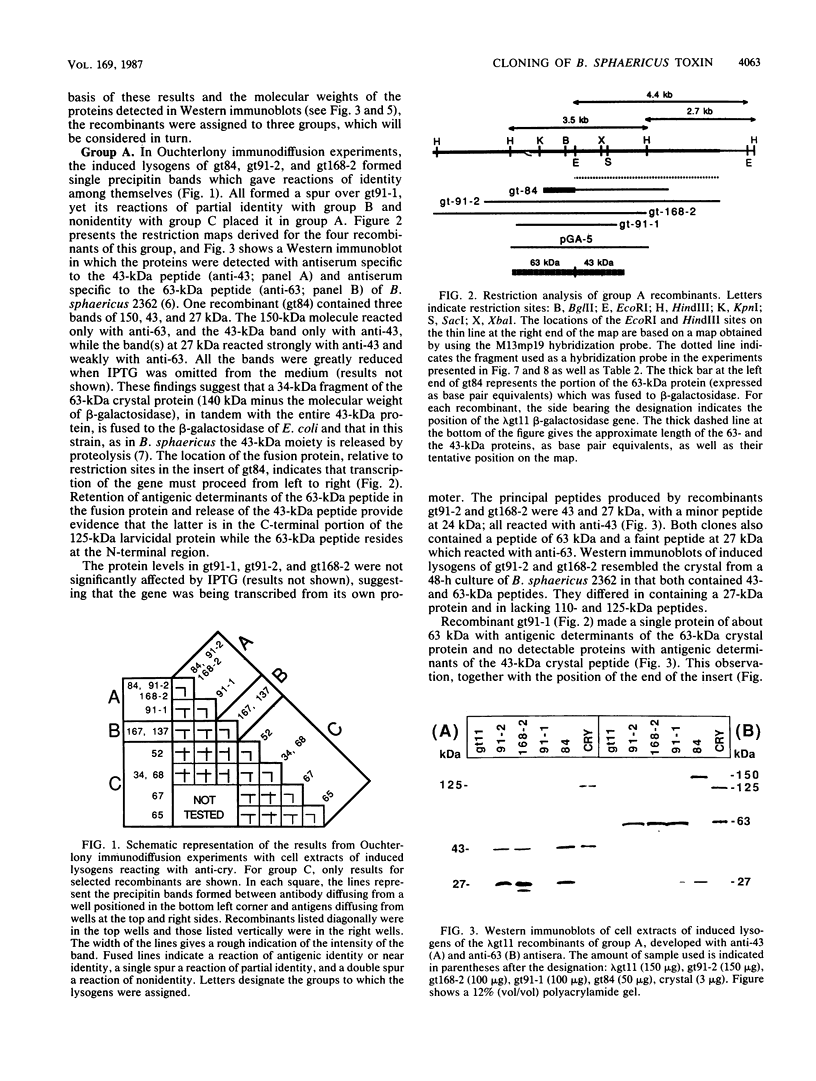
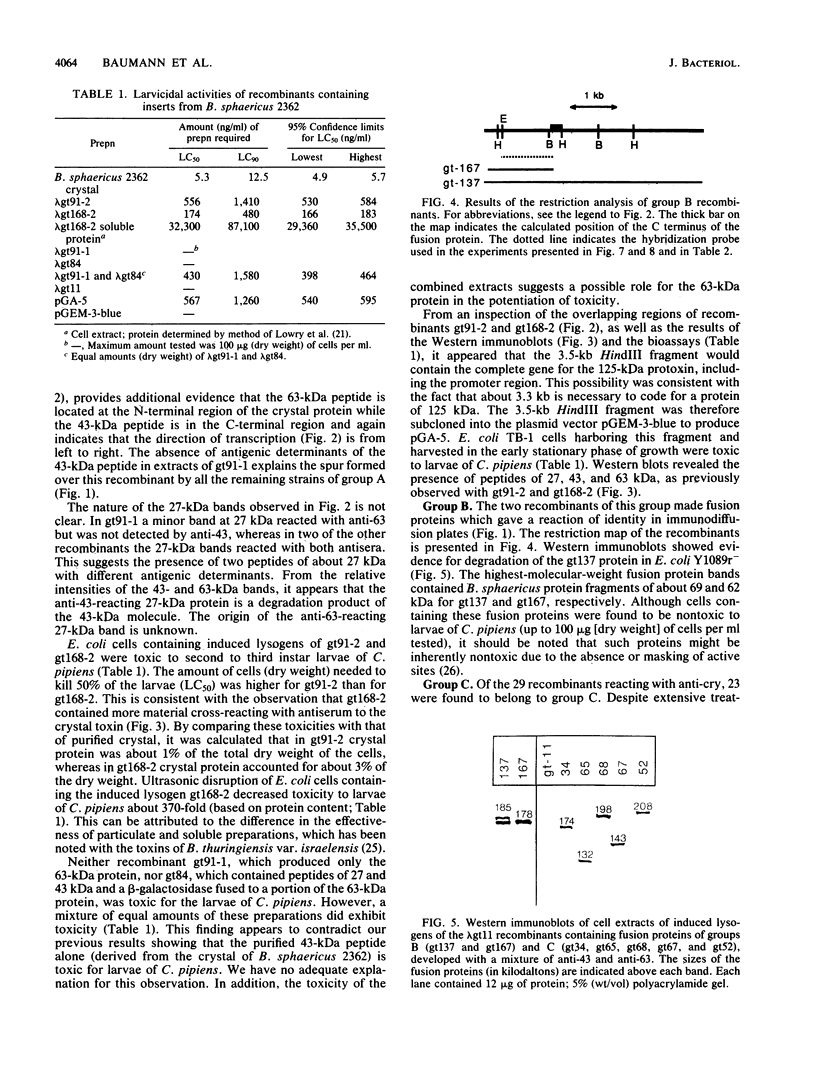
During sporulation, Bacillus sphaericus 2362 produces a parasporal crystalline protein which is toxic for the larvae of a number of mosquito species. Using the Escherichia coli cloning vector lambda gt11, in which gene products of the inserts may be fused to beta-galactosidase, we isolated 29 bacteriophages which produced peptides-reacting with antiserum to crystal protein. On the basis of restriction enzyme analyses of the recombinants and Ouchterlony immunodiffusion experiments with induced lysogens as a source of antigens, the recombinants were assigned to three groups, designated A, B, and C. Group A consisted of three clones which appeared to express all or part of the B. sphaericus toxin gene from their own promoters and one clone producing a beta-galactosidase-toxin fusion protein. The host cells of two induced recombinant lysogens of this group were toxic to larvae of Culex pipiens. A cell suspension containing 174 ng (dry weight) of the more toxic recombinant per ml killed 50% of the larvae. Both recombinants formed peptides with molecular sizes of 27, 43, and 63 kilodaltons (kDa). The antigenically related 27- and 43-kDa peptides were distinct from the 63-kDa peptide, which resembled crystals from sporulating cells of B. sphaericus in which antigenically distinct 43- and 63-kDa proteins are derived from a 125-kDa precursor. A 3.5-kilobase HindIII fragment from recombinants having toxic activity against larvae was subcloned into pGEM-3-blue. E. coli cells harboring this fragment were toxic to mosquito larvae and produced peptides of 27, 43, and 63 kDa. The distribution of the A gene among strains of B. sphaericus of different toxicities suggested that it is the sole or principal gene encoding the larvicidal crystal protein. The two recombinants of group B and the 23 of group C were all beta-galactosidase fusion proteins, suggesting that in E. coli these genes were not readily expressed from their own promoters. The distribution of these two genes in different strains of B. sphaericus suggested that they do not have a role in the toxicity of this species to mosquito larvae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson A. I., Beckman W., Dunn P. Bacillus thuringiensis and related insect pathogens. Microbiol Rev. 1986 Mar;50(1):1–24. doi: 10.1128/mr.50.1.1-24.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard R. W., Palleroni N. J., Doudoroff M., Stanier R. Y., Mandel M. Taxonomy of the aerobic pseudomonads: Pseudomonas cepacia, P. marginata, P. alliicola and P. caryophylli. J Gen Microbiol. 1970 Feb;60(2):199–214. doi: 10.1099/00221287-60-2-199. [DOI] [PubMed] [Google Scholar]
- Baumann P., Baumann L., Woolkalis M. J., Bang S. S. Evolutionary relationships in vibrio and Photobacterium: a basis for a natural classification. Annu Rev Microbiol. 1983;37:369–398. doi: 10.1146/annurev.mi.37.100183.002101. [DOI] [PubMed] [Google Scholar]
- Baumann P., Unterman B. M., Baumann L., Broadwell A. H., Abbene S. J., Bowditch R. D. Purification of the larvicidal toxin of Bacillus sphaericus and evidence for high-molecular-weight precursors. J Bacteriol. 1985 Aug;163(2):738–747. doi: 10.1128/jb.163.2.738-747.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell A. H., Baumann P. Proteolysis in the gut of mosquito larvae results in further activation of the Bacillus sphaericus toxin. Appl Environ Microbiol. 1987 Jun;53(6):1333–1337. doi: 10.1128/aem.53.6.1333-1337.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwell A. H., Baumann P. Sporulation-associated activation of Bacillus sphaericus larvicide. Appl Environ Microbiol. 1986 Oct;52(4):758–764. doi: 10.1128/aem.52.4.758-764.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. W. Effects of Bacillus sphaericus 1593 and 2362 spore/crystal toxin on cultured mosquito cells. J Invertebr Pathol. 1986 Jan;47(1):21–31. doi: 10.1016/0022-2011(86)90159-x. [DOI] [PubMed] [Google Scholar]
- Ganesan S., Kamdar H., Jayaraman K., Szulmajster J. Cloning and expression in Escherichia coli of a DNA fragment from Bacillus sphaericus coding for biocidal activity against mosquito larvae. Mol Gen Genet. 1983;189(1):181–183. doi: 10.1007/BF00326076. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacey L. A., Undeen A. H. Microbial control of black flies and mosquitoes. Annu Rev Entomol. 1986;31:265–296. doi: 10.1146/annurev.en.31.010186.001405. [DOI] [PubMed] [Google Scholar]
- Losick R., Youngman P., Piggot P. J. Genetics of endospore formation in Bacillus subtilis. Annu Rev Genet. 1986;20:625–669. doi: 10.1146/annurev.ge.20.120186.003205. [DOI] [PubMed] [Google Scholar]
- Louis J., Jayaraman K., Szulmajster J. Biocide gene(s) and biocidal activity in different strains of Bacillus sphaericus. Expression of the gene(s) in E. coli maxicells. Mol Gen Genet. 1984;195(1-2):23–28. doi: 10.1007/BF00332718. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Payne J. M., Davidson E. W. Insecticidal activity of the crystalline parasporal inclusions and other components of the Bacillus sphaericus 1593 spore complex. J Invertebr Pathol. 1984 May;43(3):383–388. doi: 10.1016/0022-2011(84)90084-3. [DOI] [PubMed] [Google Scholar]
- Schnell D. J., Pfannenstiel M. A., Nickerson K. W. Bioassay of solubilized Bacillus thuringiensis var. israelensis crystals by attachment to latex beads. Science. 1984 Mar 16;223(4641):1191–1193. doi: 10.1126/science.6701520. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Beckwith J. R. Uses of lac fusions for the study of biological problems. Microbiol Rev. 1985 Dec;49(4):398–418. doi: 10.1128/mr.49.4.398-418.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley H. R., Schnepf H. E. The molecular biology of parasporal crystal body formation in Bacillus thuringiensis. Annu Rev Microbiol. 1986;40:549–576. doi: 10.1146/annurev.mi.40.100186.003001. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yousten A. A. Bacillus sphaericus: microbiological factors related to its potential as a mosquito larvicide. Adv Biotechnol Processes. 1984;3:315–343. [PubMed] [Google Scholar]






